Abstract
Objectives
To compare use and effectiveness of bleeding avoidance strategies (BAS) by gender.
Background
Women have higher rates of bleeding following percutaneous coronary intervention (PCI).
Methods
Among 570,777 men (67.5%) and women (32.5%) undergoing PCI in the National Cardiovascular Data Registry’s CathPCI Registry® between 7/1/2009 and 3/31/2011, in-hospital bleeding rates and the use of BAS (vascular closure devices, bivalirudin, radial approach and their combinations) were assessed. The relative risk of bleeding for each BAS compared with no BAS was determined in women and men using multivariable logistic regressions adjusted for clinical characteristics and the propensity for receiving BAS. Finally, the absolute risk differences in bleeding associated with BAS were compared.
Results
Overall, the use of any BAS differed slightly between women and men (75.4% vs. 75.7%, p=0.01). When BAS were not used, women had significantly higher rates of bleeding than men (12.5% vs. 6.2%, p<0.01). Both genders had similar adjusted risk reductions of bleeding when any BAS was used (women: OR=0. 60, 95% CI 0.57–0.63; men OR=0. 62, 95% CI 0.59–0.65). Women and men had lower absolute bleeding risks with BAS; however, these absolute risk differences were greater in women (6.3% vs. 3.2%, p<0.01).
Conclusions
Women continue to have almost twice the rate of bleeding following PCI. The use of any BAS was associated with a similarly lower risk of bleeding for both genders; however, the absolute risk differences were substantially higher in women. These data underscore the importance of applying effective strategies to limit post-PCI bleeding, especially in women.
Keywords: Gender, Post-procedural bleeding, Bleeding avoidance strategies, Effectiveness
Introduction
Peri-procedural bleeding is the most common non-cardiac complication following percutaneous coronary intervention (PCI) and is associated with high morbidity and mortality(1–3). Historically, women have been at higher risk for peri-procedural bleeding following PCI compared with men(4–10). Bleeding avoidance strategies (BAS) including vascular closure devices, bivalirudin and radial access are increasingly used and have been associated with decreased rates of bleeding following PCI(8,9,11–13). In practice, however, those at the highest predicted risk for bleeding are often the least likely to receive BAS at the time of PCI suggesting a “risk-treatment paradox”(11). Whether women, who are known to be at high risk for bleeding, receive BAS during PCI as frequently as men in contemporary practice has not been determined. Furthermore, whether BAS are associated with similar reductions in peri-procedural bleeding in women compared with men is not known.
To address these gaps in knowledge, we compared the use of BAS (vascular closure devices, bivalirudin, radial access or their combinations) by gender and conducted an observational comparative effectiveness study of BAS to determine whether the lower risk of bleeding associated with BAS use was similar between women and men. This study was designed to provide a contemporary assessment of the use of BAS and the extent to which BAS may reduce the risk of this common adverse consequence in women undergoing PCI.
Methods
Data Source
Data were obtained from the National Cardiovascular Data Registry’s (NCDR) CathPCI Registry, which is an initiative of the American College of Cardiology (ACC) Foundation and the Society for Cardiovascular Angiography and Interventions. A detailed description of the registry has been published previously(14). Demographic, clinical, procedural and institutional data elements for PCI procedures are collected at more than 1400 participating centers throughout the United States (~80% of hospitals with invasive catheterization labs). Data are entered via a secure Web-based platform or via software provided by ACC-certified vendors. Data quality assurance measures include automatic system validation and reporting of data completeness, random on-site auditing of participating centers, and education and training of site data managers(15). A comprehensive description of NCDR data elements and definitions is available at http://www.ncdr.com/WebNCDR/NCDRDocuments/CathPCI_v4_CodersDictionary_4.4.pdf. Study Population
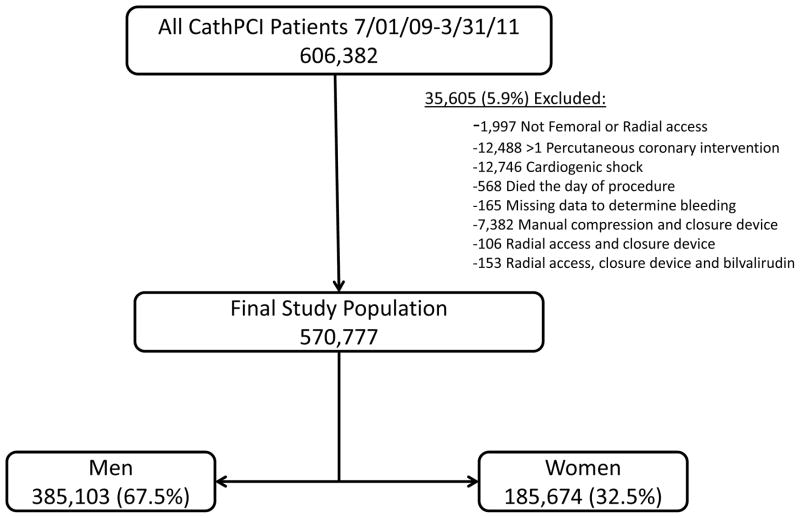
All patients within the CathPCI Registry discharged after PCI between July 1, 2009 and March 31, 2011 were candidates for inclusion (n=606,382 patients at 1232 sites). Patients whose PCI was not from either a radial or femoral approach (n=1,997) and those undergoing more than one PCI procedure during their hospital stay (n=12,488) were excluded. Patients were also excluded if they had cardiogenic shock (n=12,746), died the same day as procedure (n=568) or if they were missing data to determine a bleeding event (n=165). Patients were also excluded if they had received manual compression and a closure device (n=7,382) as it was felt these cases may reflect failed deployment of the closure device. In addition, patients who received radial access and a closure device (n=106) or bivalirudin, radial access and a closure device (n=153) were excluded as this combination of treatments was felt to reflect procedures with combined femoral and radial access which may inherently pose a higher risk for peri-procedural bleeding irrespective of the BAS strategy used. After applying exclusions (total of 5.9% excluded), 570,777 patients at 1230 sites remained. Among the final study cohort, 385,103 (67.5%) were men and 185,674 (32.5%) were women. (Figure 1)
Figure 1. Study population.
A total of 606,382 patients were in the CathPCI Registry from July 01, 2009 through March 31, 2011. After applying exclusions (total of 5.9% excluded), 570,777 patients at 1230 sites remained. Among the final study cohort, 385,103 (67.5%) were men and 185,674 (32.5%) were women.
Study Outcomes
In hospital bleeding complications following PCI were ascertained and reported by participating centers. Peri-procedural bleeding was defined according to the CathPCI V4 data definitions and included: 1) any documented bleeding event that occurred within 72 hours after PCI regardless of site (including access site bleeding, access site hematoma, retroperitoneal bleeding, gastrointestinal bleeding, genital-urinary bleeding, intra-cerebral hemorrhage); OR 2) pericardial tamponade; OR 3) any transfusion following PCI (except among patients with pre-procedure hemoglobin 8 ≤g/dL or those who underwent coronary artery bypass grafting during their hospital stay); OR 4) any absolute decline ≥3 g/dL in hemoglobin level (except for patients with pre-procedure hemoglobin > 16 g/dL)(16).
Bleeding Avoidance Strategies
Bleeding avoidance strategies studied included: 1) vascular closure devices (VCD) alone (see eTable 1 for list of specific devices included) 2) bivalirudin alone (Angiomax [The Medicines Company, Parsippany, New Jersey]); 3) bivalirudin with VCD; 4) radial access alone; and 5) radial access with bivalirudin. Patients receiving manual compression and who did not receive VCD, bivalirudin or radial access served as the referent group for effectiveness comparisons.
Pre-procedural Bleeding Risk Estimation
Estimated bleeding risk scores based upon pre-procedural patient characteristics were derived using the CathPCI bleeding risk model, version 4(17). Risk scores were generated for each patient based on the inverse logarithmic sum of the β coefficients for each of the following pre-PCI variables: gender, age, body mass index, previous cerebrovascular disease, chronic lung disease, previous PCI, peripheral vascular disease, diabetes mellitus, left ventricular ejection fraction, chronic kidney disease, PCI status (defined as elective, urgent, emergent, or salvage), ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI), cardiac arrest within 24 hours, pre-procedure New York Heart Association (NYHA) Class IV heart failure, estimated glomerular filtration rate, pre-procedure hemoglobin, pre-procedure TIMI flow, number of diseased vessels, use of fibrinolytics prior to PCI, sub-acute stent thrombosis, Society for Cardiovascular Angiography and Intervention lesion class, and lesion location (proximal left anterior descending or left main vs. other).
Statistical analysis
Baseline demographic, clinical, procedural and hospital characteristics were compared between women and men using Pearson χ2 tests for categorical variables and Kruskal-Wallis tests for continuous variables. In the primary outcomes analysis, rates of in-hospital bleeding were compared between women and men. Next, the use of each BAS strategy was compared by gender. Given prior studies demonstrating a risk treatment paradox for BAS use (i.e. lower rates of use among those at highest predicted risk for bleeding), rates of use of any BAS were compared between women and men among tertiles of bleeding risk (defined as low (<2.3%), intermediate (2.3–5.0%) and high (>5%)) according to the CathPCI bleeding risk model (11). Because female gender was a determinant of higher bleeding risk, there were a larger proportion of women in the higher tertiles of bleeding risk.
To compare the effectiveness of BAS strategies by gender, the crude relative risk ratios in bleeding for any BAS and individual BAS types vs. no BAS were compared between women and men. Next, among strata of women and men, multivariable logistic regression was used to assess the relationship between each BAS strategy (compared with none) and peri-procedural bleeding adjusting for baseline patient, procedural, and site characteristics (all Tables 1 and 2 variables except estimated bleeding risk) as well as clustering by site and the propensity to receive each BAS.
Table 1.
Demographic and clinical characteristics by gender.
| Men N=385,103 |
Women N=185,674 |
P-value | |
|---|---|---|---|
|
| |||
| Demographics | |||
| Age, years, mean (SD) | 63.3 (11.8) | 67.1 (12.3) | <0.01 |
| White | 89.5 | 86.0 | <0.01 |
|
| |||
| Clinical Characteristics | |||
| Body mass index, mean (SD) | 29.8 (5.9) | 30.4 (7.4) | <0.01 |
| Body surface area, mean (SD) | 2.1 (0.2) | 1.8 (0.2) | <0.01 |
|
| |||
| New York Heart Association class | <0.01 | ||
| I | 13.5 | 10.6 | |
| II | 31.6 | 29.3 | |
| III | 34.9 | 36.5 | |
| IV | 19.4 | 23.1 | |
|
| |||
| Coronary artery disease risk factors | |||
| Diabetes | 32.4 | 39.8 | <0.01 |
| Hypertension | 78.9 | 85.0 | <0.01 |
| Dyslipidemia | 79.2 | 78.9 | 0.04 |
| Current/Recent Smoker | 28.5 | 25.9 | <0.01 |
| Family history of coronary disease | 24.3 | 25.5 | <0.01 |
|
| |||
| Coronary artery disease history | |||
| Previous PCI | 34.9 | 31.1 | <0.01 |
| Previous CABG | 19.1 | 13.6 | <0.01 |
| Myocardial infarction | 27.8 | 23.6 | <0.01 |
|
| |||
| Other cardiovascular disease history | |||
| Congestive heart failure | 9.5 | 12.7 | <0.01 |
| Cerebrovascular disease | 10.2 | 16.1 | <0.01 |
| Peripheral vascular disease | 11.1 | 13.0 | <0.01 |
| Previous valve surgery | 1.4 | 1.4 | 0.80 |
|
| |||
| Other disease history | |||
| Chronic lung disease | 12.9 | 17.6 | <0.01 |
| Previous renal failure | 1.9 | 2.6 | <0.01 |
|
| |||
| Test information, mean (SD) | |||
| Estimated glomerular filtration rate | 78.7 (30.2) | 70.8 (30.1) | <0.01 |
| Left ventricular ejection fraction | 51.2 (12.1) | 54.8 (12.1) | <0.01 |
| Pre-procedural hemoglobin (g/dL) | 14.1 (1.8) | 12.7 (1.7) | <0.01 |
|
| |||
| Estimated pre-procedural bleeding risk* | |||
| Mean bleeding risk %, (SD) | 4.0 (3.9) | 8.0 (7.l) | <0.01 |
| Categories of risk | <0.01 | ||
| High (>5%) | 23.3 | 54.1 | |
| Intermediate (2.3–5.0%) | 30.7 | 38.9 | |
| Low (<2.3%) | 46.0 | 7.0 | |
Based on NCDR Bleeding risk model v4 which accounts for age, gender, body mass index, previous cerebrovascular disease, chronic lung disease, previous PCI, peripheral vascular disease, diabetes mellitus, left ventricular ejection fraction, chronic kidney disease, cardiogenic shock, PCI status, ST-elevation myocardial infarction, non-ST-elevation myocardial infarction, cardiac arrest within 24 hours, pre-procedure New York Heart Association Class IV, estimated glomerular filtration rate, pre-procedure hemoglobin, pre-procedure TIMI flow, number of diseased vessels, use of lytics prior to PCI, subacute stent thrombosis, Society for Cardiovascular Angiography and Intervention lesion class, and lesion location.
SD = standard deviation; PCI = percutaneous coronary intervention; CABG = coronary artery bypass grafting; TIMI = Thrombolysis in Myocardial Infarction
Table 2.
Admission and hospital characteristics by gender.
| Men N=385,103 |
Women N=185,674 |
p-value | |
|---|---|---|---|
|
| |||
| Admission Presentation | |||
| Symptoms | <0.01 | ||
| No symptoms | 9.6 | 8.0 | |
| Atypical chest pain | 3.0 | 3.5 | |
| Stable angina | 18.5 | 18.3 | |
| Unstable angina | 34.7 | 37.8 | |
| Non ST-elevation myocardial infarction | 17.8 | 19.1 | |
| ST-elevation myocardial infarction | 16.4 | 13.2 | |
| Cardiac arrest within 24 hours | 1.17 | 0.85 | <0.01 |
|
| |||
| Procedure status | <0.01 | ||
| Elective | 44.7 | 44.3 | |
| Urgent | 37.4 | 41.1 | |
| Emergency | 17.8 | 14.5 | |
| Salvage | 0.1 | 0.1 | |
| Number vessels intervened upon | <0.01 | ||
| 1 | 85.8 | 86.8 | |
| 2 | 13.1 | 12.2 | |
| 3 | 0.8 | 0.8 | |
| Lesion characteristics | |||
| Pre-procedure TIMI flow | <0.01 | ||
| Complete | 53.9 | 59.5 | |
| Partial | 19.3 | 19.4 | |
| Slow | 9.3 | 8.9 | |
| No | 17.2 | 13.0 | |
| Subacute stent thrombosis | 0.09 | 0.07 | 0.03 |
| SCAI Lesion class | <0.01 | ||
| Class I | 44.3 | 49.3 | |
| Class II | 37.8 | 37.3 | |
| Class III | 4.8 | 3.9 | |
| Class IV | 13.0 | 9.4 | |
| Lesion location | <0.01 | ||
| Proximal Left anterior descending | 15.0 | 15.2 | |
| Left Main | 1.5 | 1.5 | |
| Other | 82.9 | 82.9 | |
|
| |||
| Hospital characteristics | |||
| Region | <0.01 | ||
| West | 15.4 | 13.7 | |
| Northeast | 15.2 | 14.4 | |
| Midwest | 29.2 | 30.0 | |
| South | 40.3 | 41.9 | |
| Community type | <0.01 | ||
| Rural | 10.5 | 10.8 | |
| Urban | 89.5 | 89.2 | |
| Profit type | <0.01 | ||
| Government | 1.9 | 1.7 | |
| Private/community | 85.8 | 86.9 | |
| University | 12.3 | 11.5 | |
| Annual PCI volume, mean (SD) | 898.3 (613.7) | 904.6 (622.0) | 0.02 |
TIMI = Thrombolysis in Myocardial Infarction; SCAI= Society for Cardiovascular Angiography and Intervention; SD = standard deviation
Propensity scores for each BAS were calculated in women and men to minimize the effect of potential selection bias for BAS choice. The propensity score for receiving each BAS was derived using multiple logistic regression models. Variables used to derive these propensity scores included demographics (age, gender, race/ethnicity); clinical characteristics (body mass index, NYHA heart failure classification); coronary artery disease risk factors (diabetes, hypertension, dyslipidemia, smoking, family history of coronary artery disease); coronary artery disease history (previous PCI, coronary artery bypass graft surgery, myocardial infarction); other cardiovascular disease history (congestive heart failure, cerebrovascular disease, peripheral vascular disease); other disease history (chronic obstructive pulmonary disease, renal failure); and presenting syndrome (no symptoms, atypical chest pain, stable angina, unstable angina, STEMI, and NSTEMI) (11). Given the smaller numbers of patients within certain BAS groups (e.g. bivalirudin plus radial) and potential loss of a significant portion of the population with propensity matching, inverse probability-weighted estimators were used (18). Compared with matching and stratification, semi-parametric inverse probability-weighted estimators require few distributional assumptions about underlying data and they avoid the potential residual confounding that arises from stratification on a fixed number of strata (19). Finally, the absolute differences in bleeding risk for each BAS compared with none were determined for women and men using chi-squared tests.
All statistical analyses were performed using Statistical Analytic Systems version 9.2 (SAS Institute, Cary, North Carolina). The study was approved by the Institutional Review Board of Duke University Medical Center and was determined to meet the definition of research not requiring informed consent.
Results
Study Population Characteristics
Baseline demographic and clinical characteristics for women and men are shown in Table 1. Compared with men, women were older and more likely to have higher NYHA class, have lower estimated glomerular filtration rate and have slightly higher body mass index. Admissions symptoms, procedural details and hospital characteristics for women and men are shown in Table 2. Women more often presented with an acute coronary syndrome (NSTEMI or unstable angina) compared with men and less often underwent PCI for an emergency procedure or for an emergent indication.
According to the CathPCI Registry bleeding risk model, the mean estimated bleeding risk was significantly higher for women than men (8.0%, SD 7.1 vs. 4.0%, SD 3.9; p<0.01). Women were significantly more likely to be categorized as either high (54.1% vs. 23.3%) or intermediate (38.9% vs. 30.7%) risk for bleeding compared with men; only 7.0% of women compared with 46.0% of men were categorized as low risk for predicted bleeding (p<0.01 for all). (Table 1)
Bleeding Outcomes by Gender
Following PCI, 7.8% of women and 3.7% of men experienced a bleeding event (p<0.01). The rates of all of the components of the composite bleeding outcome were significantly higher among women compared with men with the exception of lower rates of genitourinary bleeding (0.05% vs. 0.07%, p<0.01). Both access and non-access site bleeding complications were more frequent in women. The largest contributors to bleeding events for both genders were related to post PCI transfusions and hemoglobin decreases ≥3 g/dL. (eTable 2) In the bleeding outcomes models adjusted for patient, procedural characteristics, site characteristics, and the inverse probability-weighted estimators to receive BAS, women were more than twice as likely to bleed compared with men (OR=2.23, 95% CI 2.17–2.30).
Use of BAS by Gender and Bleeding Risk
Overall, the use of any BAS strategy was statistically different in women and men but absolute differences were small (overall use of BAS in women = 75.4% vs. men =75.7%, p=0.01; absolute difference = 0.3%). Compared with men, women were more likely to receive bivalirudin (31.0% vs. 27.5%, p<0.01) and less likely to undergo a radial approach (3.0% vs. 3.5%, p<0.05) or have a closure device deployed (16.4% vs. 18.6%, p<0.01).
BAS of any type was used the least often in both women and men at highest risk for bleeding compared with those in the lower risk tertiles (men: 63.9% high vs. 76.4% intermediate and 81.2% low; women: 71.4% high vs. 79.7% intermediate and 82.4% low; p<0.01 for both). Further, the interaction of gender and risk category for receiving BAS was statistically significantly (p<0.01) suggesting that the use of BAS significantly varied by gender and risk category with largest gender differences in BAS use seen among highest risk women and men (71.4% vs. 63.9%).
BAS and Bleeding Risk by Gender
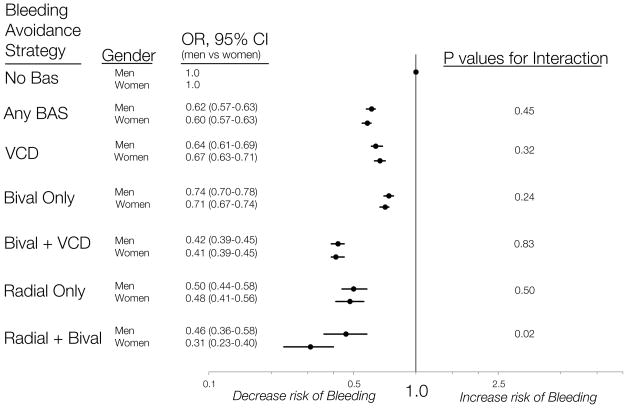
Women not receiving BAS had significantly more bleeding events following PCI compared with men not receiving BAS (crude rates of 12.6% vs. 6.2%, p<0.01). For women and men, the use of all BAS types was associated with a similarly lower relative risk of bleeding compared with no BAS. In the multivariable models adjusting for patient characteristics, procedural information, hospital data, clustering by site and the propensity to receive each BAS, both women and men had significantly lower odds of bleeding with each BAS strategy compared with no BAS. (Figure 2) There was no significant interaction between gender and specific BAS type with the exception of the radial approach plus bivalirudin group, where the relative risk ratio was significantly greater in women than in men (p=0.02 for interaction). (Figure 2)
Figure 2. The relative differences in peri-procedural bleeding risk by BAS and gender.
In the multivariable models adjusting for patient characteristics, procedural information, hospital data, clustering by site and the propensity to receive each BAS, both women and men had significantly lower odds of bleeding with each BAS strategy compared with no BAS. There was no significant interaction between gender and specific BAS type with the exception of the radial approach plus bivalirudin group, where the relative risk difference was significantly greater in women than in men (p=0.02 for interaction).
BAS indicates bleeding avoidance strategy; bival, bivalirudin; VCD, vascular closure device
Given the higher bleeding risk for women among all BAS strata, the absolute differences in bleeding associated with each BAS compared with none were greater in women (range 4.1–9.5%) than men (range 2.2–4.4%). (Figure 3) Compared with the bleeding risk associated with the use of no BAS, the largest absolute differences in bleeding risk were seen among women and men who received radial approach plus bivalirudin (9.5% absolute bleeding risk difference in women; 4.4% absolute bleeding risk difference in men). (Figure 3)
Figure 3. Peri-procedural bleeding rates and absolute differences in bleeding risk by BAS and gender.
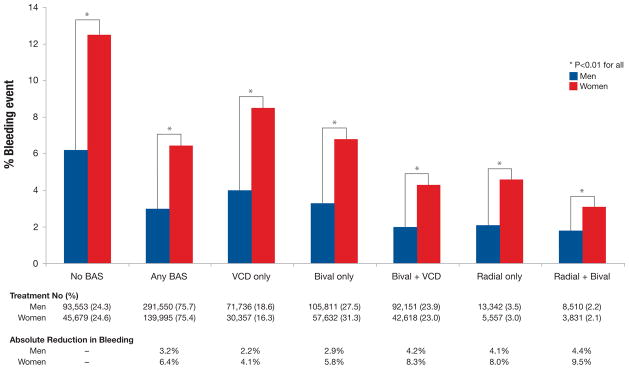
Women not receiving BAS had significantly more bleeding events following PCI compared with men not receiving BAS (crude rates of 12.6% vs. 6.2%, p<0.01). Given the higher bleeding risk for women among all BAS strata, the absolute differences in bleeding associated with each BAS compared with none were greater in women (range 4.1–9.5%) than men (range 2.2–4.4%)
BAS indicates bleeding avoidance strategy; bival, bivalirudin; VCD, vascular closure device
Discussion
In this contemporary cohort of over 500,000 patients undergoing PCI at over 1200 hospitals throughout the United States, women had twice the risk of bleeding compared with men. Although a risk treatment paradox was seen for both women and men with those at highest risk for bleeding being the least likely to receive BAS, women and men were overall similarly likely to receive any form of BAS following PCI. Finally, all BAS were associated with similar differences in bleeding risk regardless of gender; however, given higher bleeding risk in women, the absolute differences in bleeding risk associated with all BAS were almost two times higher in women compared with men.
Although others have reported gender differences in bleeding following PCI, our study expands the literature in several ways (4–10). First, our study reflects a contemporary comparison of periprocedual bleeding in women and men from 2009–2011, a time period in which advances in pharmacologic agents (i.e. the use of bivalirudin) and procedural techniques (i.e. increased use of a radial approach) have occurred (13,20,21). Second, our study includes the largest cohort of women and men undergoing PCI throughout the United States. Finally, our study employs an established and broad definition for periprocedural bleeding. The CathPCI® Registry definition of bleeding includes not only bleeding at the access site, but bleeding at non-access sites as well as post procedural transfusion or an absolute hemoglobin drop (22). This definition is similar to the bleeding definitions being used in contemporary clinical trials of PCI procedures which focus on both access related and non-access related bleeding as a primary outcome (23). Bleeding of various degrees, including minor bleeding, among patients undergoing PCI is associated with increased risk of mortality and morbidity, longer hospital stay and increased costs; therefore, a heterogeneous definition for bleeding allows a greater sensitivity to detect patients at higher risk for poor outcomes (1,24,25).
The current study demonstrates that in contemporary practice women have almost twice the rate of bleeding compared with men following PCI (7.8% vs. 3.7%, adjusted OR 2.23, 95% CI 2.17–2.30) despite the relatively frequent use of BAS. Over the last two decades, numerous studies have similarly demonstrated significantly higher rates of post procedural bleeding in women (4–10,26). Another important contribution of this study is that we found that approximately 3 out of 4 women and men undergoing PCI receive any type of BAS. Although overall BAS use was similar in women and men, our study confirms the presence of a risk treatment paradox for both women and men (11). Future studies are needed to investigate potential reasons for lower rates of BAS use among the highest risk patients (11).
One of the most important findings of our study is a comparison of the relative and absolute risk differences in bleeding associated with different types of BAS among women and men. Overall, each BAS strategy studied was associated with similarly lower relative risks of bleeding in women and men compared with the use of no BAS. Although several studies have demonstrated reductions in bleeding risk with BAS compared with none, few have evaluated these relationships by gender (4,9). Our study suggests a slightly greater benefit of the combined use of trans-radial approach and bivalirudin in women compared with men (reduction in bleeding: 69% in women vs. 54% in men, p<0.02 for interaction). However, these finding should be interpreted with caution as the numbers of women and men undergoing a radial approach and receiving bivalirudin was relatively small. Additional studies are needed to confirm whether one type of BAS will prove to be more effective in women than in men.
Although we found similarly lower relative risk differences associated with all BAS types, the absolute differences in bleeding risk for women were approximately two-fold larger than in men because of the higher baseline predicted risk of bleeding in women. For example, women who received bivalirudin and a vascular closure device had an 8.3% absolute difference in bleeding risk compared with a 4.2% absolute difference among men. Absolute differences of this magnitude would result in an estimated number needed to treat to prevent one bleed of 12 for women and 24 for men. Therefore, given their significantly higher risk of bleeding, use of BAS in women might prevent a greater proportion of bleeding events; therefore a low clinical threshold for BAS use among women should be considered.
Certain limitations should be considered when interpreting the findings of our study. First, controversy exists as to whether the BAS types examined in this study are efficacious in reducing bleeding. Specifically, studies on the safety of vascular closure devices have shown increased, decreased or neutral bleeding complications (13). Further, the extent to which bivalirudin reduces bleeding when compared to unfractionated heparin alone is not well known (13). The current study suggests that the BAS types examined are associated with similar relative differences in bleeding in women and men following PCI, however, randomized trials are needed to define the most efficacious strategy for bleeding avoidance. Second, bleeding outcomes are site-reported and may underestimate bleeding rates. However, we employed a definition for bleeding which includes documentation of a bleeding event as well as objective drops in hemoglobin which comprised the largest portion of bleeding events for women and men. Further, the definition was applied equally to women and men and reporting would not be expected to vary by gender. Third, the CathPCI Registry only captures in-hospital bleeding events and does not evaluate longitudinal outcomes following discharge. However, in multiple studies, inhospital bleeding events have been associated with longer-term morbidity and mortality (1–3). Fourth, the current analysis does not account for the sheath size which has been shown to be associated with differences in post procedural bleeding among women compared with men (4,9). However, sheath size has been associated with adverse bleeding at the access site and many of our bleeding outcomes were not access specific. Fifth, we were unable to account for possible gender differences in the dosing of either antithrombotic or antiplatelet agents. Prior studies have demonstrated that women were more likely to receive inappropriately high doses of both antithrombotic and antiplatelet agents which may partially explain higher rates of bleeding in women (27). It is not clear, however, that rates of inappropriate dosing would necessarily differ among strata of BAS use. Finally, given the observational nature of our data, the possibility of residual unmeasured confounding may explain the differences in risk associated with BAS in our study. We attempted to minimize confounding by calculating a non-parsimonious propensity score for receiving each type BAS among women and men and using inverse probability-weighted estimators.
This study has several implications for clinical care and future research. First, we have demonstrated that gender differences in bleeding following PCI are not largely due to differences in BAS use. Second, while the risk treatment paradox for BAS use is present for women and men, it did not differ markedly between genders. Third, the relative risk difference associated with each BAS was similar between women and men with the exception of slightly greater differences in bleeding risk with the combined use of radial approach and bivalirudin in women. Taken together, these findings suggest that persistent gender differences in bleeding post-PCI are not due to differences in the apparent effectiveness of BAS. Finally, the absolute reductions in bleeding risk were almost 2 times higher in women compared with men for all BAS types. Therefore, our findings underscore the importance of comparing available strategies and developing new approaches to reduce bleeding risk in women following PCI. One such trial is currently ongoing. (http://clinicaltrials.gov/ct2/show/NCT01406236?term=SAFE-PCI&rank=1).
Conclusions
In contemporary practice, women have almost twice the risk of bleeding following PCI. The use of BAS was associated with significantly lower bleeding risks for both genders; however, the absolute risk differences were higher in women. These data underscore the importance of applying effective strategies to limit post-PCI bleeding, especially in women.
Supplementary Material
Acknowledgments
Funding:
This research was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR). Dr. Daugherty is supported by Award Number K08HL103776 from the National Heart, Lung and Blood Institute. The views expressed in this manuscript represent those of the author(s), and do not necessarily represent the official views of the NCDR, its associated professional societies identified at www.ncdr.com, or the National Heart, Lung and Blood Institute. Dr. Daugherty and Dr. Kim had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
This research was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR). Dr. Daugherty is supported by Award Number K08HL103776 from the National Heart, Lung and Blood Institute. The views expressed in this manuscript represent those of the author(s), and do not necessarily represent the official views of the NCDR, its associated professional societies (www.ncdr.com), or the NHLBI.
Abbreviations
- ACC
American College of Cardiology
- BAS
Bleeding avoidance strategy
- CI
Confidence interval
- NCDR
National Cardiovascular Data Registry
- NSTEMI
Non-ST-elevation myocardial infarction
- NYHA
New York Heart Association
- OR
Odds ratio
- PCI
Percutaneous coronary intervention
- STEMI
ST-elevation myocardial infarction
- VCD
Vascular closure device
Footnotes
Disclosures:
Dr. Sunil Rao is a consultant for The Medicines Company, Terumo. Dr. Frederick Masoudi is the Senior Medical Officer for the NCDR. All other authors report no conflicts in relation to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ndrepepa G, Berger PB, Mehilli J, et al. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions - Appropriateness of including bleeding as a component of a quadruple end point. Journal of the American College of Cardiology. 2008;51:690–697. doi: 10.1016/j.jacc.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 2.Lindsey JB, Marso SP, Pencina M, et al. Prognostic Impact of Periprocedural Bleeding and Myocardial Infarction After Percutaneous Coronary Intervention in Unselected Patients Results From the EVENT (Evaluation of Drug-Eluting Stents and Ischemic Events) Registry. JACC-Cardiovasc Interv. 2009;2:1074–1082. doi: 10.1016/j.jcin.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Eikelboom JW, Mehta SR, Anand SS, Xie CC, Fox KAA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–782. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 4.Tavris DR, Gallauresi BA, Dey S, Brindis R, Mitchel K. Risk of local adverse events by gender following cardiac catheterization. Pharmacoepidemiology and Drug Safety. 2007;16:125–131. doi: 10.1002/pds.1307. [DOI] [PubMed] [Google Scholar]
- 5.Argulian E, Patel AD, Abramson JL, et al. Gender differences in short-term cardiovascular outcomes after percutaneous coronary interventions. American Journal of Cardiology. 2006;98:48–53. doi: 10.1016/j.amjcard.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 6.Duvernoy CS, Smith DE, Manohar P, et al. Gender differences in adverse outcomes after contemporary percutaneous coronary intervention: An analysis from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) percutaneous coronary intervention registry. American Heart Journal. 2010;159:677–U210. doi: 10.1016/j.ahj.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 7.Akhter N, Milford-Beland S, Roe MT, Piana RN, Kao J, Shroff A. Gender differences among patients with acute coronary syndromes undergoing percutaneous coronary intervention in the American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR) American Heart Journal. 2009;157:141–148. doi: 10.1016/j.ahj.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Tavris DR, Dey S, Weintraub WS, et al. Risk of local adverse events following cardiac catheterization by hemostasis device and gender. Journal of the American College of Cardiology. 2005;45:40A–40A. [Google Scholar]
- 9.Ahmed B, Piper WD, Malenka D, et al. Significantly Improved Vascular Complications Among Women Undergoing Percutaneous Coronary Intervention A Report From the Northern New England Percutaneous Coronary Intervention Registry. Circulation-Cardiovascular Interventions. 2009;2:423–429. doi: 10.1161/CIRCINTERVENTIONS.109.860494. [DOI] [PubMed] [Google Scholar]
- 10.Peterson ED, Lansky AJ, Kramer J, Anstrom K, Lanzilotta MJ. Effect of gender on the outcomes of contemporary percutaneous coronary intervention. Am J Cardiol. 2001;88:359–64. doi: 10.1016/s0002-9149(01)01679-4. [DOI] [PubMed] [Google Scholar]
- 11.Marso SP, Amin AP, House JA, et al. Association Between Use of Bleeding Avoidance Strategies and Risk of Periprocedural Bleeding Among Patients Undergoing Percutaneous Coronary Intervention. Journal of the American Medical Association. 2010;303:2156–2164. doi: 10.1001/jama.2010.708. [DOI] [PubMed] [Google Scholar]
- 12.Lansky AJ, Mehran R, Cristea E, et al. Impact of Gender and Antithrombin Strategy on Early and Late Clinical Outcomes in Patients With Non-ST-Elevation Acute Coronary Syndromes (from the ACUITY Trial) American Journal of Cardiology. 2009;103:1196–1203. doi: 10.1016/j.amjcard.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 13.Dauerman HL, Rao SV, Resnic FS, Applegate RJ. Bleeding avoidance strategies. Consensus and controversy J Am Coll Cardiol. 2011;58:1–10. doi: 10.1016/j.jacc.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR): building a national clinical data repository. Journal of the American College of Cardiology. 2001;37:2240–2245. doi: 10.1016/s0735-1097(01)01372-9. [DOI] [PubMed] [Google Scholar]
- 15.Messenger JC, Ho KK, Young CH, et al. The National Cardiovascular Data Registry (NCDR) Data Quality Brief: the NCDR Data Quality Program in 2012. J Am Coll Cardiol. 2012;60:1484–8. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Foundation ACoC, editor. NCDR CathPCI Registry v4.4 Coder’s Data Dictionary. 2011. [Google Scholar]
- 17.Rao SV, Kaltenbach LA, Spertus JA, Krone RJ, Singh M, Peterson ED. Contemporary Predictors of Post-Procedural Bleeding Complications Among Patients Undergoing Percutaneous Coronary Intervention (PCI): Results from the National Cardiovascular Data Registry (NCDR) Circulation. 2011;124:A13479. [Google Scholar]
- 18.Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using Inverse Probability-Weighted Estimators in Comparative Effectiveness Analyses With Observational Databases. Medical Care. 2007;45:S103–S107. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 19.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23:2937–60. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- 20.Rao SV, Ou FS, Wang TY, et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovascular interventions. 2008;1:379–86. doi: 10.1016/j.jcin.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Roe MT, Messenger JC, Weintraub WS, et al. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol. 2010;56:254–63. doi: 10.1016/j.jacc.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Mehta SK, Frutkin AD, Lindsey JB, et al. Bleeding in Patients Undergoing Percutaneous Coronary Intervention The Development of a Clinical Risk Algorithm From the National Cardiovascular Data Registry. Circulation-Cardiovascular Interventions. 2009;2:222–U107. doi: 10.1161/CIRCINTERVENTIONS.108.846741. [DOI] [PubMed] [Google Scholar]
- 23.Jolly SS, Niemelä K, Xavier D, et al. Design and rationale of the RadIal Vs. femorAL access for coronary intervention (RIVAL) trial: A randomized comparison of radial versus femoral access for coronary angiography or intervention in patients with acute coronary syndromes. American Heart Journal. 2011;161:254–260.e4. doi: 10.1016/j.ahj.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Ndrepepa G, Schuster T, Hadamitzky M, et al. Validation of the Bleeding Academic Research Consortium Definition of Bleeding in Patients with Coronary Artery Disease Undergoing Percutaneous Coronary Intervention. Circulation. 2012 doi: 10.1161/CIRCULATIONAHA.111.060871. [DOI] [PubMed] [Google Scholar]
- 25.Rao SV, O’Grady K, Pieper KS, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005;96:1200–6. doi: 10.1016/j.amjcard.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 26.Lansky AJ, Hochman JS, Ward PA, et al. Percutaneous Coronary Intervention and Adjunctive Pharmacotherapy in Women. Circulation. 2005;111:940–953. doi: 10.1161/01.CIR.0000155337.50423.C9. [DOI] [PubMed] [Google Scholar]
- 27.Alexander KP, Chen AY, Newby LK, et al. Sex Differences in Major Bleeding With Glycoprotein IIb/IIIa Inhibitors. Circulation. 2006;114:1380–1387. doi: 10.1161/CIRCULATIONAHA.106.620815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.