Abstract
Background:
Coriandrum sativum has been used in the traditional systems of medicine for management of arthritis and other inflammatory disorders.
Objectives:
In this study, we have evaluated the anti-inflammatory and anti-granuloma activities of Coriandrum sativum hydroalcoholic extract (CSHE) in experimental models.
Materials and Methods:
The anti-inflammatory activity of CSHE was evaluated using carrageenan-induced paw edema model and the anti-granuloma activity of CSHE was evaluated using the subcutaneous cotton pellet implantation-induced granuloma formation and stimulation of peritoneal macrophages with complete Freund's adjuvant. Serum tumor necrosis factor-α (TNF-α), IL-6, IL-1 β levels, and peritoneal macrophage expression of TNF-R1 were evaluated as markers of global inflammation.
Results:
CSHE at the highest dose tested (32 mg/kg) produced a significant reduction (P < 0.05) in paw edema after carrageenan administration. CSHE treatment also reduced dry granuloma weight in all treated animals. Serum IL-6 and IL-1 β levels were significantly (P < 0.05) lower in the CSHE (32 mg/kg)-treated group as compared to control. Although there was an increase in serum TNF-α level in the CSHE-treated group as compared to control, TNF-R1 expression on peritoneal macrophages was found to be reduced.
Conclusion:
Thus, the result of this study demonstrates the anti-inflammatory and anti-granuloma activities of CSHE in experimental models, and validates its traditional use for the management of arthritis and other inflammatory disorders.
Keywords: Adjuvant, carrageenan, cotton pellet granuloma, pro-inflammatory cytokines
INTRODUCTION
Coriandrum sativum L. (Family: Apiaceae) is a small, erect, branched, sweet smelling, perennial herb that is widely distributed in South Asia, the Mediterranean region, and in America. It is commonly known as coriander, phakchi, Chinese parsley, cilandrio, and cilantro. In the traditional systems of medicine, formulations containing CS seed have been used in the management of rheumatoid arthritis and other inflammatory diseases.[1–3]
Though widely used in the traditional systems of medicine, experimental studies with this plant are scarce. The anti-inflammatory activity of this plant extract has been demonstrated in carrageenan-induced paw edema in experimental animals.[4,5] In a recent study from our laboratory, we demonstrated the disease-modifying activity of Coriandrum sativum hydroalcoholic extract (CSHE) in complete Freund's adjuvant (CFA)-induced arthritis in Wistar rats.[6] Disease markers, viz., joint swelling, serum and synovial expression of pro-inflammatory cytokines (predominantly secreted by macrophages), were found to be reduced in the CSHE-treated animals as compared to arthritis control. However, in this model, there is an involvement of different types of pro-inflammatory cells in development of the disease state.[7,8] A reduction in these disease markers was also observed in the indomethacin-treated animals. Therefore, it could not be definitely concluded whether the anti-inflammatory activity or the disease-modifying activity of the test drug was primarily responsible for the observed effects.
This study was thus designed to evaluate the involvement of anti-inflammatory activity of CSHE at the doses tested. Carrageenan-induced paw edema model was used to evaluate the effect of CSHE on autacoid mediators of inflammation. Among pro-inflammatory mechanisms associated with the development and progression of granulomatous disorders such as rheumatoid arthritis, macrophage activation and dysfunction, are known to be central players.[8,9] Therefore, the disease-modifying activity of CSHE was evaluated in terms of its anti-macrophage activity. Subcutaneous cotton pellet implantation-induced granuloma formation was used for evaluation of anti-macrophage activity as this model is depictive of circulating macrophage activation and aggregation.[10]
MATERIALS AND METHODS
Animals
Adult male Wistar albino rats (150-180 g) from our institutional breeding stock were used in the study. Animals were housed at 25°C ± 2°C in clean polypropylene cages in batches of three with free access to standard pellet diet and drinking water. Experiments were carried out after approval by the Institutional Animal Ethics Committee, All India Institute of Medical Sciences, New Delhi, in accordance with “Guidelines for care and use of animals in scientific research (Indian National Science Academy 1998, Revised 2000).”
Test drug
In a previous report from our laboratory, we demonstrated the anti-arthritic activity of the test drug CSHE in experimental models.[6] The same test drug was employed in the present study. Briefly, the dried seeds of CS were obtained from the local market and authenticated by Professor Mohammad Ali, pharmacognosist and phytochemist at Jamia Hamdard University, New Delhi, India, as C. sativum L. (Family: Apiaceae), and a voucher specimen (PRL/JH/08/04) was submitted at the herbarium for future reference. The seeds were coarsely grounded and the powder was extracted by cold maceration with 50% v/v methanol for 72 h. The slurry was filtered through cotton wool to separate the suspended particles. The solvent was then evaporated under reduced pressure till a resinous extract was obtained. The extract was golden brown in color and the total quantitative yield was 13.86% w/w. CSHE was further subjected to pharmacognostical standardization for detection of secondary plant metabolites[11] and was found to contain sugars, alkaloids, tannins, resins, flavonoids, fatty acids, and sterols. Toxicity studies previously carried out with the plant extract demonstrated an oral LD50 value >2,000 mg/kg in rats.[6] No overt physiological changes were observed in any of the CSHE-treated animals after oral administration of the plant extract at a dose of 160 mg/kg for 28 days.[6]
Induction of paw edema by carrageenan administration
After acclimatization to the laboratory conditions, animals were divided into five groups (n = 6) and fasted overnight with access to water ad libitum. Baseline paw volume recording was carried out using a digital plethysmometer (Ugo Basile, Italy). Thereafter, group I received vehicle (2 mL/kg body weight 1% gum acacia) and served as the control, group II received indomethacin (3 mg/kg body weight), and groups III, IV, and V received CSHE at a dose of 8, 16, and 32 mg/kg body weight, respectively. Dose selection of the test drug was done on the basis of our previous report.[6] Sixty minutes after administration of the vehicle/drug, experimental paw edema was induced by subcutaneous administration of 0.1 mL of 1% λ-carrageenan (freshly constituted in normal saline) into the left hind paw of the animal.[12] Paw volume was again measured at 1 h, 3 h, and 6 h post-carrageenan administration. Paw edema was determined as described by us in a previous study.[13]
Induction of granuloma formation by subcutaneous cotton pellet implantation
Animals were divided into five groups (n = 6) and maintained under aseptic condition for the entire duration of the study. After overnight fasting, group I received vehicle (2 mL/kg body weight 1% gum acacia) and served as control, group II received indomethacin (3 mg/kg) and groups III, IV, and V received CSHE at a dose of 8 mg/kg, 16 mg/kg, and 32 mg/kg, respectively. Thirty minutes after administration of drug/vehicle, the animal was anaesthetized with diethyl-ether and a sterile cotton pellet (made from bleached cotton weighing 30 ± 1 mg) was implanted subcutaneously, bilaterally under the axilla.[13] This was designated as day 0. Drug/vehicle treatment of the respective groups was continued for 6 more days. On day 7, terminal blood collection was carried out and the cotton pellets were excised. Serum was separated by centrifuging at 3,000 rpm and used for estimation of circulating cytokines by enzyme linked immune sorbent assay (ELISA) and dot blot analysis. The cotton pellets were dried overnight in a hot air oven (60°C) till a constant weight was recorded at two consecutive time points. The difference between the initial and post-implantation weight was considered to be the dry weight of granuloma tissue.[14]
Estimation of serum tumor necrosis factor-α levels
Tumor necrosis factor-α (TNF-α) level was estimated in serum of treated animals using a commercially available ELISA kit (U-CyTech Biosciences, The Netherlands) as per the manufacturer's instructions.
Estimation of serum IL-6 and IL-1 β levels
Measurement of circulating IL-6 and IL-1 β protein was carried out by dot-blot analysis in serum of control, indomethacin, and highest dose CSHE (32 mg/kg) treated animals.[13] Briefly, 3 μL of serum from each sample was diluted to 10 μL with 10 mM PBS (pH 7.2) and spotted on to nitrocellulose membranes and dried at room temperature. After blocking the non-specific sites, the cytokines were detected with anti-IL-6/anti-IL-1 β polyclonal primary antibody (Santa Cruz Biotechnology, Inc, Santa Cruz, USA) followed by corresponding (Horse Radish Peroxidase) HRP-conjugated secondary antibody (Bangalore Genei, India). The blots were developed with nickel-enhanced DAB (di-aminobenzidine) substrate (Vector Labs, USA). Cytokine protein expression was measured by determining the intensity of darkness of dots by a densitometer (Alpha Imager EC Gel Doc System, CA, USA) using Alpha-View imaging software.
Stimulation of peritoneal macrophages
Three groups (n = 3) of male Wistar albino rats were used in this study. Peritoneal macrophages were stimulated according to the method described by us earlier.[13] After overnight fasting, group I was administered vehicle (2 mL/kg 1% gum acacia) and served as the control, group II was administered indomethacin (3 mg/kg), and group III was administered CSHE (32 mg/kg) by gavage. Thirty minutes post-administration of drug/vehicle, 0.1 mL of CFA (0.05% w/v dead Mycobacterium butyricum in mineral oil) was injected into the peritoneal cavity using a 26-gauge needle.[15,16] This was designated as day 0. Drug/vehicle administration was continued for 6 more days.
Isolation and culture of rat peritoneal macrophages
On day 7, animals were sacrificed by an overdose of anesthetic ether. Sterile RPMI 1640 media (10 mL) was injected into the peritoneal cavity and the abdomen was massaged for 5 min. The media was then aspirated under sterile conditions and transferred into a 15 mL centrifuge tube. The cellular content was pelleted out by centrifuging at 1,000 × g for 10 min at room temperature. The pellet was re-suspended and cultured aseptically in 60 mm culture dishes as described by us earlier.[13] The supernatant was then discarded and the adherent macrophages were washed twice with ice cold 10 mM PBS and scraped off. Culture purity of macrophages was determined according to the method of Ernist and Jones as described earlier.[13,17]
Preparation of cell lysate and western blotting
The macrophage pellet was lysed by strong vortexing in 100 μL of lysis buffer containing 50 mM Tris–HCl (pH 7.4), 300 mM NaCl, 0.5% (v/v) Triton X-100, 5 mM EDTA with 2 mM PMSF, 10 μg/mL leupeptin, and 10 U/mL aprotinin. The lysate was then kept on ice for 30 min and centrifuged at 10,000 × g for 15 min at 4°C. Supernatant was collected and protein concentration was determined by Bradford method[18] using bovine serum albumin as the standard.
Seventy-five microgram of protein was separated in 12% polyacrylamide gels (1.0 mm thick) overlaid with 4% stacking gel in SDS according to the standard procedure of Laemmli.[19] The separated proteins were then transferred onto nitrocellulose membrane under an electric potential.[20] After blocking the non-specific sites, detection of TNF-R1 and β-actin protein expression was carried out using anti-TNF-R1 or anti-β-actin polyclonal primary antibody (Santa Cruz Biotechnology, Inc, Santa Cruz, USA), HRP-conjugated polyclonal secondary antibody (Bangalore Genei, India), and femtoLUCENT detection kit (G-Biosciences, USA).[13] Protein expression was quantified by determining the intensity of darkness of bands by a densitometer (Alpha Imager EC Gel Doc System, CA, USA) using Alpha-View imaging software. Expression of TNF-R1 receptor protein was expressed as percentage integrated density value (%IDV) after normalization against β-actin.
Statistical analysis
Difference between groups was analyzed by One-way ANOVA followed by Dunnett's Multiple Comparison (GraphPad InStat; Version 3.05). P <0.05 was considered significant.
RESULTS
Coriandrum sativum hydroalcoholic extract administration reduced paw edema in carrageenan-induced paw edema model
Subplantar administration of carrageenan produced paw edema that was persistent in all the tested animals throughout the observation period. Maximum paw edema was observed at 6 h post-carrageenan administration [Figure 1]. The standard drug indomethacin produced a significant (P < 0.05) reduction in paw edema at 3 h and 6 h post-carrageenan administration. CSHE treatment produced a significant (P < 0.05) reduction in paw edema at the higher two doses (16 mg/kg and 32 mg/kg), 1 h and 3 h post-carrageenan administration. The reduction in paw edema at these two time points was greater than 40% and can be considered to be biologically significant. Although the highest dose treatment (32 mg/kg) produced a statistically significant (P < 0.05) reduction in paw edema at 6 h post-carrageenan administration, the percentage inhibition was less than 20% and therefore, could not be considered to be biologically significant.
Figure 1.
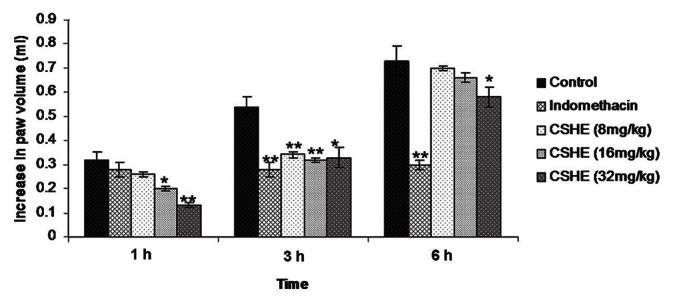
Coriandrum sativum hydroalcoholic extract treatment produced signifi cant inhibition of carrageenan-induced paw edema in rats. Each bar represents the mean±SE of six animals. Statistical analysis by One-way ANOVA followed by Dunnett's multiple comparison. *P < 0.05; **P < 0.01
Coriandrum sativum hydroalcoholic extract administration reduced cotton pellet implantation-induced granuloma formation
Implantation of sterile cotton pellets subcutaneously under the axilla led to the formation of granuloma tissue around the foreign body in all animals [Figure 2]. Both indomethacin and CSHE (all tested doses) treatment produced a significant (P < 0.05) decrease in the dry granuloma weight as compared to control animals. Maximum anti-granuloma activity was observed in the 32 mg/kg CSHE-treated group.
Figure 2.
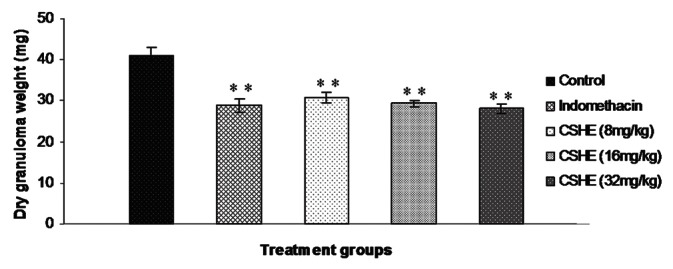
Coriandrum sativum hydroalcoholic extract treatment produced signifi cant reduction in dry granuloma weight in rats. Each bar represents the mean±SE of six animals. Statistical analysis byOne-way ANOVA followed by Dunnett's multiple comparison. *P < 0.05; **P < 0.01
Effect of Coriandrum sativum hydroalcoholic extract treatment on pro-inflammatory cytokines
Circulating TNF-α level in normal animals was not in the detectable range with the kit that was used in the study (U-CyTech Biosciences, The Netherlands). Subcutaneous cotton pellet implantation produced an increase in circulating TNF-α level in all the tested animals [Figure 3]. Indomethacin treatment produced a significant (P < 0.05) two-fold increase in circulating TNF-α level as compared to control animals. A similar increase in circulating TNF-α level was also observed in the 32 mg/kg CSHE-treated group and this increase was statistically significant as compared to the control.
Figure 3.
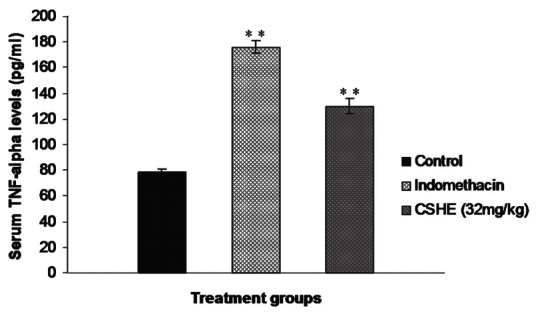
Effect of Coriandrum sativum hydroalcoholic extract treatment on serum tumor necrosis factor-α levels after subcutaneous cotton pellet implantation in Wistar rats. Each bar represents the mean±SE of six animals. Statistical analysis by One-way ANOVA followed by Dunnett's multiple comparison. *P < 0.05; **P < 0.01
A statistically significant decrease in circulating IL-6 level was observed in the indomethacin-treated group as compared to control [Figure 4]. Although there was a slight increase in IL-1 β levels in the indomethacin-treated animals as compared to control, this increase was not statistically significant [Figure 5]. CSHE (32 mg/kg) treatment produced a statistically significant decrease in circulating levels of both pro-inflammatory cytokines as compared to control.
Figure 4.
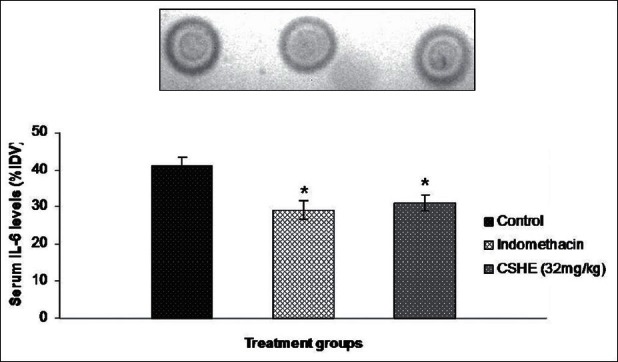
Coriandrum sativum hydroalcoholic extract treatment produced a signifi cant reduction in serum IL-6 levels after subcutaneous cotton pellet implantation in Wistar rats. Each bar represents the mean±SE of six animals. Statistical analysis by One-way ANOVA followed by Dunnett's multiple comparison. *P < 0.05; **P < 0.01
Figure 5.
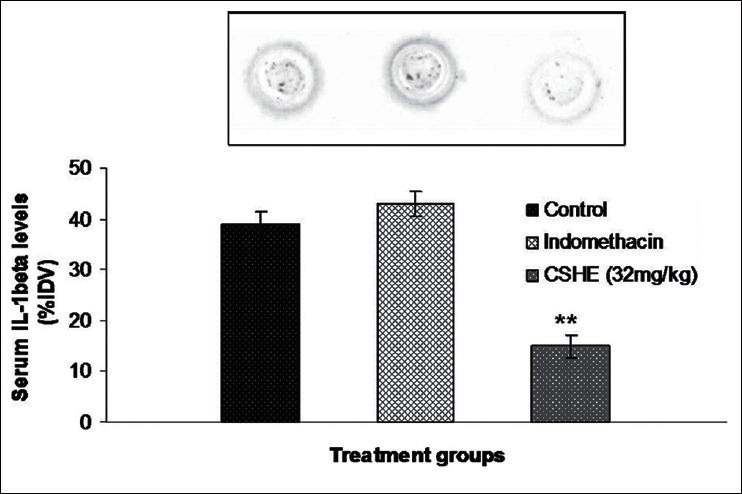
Coriandrum sativum hydroalcoholic extract treatment produced a significant reduction in serum IL-1 α levels after subcutaneous cotton pellet implantation in Wistar rats. Each bar represents the mean± SE of six animals. Statistical analysis by One-way ANOVA followed by Dunnett's multiple comparison. **P < 0.01
Coriandrum sativum hydroalcoholic extract administration decreased TNF-R1 protein expression in peritoneal macrophage
Initially, %IDV values were calculated separately for TNF-R1 and β-actin for each sample. Thereafter, normalization of TNF-R1 protein expression in each sample was carried out by determining the amount of TNF-R1 expressed per unit of β-actin ([%IDV-TNF-R1/%IDV-β-actin] ×100). Comparison of normalized data demonstrated a significant (P < 0.05) reduction in macrophage TNF-R1 protein expression in the indomethacin and CSHE (32 mg/kg)-treated groups as compared to control [Figure 6]. CSHE (32 mg/kg) treatment produced a greater inhibition of TNF-R1 expression as compared to indomethacin (3 mg/kg).
Figure 6.
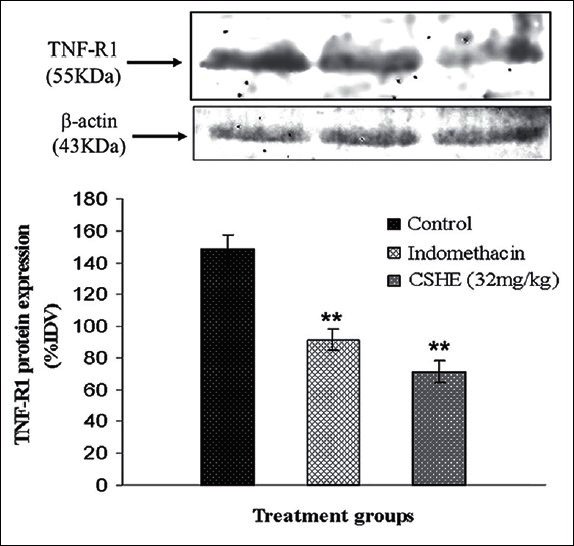
Coriandrum sativum hydroalcoholic extract treatment produced a signifi cant reduction in tumor necrosis factor-R1 expression in CFA stimulated rat peritoneal macrophages. Each bar represents the mean±SE of 3 animals. Statistical analysis by One-way ANOVA followed by Dunnett's multiple comparison. *P < 0.05; **P < 0.01
DISCUSSION
In this study, we have evaluated the pharmacological activities of CSHE in experimental models of acute inflammation and granuloma formation in rats. The carrageenan-induced paw edema model was used to evaluate the inhibitory activity of CSHE against autacoid mediators. Protection against formation of edema during the initial phase (0-2 h) is considered to depict inhibitory effect on amino acid mediators and activity during the late phase (3-6 h) is considered to be depictive of activity against arachidonic acid derivatives, mainly prostaglandins.[21] In our study, CSHE treatment at the highest dose produced a statistically significant reduction in paw edema at all observation points, indicating inhibitory activity against multiple autacoid mediators.
In cotton pellet-induced granuloma model, a significant (P < 0.05) reduction in dry granuloma weight was observed in the CSHE-treated groups as compared to control. Cotton pellet-induced granuloma formation is considered to be a reliable experimental model for evaluation of effects on macrophage dysfunction and granuloma formation,[14,22] central players in the formation, maintenance and progression of granulomas in various disease states.[9] Efficacy of CSHE in this model is therefore depictive of inhibitory activity against macrophage activation, infiltration, and aggregation. In addition, a reduction in primarily macrophage-derived pro-inflammatory cytokines, viz., IL-6 and IL-1 β, and the cytokine receptor TNF-R1 was also observed in the CSHE-treated group as compared to control animals. The effect of CSHE on cytokine profile was slightly different from that observed with indomethacin. Both drugs increased circulating TNF-α levels and decreased circulating IL-6 levels. However, while indomethacin treatment did not affect IL-1 β levels, CSHE treatment produced a statistically significant reduction in circulating cytokine level as compared to control. The effects observed with indomethacin on cytokine levels are similar to previously published reports.[6,13,23–25]
Although both test and standard drug produced an increase in circulating TNF-α levels, there was a statistically significant reduction in peritoneal macrophage TNF-R1 expression in these two groups. This phenomenon cannot be explained at the moment and mechanistic studies will be required to delineate the same. However, this finding is important as most of the pathological effects of TNF-α are mediated through TNF-R1.[26,27]
As CSHE is a compound formulation containing a large number of phytochemicals, it is difficult to attribute the observed effects to any one particular chemical moiety. However, in light of experimental evidence, at least a part of this activity could be attributed to the presence of β-sitosterol and β-sitosterolin in coriander seeds.[28] In experimental studies, these phytochemicals have been shown to reduce phologistic agent-induced paw edema and inhibit secretion of pro-inflammatory cytokines by macrophages.[29,30] In our study also, a reduction was observed in IL-6, IL-1 β, and TNF-R1 expression in treated animals as compared to control which could have contributed toward inhibition of macrophage activation, infiltration, and aggregation.
The results of our present study thus validate the traditional use of CS in the management of chronic inflammatory and granulomatous disorders.
ACKNOWLEDGMENT
The authors thank Indian Council of Medical Research, New Delhi, India, for providing financial assistance required for carrying out the research work.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Khare CP. Indian Medicinal Plants – An Illustrated Dictionary. New York: Springer; 2007. Coriandrum sativum Linn; p. 174. [Google Scholar]
- 2.Said M. Indian Medical Science Series. Delhi: Sri Satguru Publications; 1997. Hamdard Pharmacopoeia of Eastern Medicine. [Google Scholar]
- 3.Singh B, Chaurasia OP, Jadhav KL. An ethnobotanical study of Indus valley (Ladakh) J Econ Tax Bot Addl Ser. 1996;12:92–101. [Google Scholar]
- 4.Ammar NM, Al-Okbi SY, Mohamed D. Study of the anti-inflammatory activity of some medicinal edible plants growing in Egypt. J Islamic Acad Sci. 1997;10:113–22. [Google Scholar]
- 5.Mascolo N, Autore G, Capasso F. Biological screening of Italian medicinal plants for anti-inflammatory activity. Phytother Res. 1987;1:28–31. [Google Scholar]
- 6.Nair V, Singh S, Gupta YK. Evaluation of disease modifying activity of Coriandrum sativum in experimental models. Indian J Med Res. 2012;135:240–5. [PMC free article] [PubMed] [Google Scholar]
- 7.Billiau A, Matthys P. Modes of action of Freund's adjuvants in experimental models of autoimmune diseases. J Leukoc Biol. 2001;70:849–60. [PubMed] [Google Scholar]
- 8.Kinne RW, Bräuer R, Stuhlmüller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2:189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behrens EM. Macrophage activation syndrome in rheumatic disease: What is the role of the antigen presenting cell? Autoimmun Rev. 2008;7:305–8. doi: 10.1016/j.autrev.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 10.Adams DO. The granulomatous inflammatory response. Am J Pathol. 1976;84:164–91. [PMC free article] [PubMed] [Google Scholar]
- 11.Dey PM, Harborne J. London: Academic Press; 1987. Methods in Plant Biochemistry. [Google Scholar]
- 12.Winter CA, Risley EA, Nuss GW. Anti-Inflammatory and antipyretic activities of indomethacin, 1-(p-chlorobenzoyl)-5-methoxy-2-methylindole-3-acetic acid. J Pharmacol Exp Ther. 1963;141:369–76. [PubMed] [Google Scholar]
- 13.Nair V, Kumar R, Singh S, Gupta YK. Investigation into the anti-inflammatory and antigranuloma activity of Colchicum luteum Baker in experimental models. Inflammation. 2012;35:881–8. doi: 10.1007/s10753-011-9389-2. [DOI] [PubMed] [Google Scholar]
- 14.Meier R, Schuler W, Desaulles P. On the mechanism of cortisone inhibition of connective tissue proliferation. Experientia. 1950;6:469–71. doi: 10.1007/BF02154110. [DOI] [PubMed] [Google Scholar]
- 15.Chensue SW, Remick DG, Shmyr-Forsch C, Beals TF, Kunkel SL. Immunohistochemical demonstration of cytoplasmic and membrane-associated tumor necrosis factor in murine macrophages. Am J Pathol. 1988;133:564–72. [PMC free article] [PubMed] [Google Scholar]
- 16.Toth LA, Dunlap AW, Olson GA, Hessler JR. An evaluation of distress following intraperitoneal immunization with Freund's adjuvant in mice. Lab Anim Sci. 1989;39:122–6. [PubMed] [Google Scholar]
- 17.Ennist DL, Jones KH. Rapid method for identification of macrophages in suspension by acid alpha-naphthyl acetate esterase activity. J Histochem Cytochem. 1983;31:960–3. doi: 10.1177/31.7.6189884. [DOI] [PubMed] [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vinegar R, Schreiber W, Hugo R. Biphasic development of carrageenin edema in rats. J Pharmacol Exp Ther. 1969;166:96–103. [PubMed] [Google Scholar]
- 22.Wu HT, Chang CK, Tsao CW, Wen YJ, Ling SM, Cheng KC, et al. Insulin resistance without obesity induced by cotton pellet granuloma in mice. Lab Invest. 2009;89:362–9. doi: 10.1038/labinvest.2008.161. [DOI] [PubMed] [Google Scholar]
- 23.Anderson GD, Hauser SD, McGarity KL, Bremer ME, Isakson PC, Gregory SA. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J Clin Invest. 1996;97:2672–9. doi: 10.1172/JCI118717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sacerdote P, Carrabba M, Galante A, Pisati R, Manfredi B, Panerai AE. Plasma and synovial fluid interleukin-1, interleukin-6 and substance P concentrations in rheumatoid arthritis patients: Effect of the nonsteroidal anti inflammatory drugs indomethacin, diclofenac and naproxen. Inflamm Res. 1995;44:486–90. doi: 10.1007/BF01837915. [DOI] [PubMed] [Google Scholar]
- 25.Yang Z, Nandi J, Wang J, Bosco G, Gregory M, Chung C, et al. Hyperbaric oxygenation ameliorates indomethacin-induced enteropathy in rats by modulating TNF-alpha and IL-1beta production. Dig Dis Sci. 2006;51:1426–33. doi: 10.1007/s10620-006-9088-2. [DOI] [PubMed] [Google Scholar]
- 26.Feldmann M, Maini RN. Anti-TNF alpha therapy of rheumatoid arthritis: What have we learned? Annu Rev Immunol. 2001;19:163–96. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- 27.Shibata H, Yoshioka Y, Abe Y, Ohkawa A, Nomura T, Minowa K, et al. The treatment of established murine collagen-induced arthritis with a TNFR1-selective antagonistic mutant TNF. Biomaterials. 2009;30:6638–47. doi: 10.1016/j.biomaterials.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 28.Ramadan MF, Jörg-Thomas M. Oil composition of coriander (Coriandrum sativum L.) fruit-seeds. Eur Food Res Tech. 2002;215:204–9. [Google Scholar]
- 29.Bouic PJ, Etsebeth S, Liebenberg RW, Albrecht CF, Pegel K, Van Jaarsveld PP. beta-Sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: Implications for their use as an immunomodulatory vitamin combination. Int J Immunopharmacol. 1996;18:693–700. doi: 10.1016/s0192-0561(97)85551-8. [DOI] [PubMed] [Google Scholar]
- 30.Gupta MB, Nath R, Srivastava N, Shanker K, Kishor K, Bhargava KP. Anti-inflammatory and antipyretic activities of beta-sitosterol. Planta Med. 1980;39:157–63. doi: 10.1055/s-2008-1074919. [DOI] [PubMed] [Google Scholar]


