Abstract
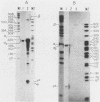
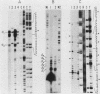
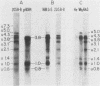
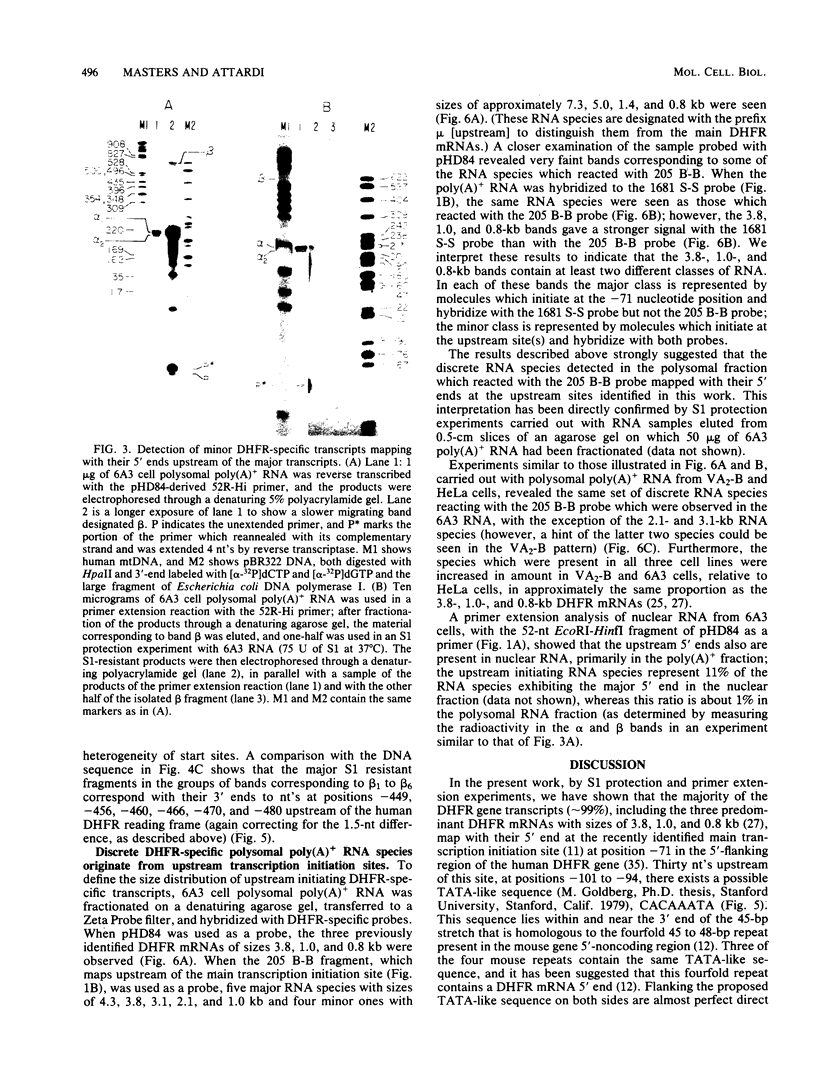
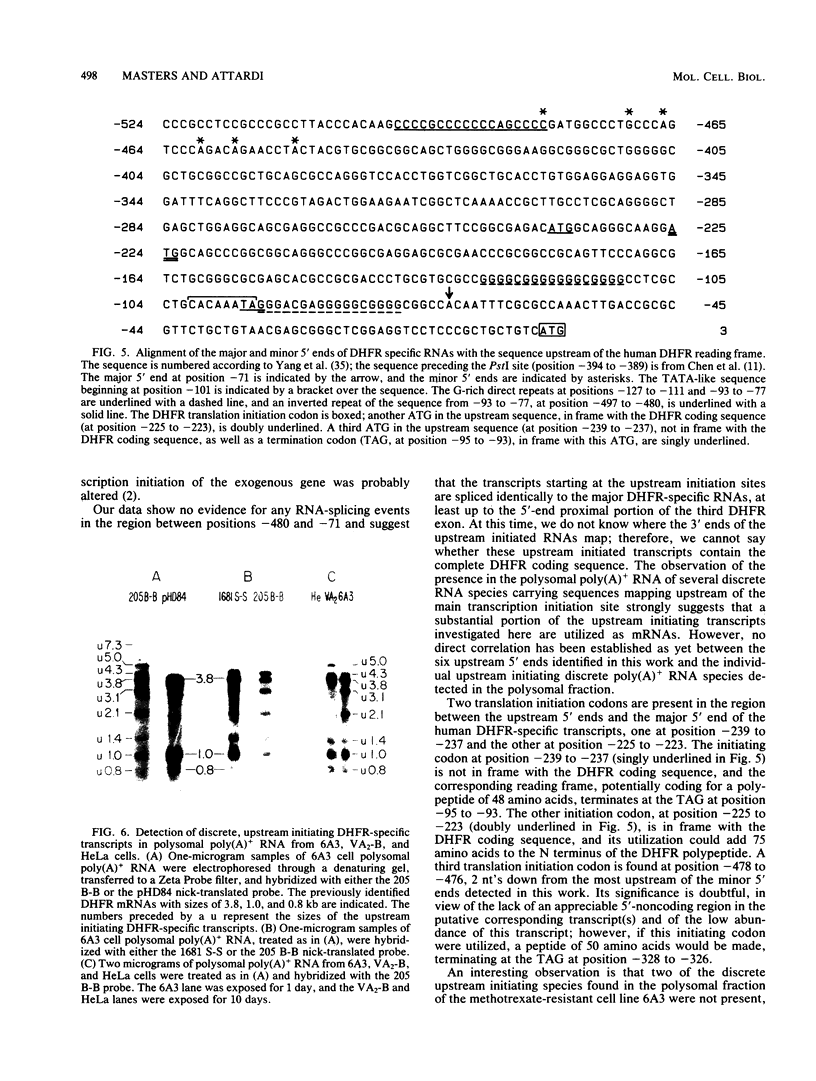
The 5' ends of dihydrofolate reductase (DHFR)-specific transcripts have been mapped in the 5'-flanking region of the amplified DHFR gene of the human methotrexate-resistant cell line 6A3 by primer extension and S1 protection experiments. The main 5' end, at position -71 relative to the first nucleotide of the DHFR reading frame, corresponds to the recently identified main transcription initiation site for the DHFR gene and pertains to transcripts representing approximately 99% of the DHFR-specific polysomal polyadenylic acid-containing RNA, and including the previously described DHFR mRNAs with sizes of 3.8, 1.0, and 0.8 kilobases. At least six other minor 5' ends have been mapped to nucleotide positions -449 to -480 upstream of the DHFR gene and pertain to approximately 1% of the DHFR-specific polysomal polyadenylic acid-containing RNA. These upstream initiating transcripts appear to include five major discrete species with sizes of 4.3, 3.8, 3.1, 2.1, and 1.0 kilobases and four minor ones with sizes of 7.3, 5.0, 1.4, and 0.8 kilobases. These species, with the exception of those of 3.1- and 2.1-kilobase sizes, also have been found in VA2-B cells, the parental line of 6A3, and in HeLa cells. The upstream initiating transcripts present in all three cell lines are increased in amount in 6A3 cells as compared with the other cell lines, in about the same proportion as the three identified DHFR mRNAs.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan M., Lanyon W. G., Paul J. Multiple origins of transcription in the 4.5 Kb upstream of the epsilon-globin gene. Cell. 1983 Nov;35(1):187–197. doi: 10.1016/0092-8674(83)90221-0. [DOI] [PubMed] [Google Scholar]
- Allan M., Zhu J. D., Montague P., Paul J. Differential response of multiple epsilon-globin cap sites to cis- and trans-acting controls. Cell. 1984 Sep;38(2):399–407. doi: 10.1016/0092-8674(84)90495-1. [DOI] [PubMed] [Google Scholar]
- Amaldi F., Attardi G. Partial sequence analysis of ribosomal RNA from HeLa cells. I. Oligonucleotide pattern of 28 s and 18 s RNA after pancreatic ribonuclease digestion. J Mol Biol. 1968 May 14;33(3):737–755. doi: 10.1016/0022-2836(68)90317-3. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson D. P., Ross J. Human beta-globin promoter and coding sequences transcribed by RNA polymerase III. Cell. 1983 Oct;34(3):857–864. doi: 10.1016/0092-8674(83)90543-3. [DOI] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Carothers A. M., Urlaub G., Ellis N., Chasin L. A. Structure of the dihydrofolate reductase gene in Chinese hamster ovary cells. Nucleic Acids Res. 1983 Apr 11;11(7):1997–2012. doi: 10.1093/nar/11.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. J., Shimada T., Moulton A. D., Cline A., Humphries R. K., Maizel J., Nienhuis A. W. The functional human dihydrofolate reductase gene. J Biol Chem. 1984 Mar 25;259(6):3933–3943. [PubMed] [Google Scholar]
- Crouse G. F., Simonsen C. C., McEwan R. N., Schimke R. T. Structure of amplified normal and variant dihydrofolate reductase genes in mouse sarcoma S180 cells. J Biol Chem. 1982 Jul 10;257(13):7887–7897. [PubMed] [Google Scholar]
- Di Segni G., Carrara G., Tocchini-Valentini G. R., Shoulders C. C., Baralle F. E. Selective in vitro transcription of one of the two Alu family repeats present in the 5' flanking region of the human epsilon-globin gene. Nucleic Acids Res. 1981 Dec 21;9(24):6709–6722. doi: 10.1093/nar/9.24.6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C., Biro P. A., Choudary P. V., Elder J. T., Wang R. R., Forget B. G., de Riel J. K., Weissman S. M. RNA polymerase III transcriptional units are interspersed among human non-alpha-globin genes. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5095–5099. doi: 10.1073/pnas.76.10.5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindlay G. J., Lanyon W. G., Allan M., Paul J. Alternative sites of transcription initiation upstream of the canonical cap site in human gamma-globin and beta-globin genes. Nucleic Acids Res. 1984 Feb 24;12(4):1811–1820. doi: 10.1093/nar/12.4.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes S. R., Jelinek W. R. Low molecular weight RNAs transcribed in vitro by RNA polymerase III from Alu-type dispersed repeats in Chinese hamster DNA are also found in vivo. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6130–6134. doi: 10.1073/pnas.78.10.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer E., Darnell J. E., Jr The primary transcription unit of the mouse beta-major globin gene. Cell. 1981 Feb;23(2):585–593. doi: 10.1016/0092-8674(81)90154-9. [DOI] [PubMed] [Google Scholar]
- Holland L. J., Wangh L. J. Efficient recovery of functionally intact mRNA from agarose gels via transfer to an ion-exchange membrane. Nucleic Acids Res. 1983 May 25;11(10):3283–3300. doi: 10.1093/nar/11.10.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopes B. C., McClure W. R. Studies on the selectivity of DNA precipitation by spermine. Nucleic Acids Res. 1981 Oct 24;9(20):5493–5504. doi: 10.1093/nar/9.20.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanteur P., Amaldi F., Attardi G. Partial sequence analysis of ribosomal RNA from HeLa cells. II. Evidence for sequences of non-ribosmal type in 45 and 32 s ribosomal RNA precursors. J Mol Biol. 1968 May 14;33(3):757–775. doi: 10.1016/0022-2836(68)90318-5. [DOI] [PubMed] [Google Scholar]
- Larsen A., Weintraub H. An altered DNA conformation detected by S1 nuclease occurs at specific regions in active chick globin chromatin. Cell. 1982 Jun;29(2):609–622. doi: 10.1016/0092-8674(82)90177-5. [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Davide J. P., Melera P. W. Selective amplification of polymorphic dihydrofolate reductase gene loci in Chinese hamster lung cells. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6961–6965. doi: 10.1073/pnas.79.22.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Colozzo M. T. Synthesis in vitro of an exceptionally long RNA transcript promoted by an AluI sequence. Nature. 1982 Nov 25;300(5890):376–379. doi: 10.1038/300376a0. [DOI] [PubMed] [Google Scholar]
- Masters J. N., Yang J. K., Cellini A., Attardi G. A human dihydrofolate reductase pseudogene and its relationship to the multiple forms of specific messenger RNA. J Mol Biol. 1983 Jun 15;167(1):23–36. doi: 10.1016/s0022-2836(83)80032-1. [DOI] [PubMed] [Google Scholar]
- Masters J., Keeley B., Gay H., Attardi G. Variable content of double minute chromosomes is not correlated with degree of phenotype instability in methotrexate-resistant human cell lines. Mol Cell Biol. 1982 May;2(5):498–507. doi: 10.1128/mcb.2.5.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Morandi C., Masters J. N., Mottes M., Attardi G. Multiple forms of human dihydrofolate reductase messenger RNA. Cloning and expression in Escherichia coli of their DNA coding sequence. J Mol Biol. 1982 Apr 15;156(3):583–607. doi: 10.1016/0022-2836(82)90268-6. [DOI] [PubMed] [Google Scholar]
- Ojala D., Attardi G. Precise localization of the origin of replication in a physical map of HeLa cell mitochondrial DNA and isolation of a small fragment that contains it. J Mol Biol. 1978 Jul 5;122(3):301–319. doi: 10.1016/0022-2836(78)90192-4. [DOI] [PubMed] [Google Scholar]
- PONTEN J., JENSEN F., KOPROWSKI H. Morphological and virological investigation of human tissue cultures transformed with SV40. J Cell Comp Physiol. 1963 Apr;61:145–163. doi: 10.1002/jcp.1030610206. [DOI] [PubMed] [Google Scholar]
- Pan J., Elder J. T., Duncan C. H., Weissman S. M. Structural analysis of interspersed repetitive polymerase III transcription units in human DNA. Nucleic Acids Res. 1981 Mar 11;9(5):1151–1170. [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Karch F., Iida S., Meyer J. The plasmid cloning vector pBR325 contains a 482 base-pair-long inverted duplication. Gene. 1981 Sep;14(4):289–299. doi: 10.1016/0378-1119(81)90161-x. [DOI] [PubMed] [Google Scholar]
- Schon E., Evans T., Welsh J., Efstratiadis A. Conformation of promoter DNA: fine mapping of S1-hypersensitive sites. Cell. 1983 Dec;35(3 Pt 2):837–848. doi: 10.1016/0092-8674(83)90116-2. [DOI] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Schimke R. T. Nucleotide sequence surrounding multiple polyadenylation sites in the mouse dihydrofolate reductase gene. J Biol Chem. 1982 May 10;257(9):5143–5147. [PubMed] [Google Scholar]
- Sollner-Webb B., Reeder R. H. The nucleotide sequence of the initiation and termination sites for ribosomal RNA transcription in X. laevis. Cell. 1979 Oct;18(2):485–499. doi: 10.1016/0092-8674(79)90066-7. [DOI] [PubMed] [Google Scholar]
- Yang J. K., Masters J. N., Attardi G. Human dihydrofolate reductase gene organization. Extensive conservation of the G + C-rich 5' non-coding sequence and strong intron size divergence from homologous mammalian genes. J Mol Biol. 1984 Jun 25;176(2):169–187. doi: 10.1016/0022-2836(84)90419-4. [DOI] [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]