Abstract
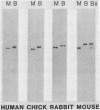
The regulation of creatine kinase (CK) induction during muscle differentiation was analyzed with MM14 mouse myoblasts. These cells withdraw from the cell cycle and commit to terminal differentiation when fed with mitogen-depleted medium. Myoblasts contained trace amounts of an isozyme of brain CK (designated BB-CK), but differentiation was accompanied by the induction of two other isozymes of muscle and brain CKs (designated MM-CK and MB-CK). Increased CK activity was detectable within 6 h of mitogen removal, 3 h after the first cells committed to differentiation and 6 h before fusion began. By 48 h, MM-CK activity increased more than 400-fold, MB-CK activity increased more than 150-fold, and BB-CK activity increased more than 10-fold. Antibodies prepared against purified mouse MM-CK cross-reacted with muscle and brain CKs (designated M-CK and B-CK, respectively) from a variety of species and were used to demonstrate that the increase in enzymatic activity was paralleled by an increase in the protein itself. CK antibodies were also used to aid in identifying cDNA clones to M-CK. cDNA sequences which corresponded to protein-coding regions cross-hybridized with B-CK mRNA; however, a subclone containing the 3'-nontranslated region was unique and was used to quantitate M-CK mRNA levels during myoblast differentiation. M-CK mRNA was not detectable in myoblasts, but within 5 to 6 h of mitogen withdrawal (6 to 7 h before fusion begins) it accumulated to about 30 molecules per cell. By 24 h, myotubes contained approximately 1,100 molecules per nucleus of M-CK mRNA.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoff S., Nadal-Ginard B. Transient induction of poly(A)-short myosin heavy chain messenger RNA during terminal differentiation of L6E9 myoblasts. J Mol Biol. 1980 Jun 25;140(2):283–298. doi: 10.1016/0022-2836(80)90106-0. [DOI] [PubMed] [Google Scholar]
- Bessman S. P., Geiger P. J. Transport of energy in muscle: the phosphorylcreatine shuttle. Science. 1981 Jan 30;211(4481):448–452. doi: 10.1126/science.6450446. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Chiu C. P., Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983 Apr;32(4):1171–1180. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Blum H. E., Deus B., Gerok W. Mitochondrial creatine kinase from human heart muscle: purification and characterization of the crystallized isoenzyme. J Biochem. 1983 Oct;94(4):1247–1257. doi: 10.1093/oxfordjournals.jbchem.a134470. [DOI] [PubMed] [Google Scholar]
- Brison O., Cambon P. A simple and efficient method to remove ribonuclease contamination from pancreatic deoxyribonuclease preparations. Anal Biochem. 1976 Oct;75(2):402–409. doi: 10.1016/0003-2697(76)90094-4. [DOI] [PubMed] [Google Scholar]
- Bulinski J. C., Kumar S., Titani K., Hauschka S. D. Peptide antibody specific for the amino terminus of skeletal muscle alpha-actin. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1506–1510. doi: 10.1073/pnas.80.6.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan A. I., Fiszman M. Y., Eppenberger H. M. Molecular and cell isoforms during development. Science. 1983 Sep 2;221(4614):921–927. doi: 10.1126/science.6348946. [DOI] [PubMed] [Google Scholar]
- Caravatti M., Minty A., Robert B., Montarras D., Weydert A., Cohen A., Daubas P., Buckingham M. Regulation of muscle gene expression. The accumulation of messenger RNAs coding for muscle-specific proteins during myogenesis in a mouse cell line. J Mol Biol. 1982 Sep;160(1):59–76. doi: 10.1016/0022-2836(82)90131-0. [DOI] [PubMed] [Google Scholar]
- Caravatti M., Perriard J. C., Eppenberger H. M. Developmental regulation of creatine kinase isoenzymes in myogenic cell cultures from chicken. Biosynthesis of creatine kinase subunits M and B. J Biol Chem. 1979 Feb 25;254(4):1388–1394. [PubMed] [Google Scholar]
- Cochet M., Perrin F., Gannon F., Krust A., Chambon P., McKnight G. S., Lee D. C., Mayo K. E., Palmiter R. Cloning of an almost full-length chicken conalbumin double-stranded cDNA. Nucleic Acids Res. 1979 Jun 11;6(7):2435–2452. doi: 10.1093/nar/6.7.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubas P., Caput D., Buckingham M., Gros F. A comparison between the synthesis of contractile proteins and the accumulation of their translatable mRNAs during calf myoblast differentiation. Dev Biol. 1981 May;84(1):133–143. doi: 10.1016/0012-1606(81)90377-8. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate accumulation of contractile protein mRNAs during myoblast differentiation. Dev Biol. 1979 Mar;69(1):202–216. doi: 10.1016/0012-1606(79)90286-0. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate regulation of contractile protein synthesis during myoblast differentiation. Cell. 1978 Apr;13(4):599–611. doi: 10.1016/0092-8674(78)90211-8. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. A practical approach for quantitating specific mRNAs by solution hybridization. Anal Biochem. 1983 Jun;131(2):385–393. doi: 10.1016/0003-2697(83)90188-4. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Perrin F., Gannon F., Palmiter R. D. Isolation and characterization of the mouse metallothionein-I gene. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6511–6515. doi: 10.1073/pnas.77.11.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dym H., Turner D. C., Eppenberger H. M., Yaffe D. Creatine kinase isoenzyme transition in actinomycin D-treated differentiating muscle cultures. Exp Cell Res. 1978 Apr;113(1):15–21. doi: 10.1016/0014-4827(78)90082-4. [DOI] [PubMed] [Google Scholar]
- EPPENBERGER H. M., EPPENBERGER M., RICHTERICH R., AEBI H. THE ONTOGENY OF CREATINE KINASE ISOZYMES. Dev Biol. 1964 Aug;10:1–16. doi: 10.1016/0012-1606(64)90002-8. [DOI] [PubMed] [Google Scholar]
- Eppenberger H. M., Dawson D. M., Kaplan N. O. The comparative enzymology of creatine kinases. I. Isolation and characterization from chicken and rabbit tissues. J Biol Chem. 1967 Jan 25;242(2):204–209. [PubMed] [Google Scholar]
- Grace A. M., Perryman M. B., Roberts R. Purification and characterization of human mitochondrial creatine kinase. A single enzyme form. J Biol Chem. 1983 Dec 25;258(24):15346–15354. [PubMed] [Google Scholar]
- Groudine M., Casimir C. Post-transcriptional regulation of the chicken thymidine kinase gene. Nucleic Acids Res. 1984 Feb 10;12(3):1427–1446. doi: 10.1093/nar/12.3.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings K. E., Emerson C. P., Jr cDNA clone analysis of six co-regulated mRNAs encoding skeletal muscle contractile proteins. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1553–1557. doi: 10.1073/pnas.79.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hørder M., Magid E., Pitkänen E., Härkönen M., Strömme J. H., Theodorsen L., Gerhardt W., Waldenström J. Recommended method for the determination of creatine kinase in blood modified by the inclusion of EDTA. The Committee on Enzymes of the Scandinavian Society for Clinical Chemistry and Clinical Physiology (SCE). Scand J Clin Lab Invest. 1979 Feb;39(1):1–5. doi: 10.3109/00365517909104932. [DOI] [PubMed] [Google Scholar]
- Keller L. R., Emerson C. P., Jr Synthesis of adult myosin light chains by embryonic muscle cultures. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1020–1024. doi: 10.1073/pnas.77.2.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konigsberg I. R. Diffusion-mediated control of myoblast fusion. Dev Biol. 1971 Sep;26(1):133–152. doi: 10.1016/0012-1606(71)90113-8. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski R. W., Schweinfest C. W., Dottin R. P. Molecular cloning and the complete nucleotide sequence of the creatine kinase-M cDNA from chicken. Nucleic Acids Res. 1984 Sep 25;12(18):6925–6934. doi: 10.1093/nar/12.18.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linkhart T. A., Clegg C. H., Hauschika S. D. Myogenic differentiation in permanent clonal mouse myoblast cell lines: regulation by macromolecular growth factors in the culture medium. Dev Biol. 1981 Aug;86(1):19–30. doi: 10.1016/0012-1606(81)90311-0. [DOI] [PubMed] [Google Scholar]
- Linkhart T. A., Clegg C. H., Hauschka S. D. Control of mouse myoblast commitment to terminal differentiation by mitogens. J Supramol Struct. 1980;14(4):483–498. doi: 10.1002/jss.400140407. [DOI] [PubMed] [Google Scholar]
- Lough J., Bischoff R. Differentiation of creatine phosphokinase during myogenesis: quantitative fractionation of isozymes. Dev Biol. 1977 Jun;57(2):330–344. doi: 10.1016/0012-1606(77)90219-6. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- McKnight G. L., McConaughy B. L. Selection of functional cDNAs by complementation in yeast. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4412–4416. doi: 10.1073/pnas.80.14.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloul D., Aloni B., Calvo J., Yaffe D., Nudel U. Developmentally regulated expression of chimeric genes containing muscle actin DNA sequences in transfected myogenic cells. EMBO J. 1984 May;3(5):983–990. doi: 10.1002/j.1460-2075.1984.tb01917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill G. F., Hauschka S. D., McKnight S. L. tk Enzyme expression in differentiating muscle cells is regulated through an internal segment of the cellular tk gene. Mol Cell Biol. 1984 Sep;4(9):1777–1784. doi: 10.1128/mcb.4.9.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Morris G. E., Head L. P. Immunoassay of muscle-specific creatine kinase with a monoclonal antibody and application to myogenesis and muscular dystrophy. Biochem J. 1983 Aug 1;213(2):417–425. doi: 10.1042/bj2130417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima Y., Fujii-Kuriyama Y., Muramatsu M., Ogata K. Alternative transcription and two modes of splicing results in two myosin light chains from one gene. Nature. 1984 Mar 22;308(5957):333–338. doi: 10.1038/308333a0. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 1978 Nov;15(3):855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- Olson E. N., Caldwell K. L., Gordon J. I., Glaser L. Regulation of creatine phosphokinase expression during differentiation of BC3H1 cells. J Biol Chem. 1983 Feb 25;258(4):2644–2652. [PubMed] [Google Scholar]
- Paterson B. M., Bishop J. O. Changes in the mRNA population of chick myoblasts during myogenesis in vitro. Cell. 1977 Nov;12(3):751–765. doi: 10.1016/0092-8674(77)90275-6. [DOI] [PubMed] [Google Scholar]
- Perriard J. C., Caravatti M., Perriard E. R., Eppenberger H. M. Quantitation of creatine kinase isoenzyme transition in differentiating chicken embryonic breast muscle and myogenic cell cultures by immunoadsorption. Arch Biochem Biophys. 1978 Nov;191(1):90–100. doi: 10.1016/0003-9861(78)90070-x. [DOI] [PubMed] [Google Scholar]
- Perriard J. C. Developmental regulation of creatine kinase isoenzymes in myogenic cell cultures from chicken. Levels of mRNA for creatine kinase subunits M and B. J Biol Chem. 1979 Aug 10;254(15):7036–7041. [PubMed] [Google Scholar]
- Rosenberg U. B., Kunz G., Frischauf A., Lehrach H., Mähr R., Eppenberger H. M., Perriard J. C. Molecular cloning and expression during myogenesis of sequences coding for M-creatine kinase. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6589–6592. doi: 10.1073/pnas.79.21.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. J., Rothblum K. N. Gene switching in myogenesis: differential expression of the chicken actin multigene family. Biochemistry. 1981 Jul 7;20(14):4122–4129. doi: 10.1021/bi00517a027. [DOI] [PubMed] [Google Scholar]
- Schweinfest C. W., Kwiatkowski R. W., Dottin R. P. Molecular cloning of a DNA sequence complementary to creatine kinase M mRNA from chickens. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4997–5000. doi: 10.1073/pnas.79.16.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockdale F. E., Baden H., Raman N. Slow muscle myoblasts differentiating in vitro synthesize both slow and fast myosin light chains. Dev Biol. 1981 Feb;82(1):168–171. doi: 10.1016/0012-1606(81)90438-3. [DOI] [PubMed] [Google Scholar]
- Sutherland W. M., Konigsberg I. R. CPK accumulation in fusion-blocked quail myocytes. Dev Biol. 1983 Oct;99(2):287–297. doi: 10.1016/0012-1606(83)90278-6. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. C., Maier V., Eppenberger H. M. Creatine kinase and aldolase isoenzyme transitions in cultures of chick skeletal muscle cells. Dev Biol. 1974 Mar;37(1):63–89. doi: 10.1016/0012-1606(74)90170-5. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Weydert A., Daubas P., Caravatti M., Minty A., Bugaisky G., Cohen A., Robert B., Buckingham M. Sequential accumulation of mRNAs encoding different myosin heavy chain isoforms during skeletal muscle development in vivo detected with a recombinant plasmid identified as coding for an adult fast myosin heavy chain from mouse skeletal muscle. J Biol Chem. 1983 Nov 25;258(22):13867–13874. [PubMed] [Google Scholar]
- Wright W. E. Induction of muscle genes in neural cells. J Cell Biol. 1984 Feb;98(2):427–435. doi: 10.1083/jcb.98.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D. Developmental changes preceding cell fusion during muscle differentiation in vitro. Exp Cell Res. 1971 May;66(1):33–48. doi: 10.1016/s0014-4827(71)80008-3. [DOI] [PubMed] [Google Scholar]