Abstract
Botanicals constitute a large part of the drugs from the traditional medicine (TM) and ethno medicine (EM) known for their history of safe use (HOSU). Phytopharmaceuticals having a base of such origin offer high advantages as they come with safety profiles, and often allow extrapolation of the HOSU data, under certain circumstances. However, while current pharmaceutical technologies are being adopted by the industry to make phytopharmaceuticals with such origin, there is a need for preformulation research and development (R and D) during formulation. Some suggestions for R and D studies in case of aqueous extracts known in Ayurveda, converted on an industrial scale to obtain a phytopharmaceutical, and formulated as a solid dosage form (granules, tablets, or capsules) are discussed.
Keywords: Ayurveda, integrative, phytopharmaceuticals, preformulation
INTRODUCTION
Traditional medicines (TMs) or ethno medicines (EMs) are known in many nations of the world. Interest in Ayurveda, Traditional Chinese medicines (TCMs) and research in the herbs and medicines of these systems of health care have led to discussions on these areas. Ayurveda, known to have about 5000 years of history, is widely used in India and few other countries. A large part of the formulations used in Ayurveda have herbs/botanicals as ingredients, although some may be made with minerals, metals, and ingredients of animal origin. Ayurvedic formulations are currently industrially prepared into products adopting large-scale technologies that pharmaceutical or food industry uses. Ayurveda mentions a number of processes and dosage forms.[1] The types of information prescribed in Ayurveda inclulde an infusion or a decoction of the botanical using water as a medium/solvent, either as a liquid or as concentrated decoction Ayurveda also advocates the use of semi-solid mass (avaleha) or dried powder, along with many other processes described in the texts. Such processed material actually forms extracts which in traditional dosages are formulated as pills. In certain cases, the infusion or decoction in water is prepared in a dilute solution of a specified herb or herbs instead of water alone, a process referred to as bhavana. Materials prepared for any botanical use such a process as prescribed in official texts of Ayurveda as listed in the First Schedule of the Drugs and Cosmetics Act, 1940 of India (or for that matter in TCM or other TM) will come with the known history of safe use (HOSU).
In contrast to the above traditional process, industry uses technology that in essence mimics the above processes but adopts requirements demanded by the technology. Such processed materials can be called as phytopharmaceuticals. Phytopharmaceuticals can be defined as “Phytopharmaceutical drug: Phytopharmaceutical drugs include processed or unprocessed standardized materials derived from plants or parts thereof or combination of parts of plants, extracts or fractions thereof in a dosage form for internal or external use of human beings or animals and intended to be used for the diagnosis, treatment, mitigation or prevention of any disease or disorder in human beings or animals but does not include administration by parenteral route.”
Phytopharmaceuticals are in essence the same as a botanical drug in common parlance and it was the US FDA (United States Food and Drug Administration) that first issued “Guidance to Industry on Botanical Drugs” that paved the way for phytopharmaceuticals to get a status of drug and marketing authorization as a drug after review by FDA US.[2] The definition of the Botanical in this Guidance document differs with the definition of the phytopharmaceutical given above. The Drugs Technical Advisory Board of Govt. of India under the Drugs and Cosmetics Regulations (DCAR) has in principle approved notification of provisions in the drugs and cosmetics rules for phytopharmaceuticals as drugs.[3] Drug development using phytopharmaceuticals that bases the leads in traditional knowledge through “Reverse Pharmacology” is gaining attention, and it offers an alternative and perhaps more quicker/economic strategy for drug development.[4]
Table 1 lists a comparison of traditionally processed botanical as per Ayurveda and pharmaceutically processed botanical extracts, adopting decoction process of herbs/botanicals as a case.
Table 1.
Ayurvedic & Pharmaceutical Processing of a botanical – A comparison
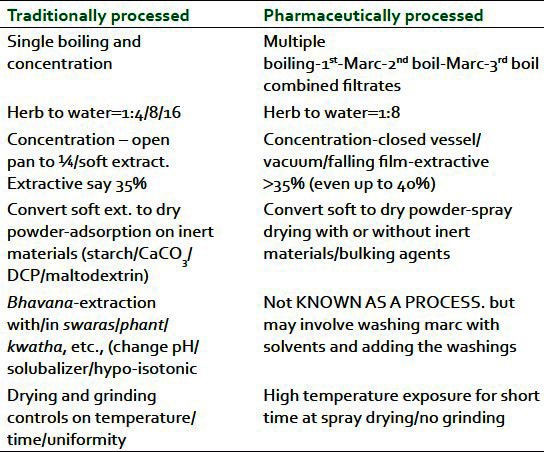
Traditionally processed as per ayurveda
One case is selected here for discussion from amongst the many dosage forms made with aqueous extracts in Ayurveda. Ayurvedic process of a herb/botanical as pure extract –swaras (fresh juice), phant (warm or hot water infusion), kwatha or kashayam (hot water decoctions) – involves normally a herb to water ratio of 1:4 or 8 or 16 depending on the soft or hard nature of the plant part used. In general, the process describes an open pan treatment and concentration is normally prescribed[1] to one-fourth of the initial amount of water or till one gets a soft extract (ghana/satwa). In some cases, such kwathas or ghana are also adsorbed on an inert material like starch, etc., to get a powder or it may be allowed to dry under sun for a number of days till it forms a powder. This process involves obviously long-time heating and drying, and the controls on this process may vary to some extent batch wise. The dried material is often either soft lumps or in many cases hard lumps which need grinding.
Pharmaceutically processed
Many industrial processors who manufacture an aqueous extract of botanicals adopt large-scale boiling with water, and the process is repeated for each herb three times by filtering the first extract and extracting the mark a second and third time. Most commonly, herb to water ratio is 1:8 during each of the three extraction steps. Combined filtrates are concentrated where a vacuum evaporation technology is adopted including use of falling film evaporators.
Conversion of soft extracts so obtained is generally achieved by spray drying where the extract is exposed to high temperature but for a short time during the spraying step and for not more than an hour during the drying in the cone of a spray dryer. If proper technology is adopted, no step of grinding may be required at the end of this spray-dried material.
Characterizing the material
Adequate scientific studies would be required with an initial step of knowing and documenting in detail all the steps, in both the above processes. While a material prepared as per Ayurvedic process briefly discussed above can be labeled as a HOSU material, it is important to generate scientific data on the pharmaceutically processed material of the same herb produced industrially as per process briefly described above. This is important as the phytopharmaceutical raw-material produced by the pharmaceutical process needs to be similar in characteristics and composition to a HOSU material and such similarity or dissimilarity data would only lead to extrapolatability of the safety profile of the HOSU material to the phytopharmaceutical. This is the first step of R and D before the phytopharmaceutical can be taken up for further formulation development.
For generation of data on the material from the above two processes, a simple conduction of the normal physicochemical parameters, thin-layer chromatographic profiles for comparison, High Performance Liquid Chromatography, HPLC analysis for either qualitative or quantitative data for analytical or biomarkers that normally are adopted by various pharmacopoeia may not be adequate. In these tests, stress is high on understanding the chemistry of processed material involving organic compounds. Many botanicals are known to contain minerals and inorganic compounds in traces to appreciable levels, and for certain pharmacological or therapeutic activities, inorganic compounds also play a great role. R and D studies need to go beyond organic chemistry alone. Process changes in the above two processes need to be carefully documented, and its impact on the nature of the material produced need to be studied. For example, heating at high temperature forms 4-deoxy-11, 12-didehydroandrographolide in Andrographis paniculata, Nees [kalmegh] that has been reported to be toxic and some pharmacopeia have a test and a limit for the same in the quality specifications.[5] Bhavana process of extraction and the impact it does on the nature and quality of extract may be change of pH of the medium/solvent. Can constituents of the bhavana herb like “phytosterols if present provide solubalizing effects, or does it add to isotonicity of the medium?”, such possibilities have not been studied or reported. If such effects are indeed seen, then the pharmaceutically processed material may also need to adopt the same in industrial scale to be similar to HOSU material.
Limiting the data generation to Thin Layer Chromatography (TLC) profiles or HPLC analytical data may be inadequate in many cases especially when the botanical processed has more compounds that are not chromophoric in nature to provide UV absorption maxima. Principal component analysis (PCA) involving a number of measurement techniques namely Infra red Spectra, Ultra Violet Spectra, Nuclear Magnetic Resonance (IR, UV, NMR) and Mass Spectra (MS) among others offer a great advantage to generate adequately large pool of data that enable to apply statistical analysis. Such an approach offers a high degree of ability to analyze and come to a fair degree of assessment of similarities and dissimilarities between the HOSU material and the phytopharmaceutical. In addition, conducting in vivo bioefficacy screening/test protocols involving small animals or organ tissue cultures or cell-line-based assays helps to generate biological efficacy comparison data.
Characterizing through fingerprinting
A good-quality fingerprint can[6]:
Provide an objective link between historical data and a proposed new material
Give an assessment of
Lab to production scalability
Raw-material variability (seasonal and regional)
Extraction robustness
Sample stability.
Techniques for fingerprint development and its analysis
HPLC is widely available although for a molecule to be detected by UV it must have a chromophores. Evaporative light-scattering detection (ELSD) and mass spectrometry (MS) offer more universal detection methods, but availability and cost can be limiting. Chromatographic resolution is essential to include as many components as possible. With the exception of MS detection, little information on component identity can be obtained. Ultra Performance Liquid Chromatography (UPLC) is an emerging technique with excellent resolving power
Fourier Transfer Infra red Spectra (FT-IR) is quick, relatively cheap, and widely available. A spectrum can give some qualitative information on the general compound classes present along with limited quantitative information. FT-IR spectra can be difficult to reproduce and are low resolution, which can result in small changes being missed.
1H-NMR has the potential to detect any molecule containing hydrogen, thus making it highly universal. High-resolution instruments(>500 MHz) have sufficient sensitivity and resolution to give quality fingerprints for complex natural products. The spectra are qualitatively and quantitatively information rich, but the technique is expensive and not widely available.
Analysis of fingerprints using principal component analysis
Analytical results from multiple techniques can be combined to create a holistic fingerprint prior to input into a PCA model. PCA modeling improves with increased sample numbers. By identifying the techniques and subsequently the data points responsible for the variance, these components can be targeted for identification or monitored as part of a specification.
Pre-formulation R and D
Once having established the similarities of the phytopharmaceutical to the HOSU material, the pre-formulation development work begins. Unlike pre-formulation studies in a pharmaceutical based on synthetic drugs where large guidance and techniques are available, the area for phytopharmaceutical is bereft of the same. Knowledge on such studies seems to be residing in industry due to obvious reasons of confidentiality of such strategy. Creative adoption of many measurement techniques is needed to study the properties of the phytopharmaceuticals, so as to know the ability to obtain quality solid dosage form formulations.
Some examples of the common attributes that need to be studied and suggestions are listed below, although these are non-exhaustive. For other tests/protocols, reference to the various editions of Ayurvedic Pharmacopoeia of India[1] is recommended.
Solubility and re-solubility/re-dispersability: The pharmaceutically processed powder (referred as powder in the paras below for brevity) materials would have undergone some changes physically that render them not easy to dissolve or re-dissolve if needed for making syrup. Generally, the authors have experienced dissolution of about 90-95% of the powder in water even with some warming. Knowledge of the extent of solubility is important to decide the need for additional steps of “adjustment to get the right quantum or, need for a step of filtration during manufacture.”
Hygroscopicity: Most herbal extracts exhibit a fair degree of hygroscopicity although the opposite phenomenon “efflorescence” is not uncommon. Hygroscopicity information and extent of the same are very important in formulation of solid dosage forms, as it will have serious implications of the manufacture steps, environmental controls on the manufacturing, design of packs and packaging, and most importantly on the stability and microbial qualities of the material and formulations.
A creative method for such a study would be to expose weighed quantities of the powders whose initial moisture contents are determined, in open Petri dishes to high humidity atmosphere. Samples from these Petri dishes can be drawn at frequent intervals and tested for moisture content and this testing can be continued till one reaches the “critical moisture content (CMC)” for that powder. CMC is that level of moisture when the powder starts to show changes in color/odor/nature (become cohesive, pulpy).
Some extracts may show opposite tendencies-lose water/volatile materials. In such cases, the same testing will provide information on the weight loss rather than weight gains of a hygroscopic powder.
One also needs to know what happens when moisture is absorbed does the material swell? Does the particle size enlarge/ grow? Observing samples of the powder-initial sample and that which has reached CMC under a microscope can provide good information.
Swelling index: Many phytopharmaceuticals exhibit swelling properties and such a behavior will have impact in the steps involving granulation, or even after the powders are filled in capsules or compressed to get tablets. Swelling of the contents of the dosage forms can lead to serious quality problems including breaking of tablets, de-shaping of capsules, change in bulkiness color, etc., of granules. Swelling property is easily testable adopting Pharmacopeial method[7] given in monograph of for Isabgol (psyllium husk).
Binding properties: Phytopharmaceutical produced by spray drying process often show varying ability to bind. The powders may vary from amorphous, fine to granular powders; vary from cohesive powders to free flowing powders, with low or high inherent binding capacities. Knowledge of the same is extremely important and measurement of bulk density –untapped/tapped can provide some information. Large difference indicates more air/intra-particle voids. Powders with such large voids may show both, good inherent binding property or sometimes exactly the opposite. An experienced scientist can gauge the binding ability by just pressing some powder between fingers.
Crystalanity and size, amorphous/crystalline: Powders may appear to be crystalline or amorphous to the naked eye. Not much information is available on this property and its impact on both pharmaceutical properties and pharmacological/therapeutic activity. Simple viewing under polarization microscopy or in specific cases, testing by powder X-ray diffraction is suggested. Determination of particle size distribution by normal methods of sieve analysis is suggested.
Interaction with Excipients: By an amendment to DCAR-Rule169, use of Permitted Excipients has been notified to assist in formulations of Ayurvedic dosage forms.[8] Selection of Excipients follow the normal dictum of compatibility with the powder. They should aid in improving the pharmaceutical properties like binding, lubricating, antisticking, anticaking, stabilizing, and solubalizing. The normal dictum that," the Excipients used should not interfere in the method of analysis" that is generally stated in the pharmacopoeia, needs a review in the context of phytopharmaceuticals as the type and nature of analysis done for phytopharmaceuticals is not the same as that which is done for a synthetic drug formulation. This applies greatly to the preservatives used with formulations involving a phytopharmaceutical. Also the recognition that all Excipients may not be necessarily “inert” is required. For example, in case of the phytopharmaceuticals formulated with magnesium oxide/magnesium carbonate as an anticaking agent, which may show slight “laxative effect or potentiate such effect if the phytopharmaceutical also is originally known for laxative effect.”
These are but few examples of the area and more need to be studied to obtain the right formulation with a phytopharmaceutical that mimics/is similar to the traditionally processed formulation, yet it is contemporary, pharmaceutically processed, and developed adopting industrial scale pharmaceutical technology. Greater funding for such research and development by the national and international agencies is the need of the hour apart from creating awareness of such research needs.
ACKNOWLEDGMENTS
Author acknowledges the numerous discussions and inputs from several scientists of Unilever Research India and Safety and Environment Assurance Centre (SEAC) of Unilever Research Colworth, UK, on many areas of this thought and process of science.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.1st ed. Part I. Vol. 8. Govt. of India: Min. of Health and F.W; 2011. The Ayurvedic Pharmacopoeia of India. [Google Scholar]
- 2.Guidance to Industry for Botanical Drugs, US FDA. 2004. [Last accessed on 2012 Jul 7]. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070491.pdf .
- 3.Shankar R. DTAB proposes to bring phyto pharmaceuticals under D and C Act soon. 2011. [Last accessed on 2012 Jul 7]. Available from: http://www.pharmabiz.com/NewsDetails.aspx?aid=65573 and sid=1 .
- 4.Patwardhan B, Vaidya AD, Chorghade M, Joshi SP. Reverse pharmacology and systems approaches for drug discovery and development. Current Bioactive Compounds. Curr Sci. 2008;4:1–12. [Google Scholar]
- 5.Indian Pharmacopeia Commission. Vol. 3. Ghaziabad UP, India: Min. of Health and F.W., Govt. of India; 2010. Indian Pharmacopeia; pp. 2514–5. [Google Scholar]
- 6.Russell P. Proceedings of the one day symposium on “Safety and risk assessment approaches for materials of herbal origin” organized by Unilever Research India, along with Ayurvedic Drug Manufacturers Association, and Indian Drug Manufacturers Association, at Bangalore, on 3 September, 2010. Toxicol Int. 2011;18:S3–19. [PMC free article] [PubMed] [Google Scholar]
- 7.Indian Pharmacopeia Commission. Vol. 3. Min. of Health and F.W., Govt. of India; 2010. Indian Pharmacopeia; p. 2513. [Google Scholar]
- 8.Deshpande SW, Nileah Gandhi. The Drugs and Cosmetics Act, 1940 and Rules 1945. 5th ed. Mumbai, India: Susmit Publications; 2009. Insertion of Rule 169 by G.S.R No 755(E) dated 23.11.2008 by Gazette Notification by Govt. of India; pp. 258–9. [Google Scholar]


