Abstract
Background:
Laghupanchamula denotes combinations of roots of five herbs. However, in Ayurvedic classics besides four common herbs viz. Kantakari, Brihati, Shaliparni, and Prinshniparni, the fifth one is either Gokshura (Laghupanchamula with Gokshura LPG) or Eranda (Laghupanchamula with Eranda LPE), and both formulations have been documented to have shothahara (anti-inflammatory) action.
Objectives:
The present study was undertaken to compare the anti-inflammatory activity of 50% ethanolic extract of LPG (LPGE) and LPE (LPEE) in rats and safety in mice.
Materials and Methods:
LPGE and LPEE were given orally, administered either just before or 60 min before experiment on mice and for 7 days to rats. Paw edema was induced by carrageenan (acute) and formalin (sub-acute), whereas granuloma pouch (sub-acute) was induced by turpentine in rats.
Results:
Both LPGE and LPEE (1.0 g/kg) at 3 h after their administration showed inhibition of formalin-induced paw edema by 46.2% and 44.3% (P < 0.001) and carrageenan-induced paw edema by 53.9% and 60.4% (P < 0.001), respectively. After 7 days of treatment, both LPGE and LPEE showed 26.3% (P < 0.01) and 32.5% (P < 0.05) inhibition, respectively, against formalin-induced paw edema, and reduced weight of turpentine-induced granuloma pouch by 42.8% and 36.1% (P < 0.001), and volume of exudates by 31.2% and 36.2% (P < 0.001), respectively. No acute toxicity was observed in mice even with a 10.0-g/kg dose of both extracts.
Conclusion:
LPGE and LPEE significantly reduced acute and sub-acute inflammation, and showed effective and similar anti-inflammatory activity. They seemed to be safe, and use of both formulations in the Laghupanchamula for their anti-inflammatory activity is, thus, authenticated.
Keywords: Anti-inflammatory activity, carrageenan, formalin, granuloma pouch, Laghupanchamula
INTRODUCTION
Charaka Samhita and Sushruta Samhita are two main original classics of Ayurveda. These texts mention thousands of single and compound formulations for alleviation of various disorders. Laghupanchamula is one of the compound formulations, which is well documented in various Ayurvedic classics for shothahara (anti-inflammatory), shulanashka (analgesic), rasayana (rejuvenator), jvarahara (antipyretic), kushtha nashaka (useful in skin disorders), and vranaropaka (wound-healing) properties. The term Laghupanchamula signifies the combination of roots of five small plants (herbs or under shrubs). However, two different classical combinations of Laghupanchamula have been mentioned in the texts. Besides the roots of four common plants, Kantakari (Solanum surratense Burm f.), Brihati (Solanum anguivi Lam.), Shaliparni (Desmodium gangeticum DC.), and Prinshniparni (Uraria picta Desv.), the first compound formulation as per the reference in Charaka Samhita,[1] and Chikitsa granthas such as Chakradatta,[2] Shargadhara Samhita, Vangasena Samhita, Yogaratnakara, and Bhaisajyasatnavali, mentions the whole plant of Gokshura (Tribulas terrestris Linn.) as the fifth plant (LPG), whereas the second compound formulation as per the reference in Sushruta Samhita,[3] Kashyapa Samhita,[4] and Siddhasara Samhita[5] mentions the use of roots of Eranda (Ricinus communis Linn.) as the fifth plant (LPE). The plants included under Laghupanchamula have been explored individually for various pharmacological properties. S. surratense Burm f. juice mixed with whey, Chirata, and ginger has been reported to be useful in fever. The juice of leaves, mixed with black pepper, has been prescribed in rheumatism.[6] S. anguivi Lam. root has been reported to have hepatoprotective, anti-inflammatory, and wound-healing activities.[7] D. gangeticum root has been documented for therapeutic value in treating typhoid, piles, inflammation, asthma, bronchitis, and dysentery.[8] U. picta Desv. root has been reported for its analgesic, anti-inflammatory, antioxidant, aphrodisiacs, and fracture-healing properties.[9] T. lanuginosus Lann. has been reported in folk medicine as tonic, aphrodisiac, analgesic, astringent, stomachic, anti-hypertensive, diuretic, and urinary anti-infective.[10] R. communis Linn seeds, seed oil, leaves, and root have been reported for treatment of inflammation and liver disorder.[11]
However, there is no scientific evidence for the pharmacological activity of the above two compound formulations of Laghupanchamula, that is, LPG and LPE. The objective of the present study was to do a comparative evaluation of 50% ethanolic extract the above two classical dosage forms of Laghupanchamula, LPG (LPGE) and LPE (LPEE), for their anti-inflammatory activity against carrageenan (acute inflammation)-and formalin-(sub-acute)-induced paw edema and turpentine-induced granuloma pouch inflammation (sub-acute) in CF strain albino rats. Acute toxicity study was also done in Swiss albino mice to see the safety profile of the test substances, LPGE and LPEE.
MATERIALS AND METHODS
Plant materials
Roots of S. surratense Burm f., S. anguivi Lam., D. gangeticum DC., U. picta Desv., and R. communis Linn., and whole plant of T. terrestris Linn., were collected from Rajiv Gandhi South Campus, Banaras Hindu University (B.H.U.), Mirzapur, Uttar Pradesh, in the months of November-December and verified with the specimen preserved at Department of Dravyaguna, Faculty of Ayurveda, Institute of Medical Sciences (IMS), Banaras Hindu University. A sample specimen for each plant was deposited at Department of Dravyaguna, IMS, B.H.U., Varanasi (LP/DG/12-13/206).
Preparation of extracts
The collected roots and whole parts of the above mentioned plants were shade-dried and reduced to coarse powder using a mechanical grinder and stored in air-dried containers. A 200-g weight each of S. surratense, S. anguivi, D. gangeticum, U. picta plus either whole parts of T. terrestris (LPG,first dosage formulation) or root of R. communis (LPE, second dosage formulation) were taken. Ethanolic extracts (50%) of the above-mentioned two formulations of Laghupanchamula plants (1 kg each) named as LPGE and LPEE, respectively, were prepared separately following standard procedures. The percentage yield of LPGE and LPEE was 10.92% and 10.78%, respectively.
Animals
CF strain albino rats (180-200 g) and Swiss albino mice (20-30 g) of either sex were obtained from the Central Animal House of the Institute, Banaras Hindu University, Varanasi. They were kept in the departmental animal house at 26 ± 2°C and 44-56% relative humidity, and light and dark cycles of 10 and 14 h, respectively, for 1 week before and during the experiments. The animals were provided standard rodent pellet diet. The Principles of Laboratory Animal Care (NIH publication no. 82-23, revised 1985) guidelines were followed. Ethical permission for investigation on animals used in experiments was obtained from the Animal Ethics Committee of the Institute (notification no. Dean/2006-07/810, dated 25-11-2006).
Chemicals and drugs
Carrageenan (Sigma-Aldrich, St Louis, MO, USA), formaldehyde (Merck Limited, Mumbai, India), turpentine oil (Loba Chemie, Mumbai, India), and diclofenac sodium (DFC) (Jagsonpal Pharmaceuticals, Mumbai, India) were used for the study.
Dose selection and treatment protocol
In the classical texts, a 60-ml dose of kvatha (decoction), prepared from ~ 50 g of the powdered drug, has been mentioned per day for an adult human of 60 kg. Considering the dose in humans and the extract yield (≈10%), the extract dose in rat (as per surface area in relation to man) was calculated as 1 g/kg approximately. Therefore, graded doses of both LPGE and LPEE (0.5, 1.0, and 1.5 g/kg) were tested to find an optimal effective anti-inflammatory dose against formalin-induced pedal edema. The test extracts/standard drug, DFC were suspended in 0.5% carboxymethylcellulose (CMC) and administered orally in a volume of 1 ml/100 g body weight of animal, once daily with the help of an orogastric tube. Details of dose, time of administration, and duration of treatment have been mentioned for each experimental paradigm studied.
Formalin-induced pedal edema
The animals were divided into eight groups (n = 6). A sub-planter injection of 0.1 ml of 2% v/v formalin was administered to the hind paw of each rat on the first and third day of experiment. The control and 1st group received 0.5% CMC, whereas the treated groups from 2nd to 4th received LPGE (500, 1000, and 1500 mg/kg) and from 5th to 7th received LPEE (500, 1000, and 1500 mg/kg), and the 8th group received DFC (10 mg/kg), respectively. The First dose of treatment was given 60 min before formalin injection and continued till 7 days. Paw thickness was measured using a plethysmograph at 0 and 3 h and seventh day after treatment following the method described by Singh et al.[12] Percent inhibition in paw volume between treated and control groups was calculated as follows:
Percent inhibition = (1 − VT/VC) × 100
Where, VT and VC are the mean paw volume of the treated and control groups, respectively, at 3 h or on seventh day.
Carrageenan-induced pedal edema
A total of four groups of rats having six animals each were used for the study. The control group received 0.5% CMC and the treated groups received LPGE and LPEE (1000 mg/kg) and DFC (10 mg/kg) orally 60 min before carrageenan administration in 18-h fasted rats. Carrageenan suspension was prepared as a homogenous 1.0% suspension of the powder in 0.9% sodium chloride solution (sterile normal saline). The volume of rat hind paw up to the ankle joint was measured plethysmographically by the mercury displacement method just after administration of 0.1 ml of 1% carrageenan (0 h) in all the above treated groups.[12] Paw volume was further recorded in a successive interval of 1, 2, 3, and 24 h, respectively. Percent inhibition in paw volume between treated and control groups was calculated as follows:
Percent inhibition = (1 − VT/VC) × 100
Where, VT and VC are the mean paw volume of the treated and control groups, respectively, at 1, 2, 3, or 24 h.
Turpentine oil-induced granuloma pouch
A subcutaneous dorsal granuloma pouch was made in ether-anaesthetized rats by injecting 2 ml of air, followed by injection of 0.5 ml of turpentine oil into it.[13] All drugs were administered orally 60 min prior to turpentine oil injection and continued for seven consecutive days. On day 7, 60 min after the last dose, the pouch was opened under anesthesia, the amount of exudates was taken out with a syringe, and the weight of the granuloma pouch and the volume of exudates were measured and expressed as gram and ml per 100 g body weight of animal. Percent inhibition in granuloma pouch weight and exudates volume between the treated and control groups was calculated as follows:
Percent inhibition = (1 − VT/VC) × 100
Where, VT and VC are the mean weight and volume of the treated and control groups, respectively, on seventh day.
Acute toxicity study
Adult Swiss albino mice of either sex, weighing between 20 and 25 g and fasted overnight, were used for toxicity study. A suspension of LPGE and LPEE was administered orally at a 10-g/kg stat dose (10 times the optimal effective dose of 1 g/kg) to mice. Subsequent to extract administration, the animals were observed closely for the first three hours for any toxicity manifestation such as increased motor activity, salivation, convulsion, coma, and death. Subsequently observations were made at regular intervals for 24 h. The animals were under further investigation up to a period of 2 weeks.[14]
Statistical analysis
Statistical comparison was performed using one-way analysis of variance (ANOVA) and multiple comparisons between treated groups and control group were done by Dunnett's test.
RESULTS
Anti-inflammatory
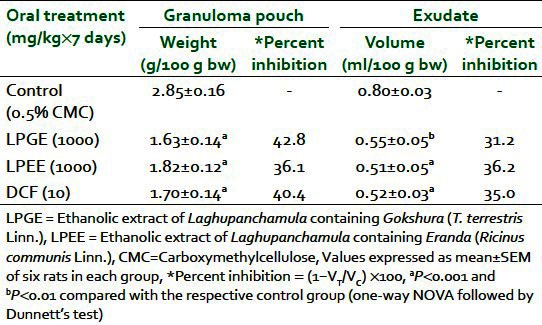
Dose-dependent inhibition of formalin-induced paw edema at 3 h and on seventh day in rats was observed after oral administration of both LPEE and LPGE (500, 1000, and 1500 mg/kg). Percent inhibition ranged from 33.5% to 52.5% at 3 h and 17.5% to 36.3% on seventh day with LPGE, whereas LPEE showed inhibition of 27.2% to 50.6% at 3 h and 14.4% to 32.5% on seventh day, respectively. A 1000-mg/kg dose of both extracts produced a significant reduction in paw volume in comparison with the control group and was used for further studies [Table 1]. LPGE (1000 mg/kg) was found to inhibit carrageenan-induced paw edema by 19.6%, 44.1%, 53.9%, and 56.0% whereas LPEE (1000 mg/kg) reduced edema by 21.6%, 46.1%, 60.4%, and 60.0% at the 1st, 2nd, 3rd, and 24th hour respectively [Table 2]. Both LPGE and LPPE after their 7 days administration reduced the weight of turpentine-induced granuloma pouch by 42.8% and 36.1% and the volume of exudates by 31.2% and 36.2%, respectively, when expressed per 100 g body weight of animal [Table 3]. The result of the test extracts on the above parameters was comparable with the standard anti-inflammatory drug, DFC.
Table 1.
Anti-inflammatory effect of LPEE and LPGE against formalin-induced paw edema in rats
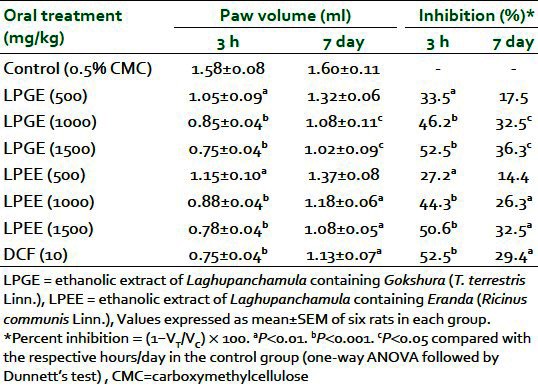
Table 2.
Anti-infl ammatory effect of LPEE and LPGE against carrageenan-induced paw edema in rats
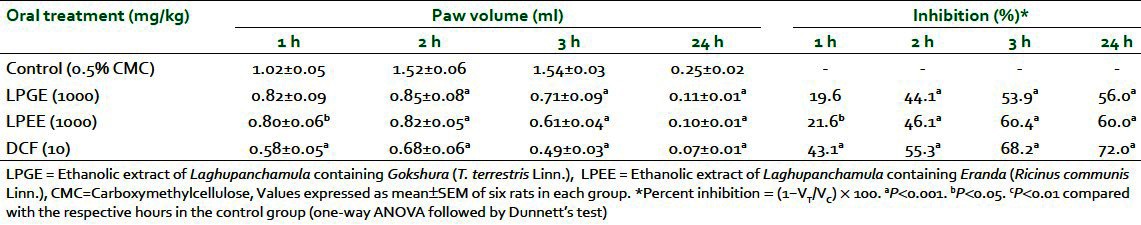
Table 3.
Effect of LPEE and LPGE on turpentine oil-induced granuloma pouch in rat
Acute toxicity
LPEE and LPGE did not produce any mortality during a 72-h period at a 10-g/kg oral dose. Mice showed no stereotypical symptoms associated with toxicity, such as convulsion, ataxia, or increased diuresis.
DISCUSSION
Two different classical combinations of Laghupanchamula have been advocated containing roots of four common plants Kantakari, Brihati, shaliparni, and Prinshniparni, and the fifth, either Gokshura or Eranda. Sushruta Samhita,[3] Kashyapa Samhita,[4] and Siddhasara Samhita[5] mentioned roots of Eranda (R. communis Linn.) instead of Gokshura (T. terrestris Linn.). The great Ayurvedic Physicians might have replaced Gokshura with Eranda either for increasing effectiveness or as an alternative to the earlier combination. The plants included under Laghupanchamula have been explored individually for various pharmacological properties, but no such comparative study has been done earlier with these two formulations of Laghupanchamula. Presently, we have compared the anti-inflammatory activity of these formulations using acute (carrageenan-induced paw edema) and sub-acute (formalin-induced paw edema and turpentine-induced granuloma pouch) models of inflammation in rats. Carrageenan-induced inflammation model is a significant predictive test for anti-inflammatory agents acting by mediators of acute inflammation.[15] Development of edema induced by carrageenan is commonly correlated with early the exudative stage of inflammation, involving release of histamine and serotonin from mast and basophil cells, and is characterized by increase in vascular permeability. Later there is release of bradykinins (an important chemical mediator of both pain and inflammation), prostaglandins, and cyclooxygenases products, and marked increase in cellular infiltration and subsequent release of acute inflammatory mediators such as myeloperoxidase and cytokines (interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, etc.) at the inflammatory site.[16] Further, neutrophils, macrophages, endothelial, and other cells at the site of inflammation may produce reactive oxygen species (ROS) and reactive nitrogen species, which play a modulating role in the inflammatory response. LPEE and LPGE were found to inhibit the edema induced by carrageenan injection from 1st hour onwards, showing good inhibitory effects at 3 h and even after 24 hr (19.6% to 60.0% inhibition) indicating that the anti-inflammatory property of LPEE and LPGE could be due to their effect both on the early and late phases of inflammation. The above effects could be due to their stabilizing effects on basophils and mast cells, with decrease in cellular infiltration and release of histamine, serotonin, bradykinins, prostaglandins, and cytokines at the inflammatory site.
The formalin-induced paw edema assay closely resembles human arthritis and is one of the most suitable methods to screen anti-arthritic and anti-inflammatory agents.[17] It involves infiltrations of neutrophils, macrophages, and proliferation of fibroblasts.[18] Both LPEE and LPGE (500, 1000, and 1500 mg/kg) decreased formalin-induced edema dose dependently from 27.2% to 52.5% at 3 h and 14.4% to 36.3% on day 7, indicating their usefulness both in acute and chronic inflammation, and that they could be useful for treatment of chronic inflammatory disease such as arthritis. The turpentine oil-induced granuloma pouch offers a model for exudative type of inflammation. Kinin is considered to be the main mediator of granuloma as it causes vasodilatation and increases vascular permeability in the early stages of inflammation.[19] The extracts showed significant reduction in granuloma pouch weight and exudative fluid (31.2% to 42.8% inhibition). The pattern of anti-inflammatory activity exhibited by these extracts was similar to that of the standard drug, diclofenac, in all the above three models, which suggests that the plants’ activity may partly be mediated by cyclooxygenase inhibition.
Phytochemical analysis of D. gangeticum has been reported to show flavonoids, glycosides, pterocarpanoides, lipids, glycolipids, and alkaloids,[20] whereas isoflavanones, triterpenes, and steroids were isolated from the roots of U. picta. β-Sitosterol, β-sitosterol glucoside, dioscin, methyl protodioscin, and protodioscin were isolated from S. anguivi and S. surratence have high concentration of solasodine, a starting material for manufacture of cortisone and sex hormone.[21] T. terrestris has been reported to contain steroidal saponins, and acts as a natural testosterone enhancer.[22] Phytochemical screening of LPGE and LPEE in our present study demonstrated the presence of flavonoids, tannins, alkaloid, and saponins. Analgesic and anti-inflammatory effects have been observed in flavonoids and tannins.[23] Tannins are further reported to have potent cyclooxygenase-1-inhibiting action and anti-edematous effect.[17] The anti-inflammatory effect of the extracts may, therefore, be due to the presence of flavonoids, tannins, alkaloid, and saponins. This study has, thus, shown that both LPEE and LPGE have significant anti-edematogenic effect on paw edema induced by carrageenan and formalin, and turpentine-induced exudation and fibroblast formations. Both formulations were found to be safe.
Hence, replacement of T. terrestris by R. communis, as advocated by Sushruta, Kashyap, and Ravigupta, did not alter the anti-inflammatory effect of the formulation; therefore, their uses in the treatment of inflammation mentioned in the Ayurvedic text seemed to be authenticated.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Trikamji J, editor. 1st ed. chap. 1, Verse 41-57. Varanasi: Chowkhambha Prakashan; 2007. Charaka Samhita of Agnivesha, Chikitsa Sthana; Abhaya Amalaki Rasayana Pada; pp. 378–9. [Google Scholar]
- 2.Sharma PV, editor. 1st ed. chap. 1, Verse 169. Varanasi: Chowkhambha Orientalia; 1994. Chakradatta of Chakrapanidatta; Jvara Chikitsa; p. 26. [Google Scholar]
- 3.Trikamji J, editor. 7th ed. chap. 38, Verse 66-67. Varanasi: Chowkhambha Orientalia; 2002. Sushruta Samhita of Sushruta, Sootra Sthana; Dravyasangrhniya Adhyaya; p. 169. [Google Scholar]
- 4.Tiwari PV, editor. 1st ed. chap. 10, Verse 83-87. Varanasi: Chowkhambha Vishvabharti; 2008. Kashyap Samhita by Kashyap, Khila Sthana; Antervartani Chikitsha Adhyaya; p. 563. [Google Scholar]
- 5.Emmerich RE, editor. 1st ed. Verse 2.29. Wiesbaden: Franz Steiner Verlag GmbH; 1980. Siddhasara of Ravigupta; p. 29. [Google Scholar]
- 6.Chopra RN, Nayar SL, Chopra IC. New Delhi, India: Publication and Information Directorate; 1956. Glossary of Indian Medicinal Plants; p. 229. [Google Scholar]
- 7.Asha VV, Pushpangadan P. Preliminary evaluation of the antihepatotoxic activity of Phyllanthus kozhikodianus, Phyllanthus maderaspatensis and Solanum indicum. Fitoterapia. 1998;69:255–9. [Google Scholar]
- 8.Dharmani P, Palit G. Exploring Indian medicinal plants for antiulcer activity. Indian J Pharmacol. 2006;38:95–9. [Google Scholar]
- 9.Rahman MM, Gibbons S, Gray AI. Isoflavanones from Uraria picta and their antimicrobial activity. Phytochemistry. 2007;68:1692–7. doi: 10.1016/j.phytochem.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Balasubramani SP, Murugan R, Ravikumar K, Venkatasubramanian P. Development of ITS sequence based molecular marker to distinguish, Tribulus terrestris L.(Zygophyllaceae) from its adulterants. Fitoterapia. 2010;81:503–8. doi: 10.1016/j.fitote.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Ilavarasan R, Mallika M, Venkataraman S. Toxicological assessment of Ricinus communis Linn. root extracts. Toxicol Mech Methods. 2011;21:246–50. doi: 10.3109/15376516.2010.538752. [DOI] [PubMed] [Google Scholar]
- 12.Singh M, Kumar V, Singh I, Gauttam V, Kalia AN. Anti-inflammatory activity of aqueous extract of Mirabilis jalapa Linn. leaves. Pharmacogn Res. 2010;2:364–7. doi: 10.4103/0974-8490.75456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shastry VM, Patil VR, Chaudhari RY. Acute and chronic anti-inflammatory activity of Pergularia daemia whole plant in various experimental animal models. Int J Pharm Pharm Sci. 2011;3:241–4. [Google Scholar]
- 14.Gautam MK, Singh A, Rao CV, Goel RK. Toxicological evaluation of Murraya paniculata (L.) leaves extract on rodents. Am J Pharmacol Toxicol. 2012;7:62–7. [Google Scholar]
- 15.Sawadogo WR, Boly R, Lompo M, Some N. Anti-inflammatory, analgesic and antipyretic activities of Dicliptera verticillata. Int J Pharmacol. 2006;2:435–8. [Google Scholar]
- 16.Guang QZ, Huang XD, Wang H, Leungb AK, Chan CL, Fong DW, et al. Anti-inflammatory and analgesic effects of the ethanol extract of Rosa multiflora Thunb. Hips. J Ethnopharmacol. 2008;118:290–4. doi: 10.1016/j.jep.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Beg S, Swain S, Hasan H, Barkat MA, Hussain MS. Systematic review of herbals as potential anti-inflammatory agents: Recent advances, current clinical status and future perspectives. Pharmacogn Rev. 2011;5:120–37. doi: 10.4103/0973-7847.91102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dutta S, Das S. A study of the anti-inflammatory effect of the leaves of Psidium guajava Linn. on experimental animal models. Pharmacognosy Res. 2010;2:131–17. doi: 10.4103/0974-8490.72331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banerjee TK, Mandal SS, Das PC, Sikdar S. Assessment of the anti-inflammatory effect of Swertia chirata in acute and chronic experimental models in male albino rats. Indian J Pharmacol. 2005;32:21–4. [Google Scholar]
- 20.Rastogi S, Pandey MM, Rawat AK. An ethanomedicinal, phytochemical and pharmacological profile of Desmodium gangeticum (L.) DC. and Desmodium adscendens (Sw.) DC. J Ethanopharmacol. 2011;136:283–96. doi: 10.1016/j.jep.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 21.Kumar N, Prakash D, Kumar P. Wound healing activity of Solanum xanthocarpum Schrad. and Wendl. fruits. Indian J Nat Prod Resour. 2010;1:470–5. [Google Scholar]
- 22.Singh S, Nair V, Gupta YK. Evaluation of the aphrodisiac activity of Tribulus terrestris Linn. in sexually sluggish male albino rats. J Pharmacol Pharmacother. 2012;3:43–7. doi: 10.4103/0976-500X.92512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmadiani A, Hosseiny J, Semnanian S, Javan M, Saeedi F, Kamalinejad M, et al. Antinociceptive and anti-inflammatory effects of Elaeagnus angustifolia fruit extract. J Ethnopharmacol. 2000;72:287–92. doi: 10.1016/s0378-8741(00)00222-1. [DOI] [PubMed] [Google Scholar]


