Abstract
BACKGROUND
The majority of thyroid cancer diagnoses in the United States are Stage I well-differentiated cancer. The use of radioactive iodine (RAI) in these low-risk patients has increased over time. The role of surgeon training in decision making regarding treatment with RAI is unknown.
METHODS
Thyroid surgeons affiliated with 368 hospitals associated with the US National Cancer Database (NCDB) were surveyed. Survey data were linked to the NCDB data. A multivariable weighted analysis controlling for surgeon and hospital characteristics was conducted to examine the relationship between surgeon training, continuing education and hospital-level RAI use for Stage I well-differentiated thyroid cancer.
RESULTS
The response rate was 70% (560/804). In both univariate and multivariable analysis controlling for hospital case volume, practice setting and surgeon specialty, training with a thyroid surgeon was associated with less RAI use for Stage I thyroid cancer (P= 0.022 and 0.028 respectively). Attending one or more professional society meetings a year was associated with a lower rate of hospital-level RAI use in univariate analysis (P= 0.044) but not multivariable analysis.
CONCLUSIONS
Training with a surgeon or group of surgeons who focus on thyroid surgery was associated with a lower proportion of Stage I thyroid cancer patients receiving RAI post total thyroidectomy. This study emphasizes the importance of surgeon training in hospital practice patterns.
INTRODUCTION
The increased incidence of thyroid cancer in the United States has been well-documented.[1, 2] According to the National Cancer Institute (NCI) Surveillance Epidemiology and End Results (SEER), thyroid cancer diagnoses have increased at a rate of 6.6 percent a year from 1997 to 2009[3] and the majority of these new cases are low-risk Stage I well-differentiated thyroid cancer.[1] Treatment for thyroid cancer typically consists of surgery, often followed by radioactive iodine (RAI). Recent clinical guidelines have recommended against RAI use in very-low risk patients (intrathyroidal cancer ≤ 1 cm) but still leave RAI use up to the provider for the majority of low-risk patients.[4] Since most peer-reviewed studies have found no statistically significant improvement in mortality or disease-specific survival in low-risk patients treated with RAI,[5] factors that affect RAI use require more study.
Although the rise in RAI use for thyroid cancer over time is well documented,[6, 7] it is not known if surgeon training and continuing education influence its use. Our previous study shows that 74% of surveyed surgeons are involved in RAI decision making and when the surgeon or endocrinologists is the primary decision maker there is less RAI use for low-risk patients than when the primary decision maker is in another specialty.[8] Prior studies suggest that surgeon training affects surgical outcomes and management.[9, 10] In addition, surgeon training has been shown to affect extent and choice of treatment.[11] We hypothesized that the knowledge and confidence obtained during surgeon training would also influence the subsequent downstream medical management of thyroid cancer patients.
To assess the relationship between surgeon training/continuing education and hospital-level RAI use for low-risk thyroid cancer, we linked surgeon survey data to data on hospital RAI use for Stage I well-differentiated thyroid cancer from the National Cancer Database (NCDB).
METHODS
Data Source and Study Population
Between 2004–2008, there were 1282 Commission on Cancer-accredited hospitals that treated thyroid cancer patients. Of these, we selected the 1159 hospitals that reported to the American College of Surgeons’ Commission on Cancer four of the five specified years. We eliminated the 235 hospitals that treated 6 or less cancer patients a year. We then randomly sampled 589 hospitals across quartiles of case volume and RAI use.
We contacted the hospital registrar at the 589 hospitals as well as searched hospital websites to identify the surgeons who performed the majority of the thyroid cancer operations at each hospital. We identified 850 surgeons affiliated with the 589 hospitals.
Prior to administering the survey, the instrument was designed and piloted in a multidisciplinary group of providers. The instrument contained both survey questions and clinical vignettes. The survey was then administered between February and June 2011. The Dillman survey method was used to encourage survey response.[12] This three wave method consists of the following: (1) an initial mailing of an introductory letter, the survey instrument, a postage-paid return envelope, and a gift; (2) a postcard reminder; (3) a second identical survey with a postage-paid return envelope to all non-responders.
Data from the returned surveys were scanned and verified. The surgeon survey responses were then linked to the NCDB, a joint project of the American College of Surgeons and the American Cancer Society.
All surgeon data was deidentified and reported in summary form only. Exemption was granted for this study by the University of Michigan Institutional Review Board.
Measures
Responses regarding residency program type, whether or not there was “a surgeon or group of surgeons who focused on thyroid surgery” in his or her training program, professional society membership, attendance at national meetings, practice setting, surgeon specialty, and awareness of the 2006 American Thyroid Association (ATA) and/or 2007 National Comprehensive Cancer Network (NCCN) clinical guidelines were obtained from the survey data. The dependent variable (the hospitals’ proportion of Stage I well-differentiated thyroid cancer patients treated with RAI after total thyroidectomy between 2004–2008) and one independent variable (hospital thyroid cancer case volume) were obtained from the NCDB.
Hospital case volume was categorized into quintiles as described by Haymart et al.5 The lower quintile was excluded as described above. The remaining four case volume categories were the following: Low (7–11 cases/year), Low-Medium (12–19 cases/year), Medium (20–34 cases/year), and High (≥ 35 cases/year). For the question pertaining to practice setting, since surgeons could select more than one choice, an algorithm described by Alderman et al. was used.[13] If academic tertiary care center was selected (including when community based academic affiliate and private practice were also selected) then the practice setting was classified as academic. When community based academic affiliate was selected (including when private practice was selected) then the practice setting was classified community. If only private practice was selected, then the practice setting was classified as private practice. A similar algorithm was employed for the question regarding type of residency program. Respondents who selected multidisciplinary cancer center and another residency program were classified as multidisciplinary cancer center. Those who selected academic medical center and community hospital were classified as academic medical center. If only community hospital was selected, then the residency program was classified as community.
Statistical Analysis
When more than one surgeon responded from the same hospital, the surveys were weighted by reported surgeon case volume. Surgeon case volume was categorized as 1, 5, 25, 50, or 100 based on the lower limit of the selected thyroid cancer case volume range per year (from the 0–4 interval, the assigned value was 1).
Univariate analysis was used to compare training and continuing education with RAI use for Stage I thyroid cancer. The surgeons were compared across specialization, training, professional membership, national meeting attendance, and clinical guidelines awareness. In addition, we looked at associations between surgeon specialty and training with a thyroid surgeon.
Multivariable weighted regression controlling for hospital case volume, practice setting, and surgeon specialty was used to analyze the relationship between training with a thyroid surgeon and RAI use. We repeated the multivariable analysis using only those surgeons who reported being involved in RAI decision making (413/560, 74%) and the findings did not change. Finally, we evaluated statistical interaction between training with a thyroid surgeon and reading the clinical guidelines.
All statistical tests were performed using SAS 9.2 (SAS Institute Inc., Cary, North Carolina). Two sided tests were used with P<0.05 considered statistically significant.
RESULTS
Forty-six of the 850 surgeons were found to be ineligible because deceased, ill, retired, no longer treating thyroid cancer patients, or incorrect mailing address. Of the 804 response eligible surgeons, 560 (70%) completed the survey. The 560 surgeons represent 368 of the sampled hospitals (62%).
Respondent Characteristics
Table 1 shows the respondent characteristics. Of the 560 survey respondents, 90% were male and 10% female. The mean surgeon age was 51 years and the surgeons averaged 19 years in practice. For practice setting, 23% currently practice at academic medical centers, 16% at a community-based academic affiliate, and 61% are in private practice. General surgeons comprised 39% of the respondents, 9% were classified as endocrine surgeons, 44% otolaryngologists, and 8% in another surgical specialty.
TABLE 1.
Surgeon characteristics (N=560)
| Characteristic | No. (%) |
|---|---|
| Gender | |
| Male | 499 (90%) |
| Female | 58 (10%) |
| Race | |
| White | 453 (83%) |
| African-American | 9 (2%) |
| Am. Indian/Alaska Native | 1 (0.2%) |
| Asian | 67 (12%) |
| Other | 18 (3%) |
| Ethnicity | |
| Hispanic | 19 (3%) |
| Practice Setting | |
| Academic | 122 (23%) |
| Community based academic affiliate | 86 (16%) |
| Private practice | 323 (61%) |
| Mean years in practice ± SD | 18.9 ± 9.6 |
| Mean age ± SD | 51.2 ± 9.5 |
Univariate Analysis
Univariate analysis of training/continuing education and RAI use between 2004–2008 for Stage I thyroid cancer is displayed in Table 2. A majority of respondents attended one or more professional society meetings per year (63%). Our analysis showed that physicians attending one or more professional society meetings per year were significantly less likely to utilize post-operative radioactive iodine for Stage I thyroid cancer (p=0.044). Three-quarters of surgeons reported having a surgeon or group of surgeons who focused on thyroid surgery in their training program. There was a statistically significant inverse relationship between training with a surgeon or group of surgeons who focused on thyroid surgery and hospital-level RAI use (p=0.022). There was no statistical interaction between training with a thyroid surgeon and attending one or more national meetings per year.
Table 2.
Univariate analysis of training/continuing education and radioactive iodine use for Stage I thyroid cancer (N=560)
| No. (%) | Mean RAI% ± SD | P value | |
|---|---|---|---|
| Residency program | 0.455 | ||
| Academic medical center | 437 (79%) | 45.41 ± 21.40 | |
| Community hospital | 66 (12%) | 47.96 ± 21.19 | |
| Multidisciplinary cancer center | 50 (9%) | 48.48 ± 19.80 | |
| Thyroid surgeon in training program | 0.022 | ||
| Yes | 414 (75%) | 44.78 ± 21.15 | |
| No | 140 (25%) | 49.51 ± 21.01 | |
| Specialization | |||
| General Surgery | 216 (39%) | 47.7 ± 21.2 | 0.387 |
| Endocrine Surgery | 48 (9%) | 44.8 ± 17.2 | |
| Otolaryngology | 241 (44%) | 44.4 ± 22.4 | |
| Other | 44 (8%) | 44.7 ± 17 | |
| Professional membership | |||
| The American College of Surgeons | 429 (77%) | 45.84 ± 20.52 | 0.809 |
| The Endocrine Society | 14 (3%) | 42.54 ± 14.34 | 0.541 |
| The American Association of Clinical Endocrinologists | 26 (5%) | 41.43 ± 16.66 | 0.264 |
| The American Thyroid Association | 46 (8%) | 44.77 ± 17.03 | 0.692 |
| The American Association of Endocrine Surgeons | 61 (11%) | 42.02 ± 17.73 | 0.124 |
| The Society of Nuclear Medicine | 0 (0%) | NA | NA |
| The American Academy of Otolaryngology-Head and Neck Surgery | 247 (44%) | 44.48 ± 21.96 | 0.142 |
| Other | 113 (20%) | 43.75 ± 19.51 | 0.214 |
| Number of national meetings attended/year | 0.044 | ||
| ≥ 1/yr. | 349 (63%) | 44.60 ± 20.34 | |
| < 1/yr. | 205 (37%) | 48.36 ± 22.47 | |
| Clinical guidelines read | |||
| ATA 2006 | 292 (52%) | 45.52 ± 20.49 | 0.613 |
| NCCN 2007 | 156 (28%) | 47.78 ± 19.71 | 0.207 |
ATA 2006= Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2006;16(2):109–141. NCCN 2007= Sherman SI, Angelos P, Ball DW, et al. Thyroid carcinoma: Clinical practice guidelines in oncology. J National Comprehensive Cancer Network 2007; 5(6):568–621.
The majority of surgeons completed their residency at an academic medical center (79%), with the remainder doing so at a community hospital (12%) or multidisciplinary cancer center (9%). A little over half of respondents reported reading the 2006 ATA guidelines and 28% had read the 2007 NCCN guidelines (clinical guidelines applicable during the 2004–2008 period of RAI use). The majority of respondents belonged to either the American College of Surgeons (77%) and/or the American Academy of Otolaryngology-Head and Neck Surgery (44%). Far fewer reported membership in the American Association of Endocrine Surgeons (11%) or the American Thyroid Association (8%). There was not a statistically significant relationship between surgeon specialty, type of residency program, professional membership, or reading the clinical guidelines and hospital-level RAI use.
Multivariable Analysis
Multivariable weighted analysis (Table 3) confirmed the significantly lower hospital-level RAI use when the affiliated surgeon trained with a surgeon or group of surgeons who focused on thyroid surgery (p = 0.028). As previously documented,[14] hospital case volume and general surgeon specialty were independently associated with a greater hospital-level use of RAI for stage I disease. In this multivariable analysis practice setting and attending one or more national meeting a year were not associated with a statistically significant difference in RAI use.
Table 3.
Multivariable analysis of hospital and training/continuing education characteristics associated with radioactive iodine use in Stage I thyroid cancer (N=560)
| Proportion treated with RAI at hospital (Mean % ± SD) | Multivariable P value | |
|---|---|---|
| Hospital characteristics | ||
| Case volume | ||
| Low | 40.13 ± 25.66 | <0.001* |
| Low-Mod | 48.63 ± 21.97 | 0.222 |
| Moderate | 44.29 ± 20.14 | 0.017* |
| High | 48.58 ± 17.75 | Ref. |
| Practice Setting | ||
| Academic | 44.91 ± 17.34 | Ref. |
| Community | 44.09 ± 19.52 | 0.354 |
| Private | 46.48 ± 22.92 | 0.283 |
| Surgeon specialty | ||
| General surgeon | 47.73 ± 21.23 | 0.015* |
| Endocrine surgeon | 44.78 ± 17.24 | 0.536 |
| Other | 44.67 ± 16.95 | 0.619 |
| Otolaryngology | 44.44 ± 22.44 | Ref. |
| Training/continuing education | ||
| Train with thyroid surgeon | ||
| Yes | 44.78 ± 21.15 | 0.028* |
| No | 49.51 ± 21.01 | |
| Attend ≥ 1 national meeting/year | ||
| Yes | 44.60 ± 20.34 | 0.905 |
| No | 48.36 ± 22.47 |
Sub-group Analysis
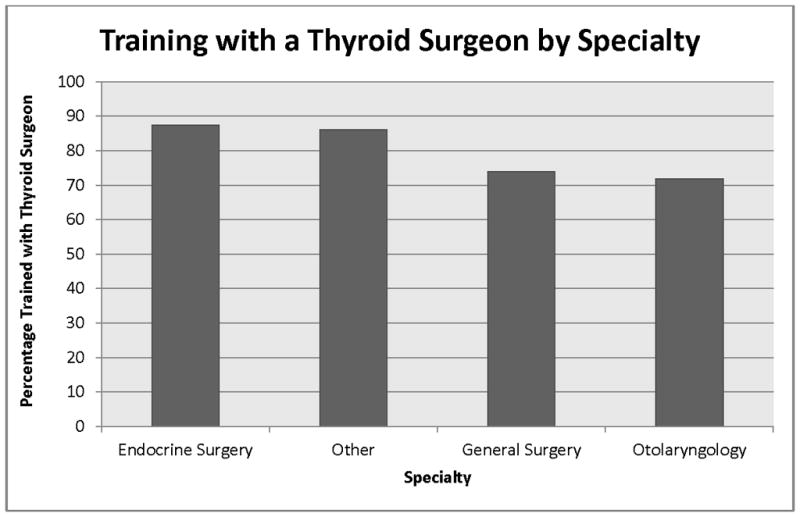
We found an association between surgeon specialty and likelihood of training with a surgeon or group of surgeons who focused on thyroid surgery (p=0.042). While 88% of endocrine surgeons trained with a surgeon or group of surgeons with thyroid cancer expertise, 72% and 74% of otolaryngologists and general surgeons, respectively, trained with a thyroid surgeon (Figure 1).
Figure 1.
Discussion
The results of this study improve our understanding of the relationship between surgeon training/continuing education and hospital RAI use. In univariate analysis, attending one or more professional society meetings per year was associated with less hospital-level RAI use for Stage I thyroid cancer. In both univariate and multivariable analysis, training with a surgeon or group of surgeons who focused on thyroid surgery was associated with lower hospital rates of RAI use for Stage I thyroid cancer. Otolaryngologists and endocrine surgeons were least and most likely, respectively, to have a surgeon or group of surgeons who focused on thyroid surgery at their training program.
As described in Haymart et al., between 1990 and 2008, patients diagnosed with low risk thyroid cancer were being treated with RAI at an increasing rate after total thyroidectomy.[6] There was wide variation in RAI use with a large proportion of it being attributed to unexplained hospital characteristics.[6] Within the hospital are physician decision makers. As one of the primary decision makers in the care of thyroid cancer patients, the surgeon’s attitudes toward treatment decisions are very important. This current study sheds light on how those attitudes are formed.
Previous studies have evaluated the relationship between surgeon specialization or thyroid surgical volume and surgical outcomes.[9, 10, 15] In addition, studies have evaluated the role of surgical training on surgeon competence and confidence.[16–18] Our study is novel since it evaluates surgical training and subsequent medical management. This study suggests that surgeon training either directly influences surgeon decision-making on medical management post-surgery or alternatively is associated with hospital-wide practice patterns.
One plausible explanation for our findings in this study is that training informs the manner in which a surgeon is likely to continue to practice. Recent data from general surgery show that surgeons in residency training have variable, and often limited, exposure to some procedures that program directors believe they should be competent to perform independently at the completion of residency.[19] This variation in experience provided by training programs may explain why some surgery residents worry that they will not feel confident performing procedures independently and believe that they must complete further specialty training to be fully competent.[16] The presence of an endocrine surgery program in a specific general surgery training program can enhance the technical skill and experience of the finishing residents.[17] Although the increased number of endocrine procedures performed in the United States has resulted in both general surgery and otolaryngology residents gaining more experience during residency, otolaryngology residents participate in nearly twice as many thyroid and parathyroid procedures as general surgery residents.[20] However, there is substantial variability among training programs in both general surgery and otolaryngology, as the maximum number of thyroid cases performed during residency is more than five times the mean.[20] Thus, it seems possible that training with a thyroid surgeon provides increased focused experience that instills greater confidence in surgical skills and a more sophisticated view of thyroid disease management. This could allow a surgeon to feel less dependent on RAI, and to have a more nuanced understanding of the indications for RAI. In addition, it is also possible that the confidence of other colleagues in the adequacy of surgical resection influences hospital RAI use such that a well-trained surgeon and colleagues involved in decision making are less reliant on RAI.
The strengths of this study include a large sample size, a diverse group of surgeon specialties, a high response rate among surgeons, a novel research design, and data on hospital-level RAI use. There are also limitations. First, nonresponse bias is a known limitation of survey studies. Next, while survey questions are a common method of assessing provider practice patterns,[13, 21, 22] we cannot be sure that provider report alone is adequate. Third, we cannot verify that the respondents treated all patients at the affiliated hospital. To remediate this potential limitation, we requested the hospital registrars provide us with the names of the highest volume thyroid surgeons and as previously described in Methods, and if more than one surgeon was surveyed from the same hospital, we then weighted the answers according to surgeon reported case volume.
In conclusion, as the use of RAI in stage I well-differentiated thyroid cancer patients increases[6, 7] and its appropriateness is debated, [5, 23, 24] it is important to identify the characteristics of the decision-makers that are associated with a lesser or greater likelihood of treating with RAI after thyroidectomy. This study finds a significant association between training with a thyroid surgeon during residency or fellowship and lower hospital-level use of RAI for Stage I thyroid cancer. The results suggest that practice patterns are formed early since training with a thyroid surgeon had more influence on treatment patterns than meeting attendance, professional society membership, and clinical guideline awareness. These findings have implications for both future intervention studies and for surgeon training. These results suggest that to initiate change in hospital practice patterns with potential for improved adherence to clinical guidelines; we need to focus on physician training.
Synopsis.
Use of radioactive iodine (RAI) in low-risk thyroid cancer patients has increased over time. We found a significant relationship between training with a thyroid surgeon during residency or fellowship and lower hospital-level use of RAI for Stage I thyroid cancer.
Acknowledgments
We would like to thank Brittany Gay, Barbara Salem, and Ashley Gay who participated in data collection and processing. Cornell University Survey Research Institute scanned the surveys for data file. Dr. Haymart is funded by grant K07CA154595-02 from the National Institutes of Health, the University of Michigan Comprehensive Cancer Center Idea Award, the Cancer Surveillance and Outcomes Research Team (CanSORT) Pilot of Feasibility Fund, and the Elizabeth Caroline Crosby Fund.
Footnotes
The authors have nothing to disclose.
Contributor Information
Kathryn M. Schuessler, Department of Health Management & Policy, University of Michigan.
Mousumi Banerjee, Department of Biostatistics, University of Michigan.
Di Yang, Department of Biostatistics, University of Michigan.
Andrew K. Stewart, Senior Manager, National Cancer Database, Commission on Cancer, The American College of Surgeons.
Gerard M. Doherty, Department of Surgery, Boston University.
Megan R. Haymart, Metabolism, Endocrinology, and Diabetes and Hematology/Oncology, Department of Medicine, University of Michigan Health System.
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 3.SEER Stat Facts Sheet: Thyroid. 2012 [Google Scholar]
- 4.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 5.Sacks W, Fung CH, Chang JT, et al. The effectiveness of radioactive iodine for treatment of low-risk thyroid cancer: a systematic analysis of the peer-reviewed literature from 1966 to April 2008. Thyroid. 2010;20:1235–1245. doi: 10.1089/thy.2009.0455. [DOI] [PubMed] [Google Scholar]
- 6.Haymart MR, Banerjee M, Stewart AK, et al. Use of radioactive iodine for thyroid cancer. JAMA. 2011;306:721–728. doi: 10.1001/jama.2011.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyer NG, Morris LG, Tuttle RM, et al. Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer. 2011;117:4439–4446. doi: 10.1002/cncr.26070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haymart MR, Banerjee M, Yang D, et al. The role of clinicians in determining radioactive iodine use for low-risk thyroid cancer. Cancer. 2012 Jun 28; doi: 10.1002/cncr.27721. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilimoria KY, Phillips JD, Rock CE, et al. Effect of surgeon training, specialization, and experience on outcomes for cancer surgery: a systematic review of the literature. Ann Surg Oncol. 2009;16:1799–1808. doi: 10.1245/s10434-009-0467-8. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhury MM, Dagash H, Pierro A. A systematic review of the impact of volume of surgery and specialization on patient outcome. Br J Surg. 2007;94:145–161. doi: 10.1002/bjs.5714. [DOI] [PubMed] [Google Scholar]
- 11.Wu AW, Wang MB, Nguyen CT. Surgical practice patterns in the treatment of papillary thyroid microcarcinoma. Arch Otolaryngol Head Neck Surg. 2010;136:1182–1190. doi: 10.1001/archoto.2010.193. [DOI] [PubMed] [Google Scholar]
- 12.Dillman DA. Mail and Internet Surveys: The Tailored Design Method. 2. New York: Wiley; 2007. Edition New York. [Google Scholar]
- 13.Alderman AK, Hawley ST, Waljee J, et al. Correlates of referral practices of general surgeons to plastic surgeons for mastectomy reconstruction. Cancer. 2007;109:1715–1720. doi: 10.1002/cncr.22598. [DOI] [PubMed] [Google Scholar]
- 14.Haymart M, Banerjee M, Yang D, Stewart A, Doherty G, Koenig R, Griggs J. The Relationship between Extent of Thyroid Cancer Surgery and Use of Radioactive Iodine. Ann Surg. 2012 doi: 10.1097/SLA.0b013e31826c8915. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sosa JA, Bowman HM, Tielsch JM, et al. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg. 1998;228:320–330. doi: 10.1097/00000658-199809000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeo H, Viola K, Berg D, et al. Attitudes, training experiences, and professional expectations of US general surgery residents: a national survey. JAMA. 2009;302:1301–1308. doi: 10.1001/jama.2009.1386. [DOI] [PubMed] [Google Scholar]
- 17.Wiseman JE, Ituarte PH, Ro K, et al. The effect of a dedicated endocrine surgery program on general surgery training: a single institutional experience. Am J Surg. 2011 doi: 10.1016/j.amjsurg.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Solorzano CC, Sosa JA, Lechner SC, et al. Endocrine surgery: where are we today? A national survey of young endocrine surgeons. Surgery. 2010;147:536–541. doi: 10.1016/j.surg.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 19.Bell RH, Jr, Biester TW, Tabuenca A, et al. Operative experience of residents in US general surgery programs: a gap between expectation and experience. Ann Surg. 2009;249:719–724. doi: 10.1097/SLA.0b013e3181a38e59. [DOI] [PubMed] [Google Scholar]
- 20.Zarebczan B, McDonald R, Rajamanickam V, et al. Training our future endocrine surgeons: a look at the endocrine surgery operative experience of U.S. surgical residents. Surgery. 2010;148:1075–1080. doi: 10.1016/j.surg.2010.09.032. discussion 1080–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz SJ, Hawley ST, Abrahamse P, et al. Does it matter where you go for breast surgery?: attending surgeon’s influence on variation in receipt of mastectomy for breast cancer. Med Care. 2010;48:892–899. doi: 10.1097/MLR.0b013e3181ef97df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jagsi R, Abrahamse P, Morrow M, et al. Coordination of Breast Cancer Care Between Radiation Oncologists and Surgeons: A Survey Study. Int J Radiat Oncol Biol Phys. 2012;82:2072–2078. doi: 10.1016/j.ijrobp.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hay ID. Managing patients with a preoperative diagnosis of AJCC/UICC stage I (T1N0M0) papillary thyroid carcinoma: East versus West, whose policy is best? World J Surg. 2010;34:1291–1293. doi: 10.1007/s00268-010-0469-5. [DOI] [PubMed] [Google Scholar]
- 24.Mazzaferri EL. What is the optimal initial treatment of low-risk papillary thyroid cancer (and why is it controversial)? Oncology (Williston Park) 2009;23:579–588. [PubMed] [Google Scholar]


