Abstract
Idiopathic pulmonary fibrosis (IPF) is a chronic interstitial pneumonia with a median survival of 3 years after diagnosis. Acute exacerbation of IPF (AE-IPF) is now identified as a life-threatening complication. It presents as worsening dyspnea with new ground glass opacities superimposed upon a radiographic usual interstitial pneumonia (UIP) pattern. It is a diagnosis of exclusion. The prognosis of AE-IPF is poor and treatment strategies lack standardization. In order to rule out any reversible etiology for an acute decompensation of a previously stable IPF patient diagnostic modalities include computerized tomographic angiogram (CTA) coupled with high-resolution computerized tomography (HRCT) imaging of the chest, bronchoalveolar lavage (BAL) and echocardiogram with bubble study. Avoiding risk factors, identifying underlying causes and supportive care are the mainstays of treatment. Anti-inflammatory and immunosuppressant medications have not shown to improve survival in AE-IPF. Most of the patients are managed in a critical care setting with mechanical ventilation. Lung transplantation is a promising option but most institutions are not equipped and not every patient is a candidate.
Keywords: Acute exacerbation of idiopathic pulmonary fibrosis, bronchoalveolar lavage, chest roentgenogram, computerized tomographic angiogram, high resolution computer tomography, idiopathic pulmonary fibrosis, usual interstitial pneumonia
Idiopathic pulmonary fibrosis ( Idiopathic pulmonary fibrosis IPF) is described as a progressive, irreversible chronic lung disease. It usually presents with progressive dyspnea, reduced lung volumes, bilateral lower lobe reticular opacities, and a usual interstial pneumonia UIP patter on histology. There is no definitive treatment and median survival is approximately 3 years.[1,2] The natural history of IPF was thought to be a steady decline in lung function but recent literature has demonstrated that the decline in lung function may be more stepwise and often accompanied by acute exacerbations hastening the fibrosing process and ultimately resulting in death.[3] There is no consensus on an established definition of acute exacerbation of IPF (AE-IPF). Most of the studies defined AE-IPF as a combination of symptoms, radiographic findings, blood gas parameters, and an exclusion of any alternative causes of this clinical scenario. AE-IPF was described as a separate entity in 1993 by Kondoh and colleagues;[4] however, it was in 2007 that Collard et al. proposed a definition describing AE-IPF as “an unexplained new or worsening shortness of breath within the past 30 days, along with new lung infiltrates and exclusion of any reversible and recognizable etiology causing lung injury”[5] [Table 1]. Understanding about pathogenesis of development AE-IPF has been enhanced with study of biomarkers and better imaging modalities.
Table 1.
Diagnostic criteria for AE-IPF*
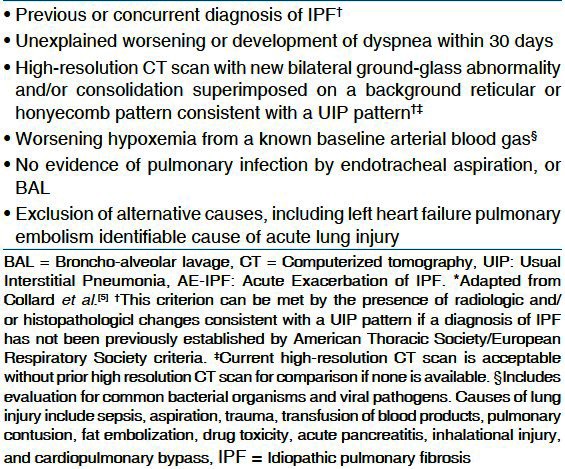
Most of the times, the clinician is faced with a patient with known IPF who has a rapid deterioration without an obvious cause. Diagnosing the condition in time is a challenge. The diagnostic dilemma is not only differentiating this entity from acute lung injury and acute respiratory distress syndrome (ARDS) but also ruling out worsening of coexisting entities such as pulmonary hypertension and heart failure. Currently, there is no definitive long-term treatment proven effective for IPF and there is even less data on prevention and treatment of AE-IPF.
With limited pharmacological options, mechanical ventilation is instituted earlier rather than later in the disease course. As of now lung transplantation seems to be the only viable treatment option; however, it is both costly and not uniformly available.[6]
Epidemiology and Risk Factors of AE-IPF
The incidence of AE-IPF is highly variable among different studies. It is certain that a lack of a standardized definition of AE-IPF and different study designs play a major role in the variability of frequencies reported. Kim et al. reported a 1-year frequency of 8.5% and a 2-year frequency of 9.6%; however, they admitted that the actual frequency of AE-IPF could be higher due to the loss of follow-up, variable definitions, and diagnostic uncertainty[7] [Tables 2 and 3]. Data from retrospective ICU studies report the incidence of AE-IPF to be as high as 60% and mortality between 69% and 96%.[17–20] Okamoto and colleagues followed 112 patients diagnosed with IPF for 10 years. Of the 112 patients, 56 with IPF died during this time due to AE-IPF. They found the incidence of AE-IPF to be as high as 25% and reported a median survival time of 3.1 years for all IPF patients after diagnosis but after the onset of AE-IPF it was 0.9 months.[21] In a recent study, authors reported 1- and 3-year incidence of AE-IPF as 14.2% and 20.7% among 461 patients with IPF.[10] AE-IPF has not been shown to be related to the degree of disease severity, age, or immunosuppressant therapy;[17,22] however, Japanese studies have suggested a genetic influence.[21,23]
Table 2.
Incidence of AE-IPF in the literature
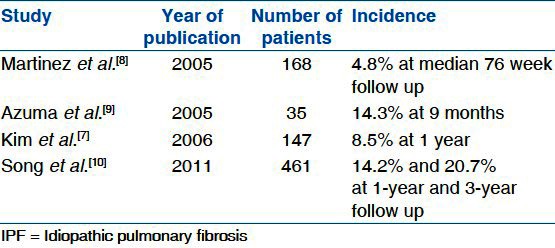
Table 3.
Risk factors reported in literature for development of AE-IPF
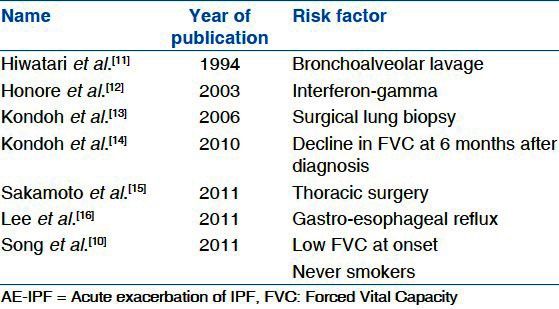
There have been anecdotal reports suggesting that broncho-alveolar lavage (BAL) and interferon therapy may have played a role as being the inciting factor for AE-IPF but the exact triggers are unknown.[11,24] There is also evidence in literature of AE-IPF developing after lung biopsy. Kondoh et al. showed that the incidence of post-operative exacerbation was 2.1% (5/236 patients) after surgical lung biopsy.[13] Similarly, surgical lung resection has also been identified as a precipitating factor for AE-IPF;[12,25] however, surgical approaches such as conventional thoracotomy, muscle sparing thoracotomy, or video-assisted thoracoscopic surgery have not been reported to influence the occurrence of post-operative AE-IPF.[26] Sakamoto and colleagues reported that 3 of 39 patients with IPF developed AE-IPF after surgical resection secondary to lung cancer (2 lobectomy, 1 biopsy).[15] All three patients died of respiratory failure within 12-82 days after the onset of AE-IPF. They postulated that this was related to oxygen supplementation at a high concentration and/or prolonged mechanical ventilation.
Gastro-esophageal reflux has been viewed with much interest as one possible causes of AE-IPF.[5,7] Lee et al. compared BAL pepsin level of 24 AE-IPF cases and 30 stable IPF controls, and found that pepsin level was an indicator of acute exacerbation status (P = 0.04).[16] However, this difference was driven by only a subgroup of eight patients (33%) with pepsin levels > 70 ng/ml, providing modest evidence that occult aspiration is associated with AE-IPF.
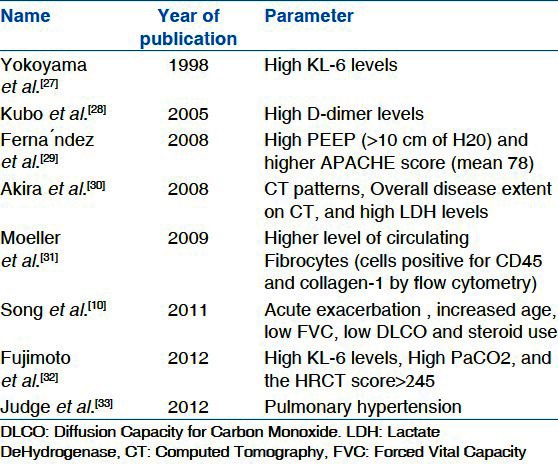
In the literature certain risk factors have shown to be associated with high mortality in patients who develop AE-IPF. These factors range from molecular level to gross clinical findings [Table 4]. As the knowledge base about this new entity expands, a clear relationship between different parameters which define its morbidity and mortality will become evident.
Table 4.
Parameters predicting mortality in AE-IPF
Pathogenesis of AE-IPF
It is not clear whether AE-IPF is secondary to acceleration of the primary disease process or represents a clinically silent trigger such as a viral infection or silent aspiration. Animal models of AE-IPF have shown that viruses have led to worsening of stable disease state.[34,35] Recent literature has questioned viruses as a trigger for AE-IPF. Wootton and colleagues, using standard polymerase chain reaction (PCR) in BAL fluid, found common respiratory viruses in 4 of 43 patients with AE-IPF; however, advanced PCR testing (multiplex PCR, pan-viral microarray, and high-throughput cDNA sequencing) revealed the presence of viruses in 15 more samples. The most common virus detected in the BAL of AE-IPF patients was torque teno virus [TTV] (28%). However, 24% of BAL samples from acute lung injury controls were also TTV-positive.[36] Likewise Huie et al. only found viruses in 5 of 27 cases with AE-IPF.[37]
Recent research on gene expression has remarkably enhanced our understanding about AE-IPF pathogenesis. Using gene expression microarray, Konishi et al. studied 23 stable IPF patients and eight patients with AE-IPF. Gene expression of CCNA2 and α-defensins were up-regulated in AE-IPF patients when compared with IPF patients and their expression was localized to the alveolar epithelium. This suggests a central role of the pulmonary epithelium in AE-IPF and possible role of α-defensins as a biomarker.[38] Similarly, a biomarker study compared patients with stable IPF (n = 20), AE-IPF (n = 47), and acute lung injury (n = 20) and also biomarkers of type I alveolar epithelial cell injury/proliferation [receptor for advanced glycation end products (RAGE)] versus markers of type II alveolar epithelial cell injury (KL-6 and SP-D).[39] They found that KL-6 and SP-D levels were significantly elevated (P = 0.0003 and 0.01, respectively) in AE-IPF compared with stable IPF. RAGE levels were not different between groups (P = 0.79). Levels of Von-willibrand factor vWF and interleukin (IL-6), total protein C, thrombomodulin, and plasminogen activator inhibitor-1PAI-1 levels were significantly higher in AE-IPF compared with stable IPF. In comparison to acute lung injury, AE-IPF demonstrated higher levels of Krebs von den Lungen-6 (KL-6) and surfactant protein D (SP-D) and lower levels of RAGE, vWF, and IL-6. The study highlighted the fact that the AE-IPF is characterized by increased type II alveolar epithelial cell injury and/or proliferation, endothelial cell injury, and coagulation abnormalities which is consistent with the hypothesis that AE-IPF represents the acceleration of the underlying primary disorder.
Diagnostic Approach to AE-IPF
History and physical examination
AE-IPF is a diagnosis of exclusion. An integrated approach is imperative. The main theme is to rule out any antecedent reversible factors that may have caused the decompensation. A patient with IPF who is in acute exacerbation presents with pronounced dyspnea, tachycardia, and hypoxemia when compared with baseline. Worsening hypoxemia in a patient diagnosed with IPF could be secondary to pneumonia, pulmonary embolism and heart failure [Table 1]. The physician should focus on current smoking status, use of prescribed and non-prescribed medications, immunosuppression, and undiagnosed reflux disease.
Laboratory tests
Increases in white blood cell count, C-reactive protein, and lactate dehydrogenase can be demonstrated in AE-IPF but these are non-specific and can be elevated in other entities such as Acute interstial pneumonitis AIP.[40,41] An Electro cardio gram EKG should be assessed for any arrhythmias and an arterial blood gas should be obtained to not only ascertain the degree of hypoxemia and hypercapnea but also to anticipate any need for mechanical ventilation.
Biomarkers
Neutrophil elastase, KL-6, lactate dehydrogenase levels, and several other serum markers have been studied for AE-IPF. Tajima et al.[42] reported significantly higher serum levels of soluble ST2 protein in patients with AE-IPF when compared with stable IPF group whereas Moeller and colleagues[31] reported that levels of circulating fibrocytes were elevated in patients with stable IPF which were further increased in AE-IPF patients. α-Defensin and ST-2 protein levels in serum have been shown to be significantly high in patient with AE-IPF.[38,40] Similarly, Kurosu and colleagues found that antibody to annexin-1 was detected in 47% of the sera and 53% of the BAL fluid from patients with AE-IPF.[43] Currently, there is not enough data to justify widespread use of these tests.
Radiological assessment
While the chest roentgenogram (CXR) is an efficient way of diagnosing any overt parenchymal or pleural abnormalities; however, it is very difficult to determine the presence of any subtle changes in a patient with IPF who has a baseline of extensive reticulonodular opacities. An attempt should be made to compare the current CXR with previous imaging to look for any new infiltrate. If the CXR is not diagnostic for the cause of the deterioration then a high resolution computerized tomogram (HRCT) is the next most important test. Diagnosing a pulmonary embolism with a CT angiogram is also of paramount importance.[5,44]
HRCT shows new ground glass abnormalities superimposed upon the baseline UIP pattern and can also help to evaluate the possibility of any potential infectious etiology for deterioration.[5] Akira and colleagues were able to show that the appearance of new extensive ground glass abnormalities on HRCT against a background of basilar honeycombing is a sign of AE-IPF [Figure 1]. They demonstrated three distinct radiographic patterns of AE-IPF in their study of 58 patients including peripheral, diffuse, and multifocal patterns of new ground glass infiltrates. The peripheral pattern was by far the most common pattern but worse survival was associated with the diffuse pattern.[30] Silva et al.[45] studied 24 patients with AE-IPF and found that the main HRCT findings consisted of bilateral ground-glass opacities (100%) and consolidation (71%) both AE-IPF from AIP, which is a major diagnostic consideration. Both diseases present with respiratory failure and new bilateral infiltrates but the ground glass opacities seen in IPF have a background of a UIP pattern that is absent in AIP.[46] Having a trained chest radiologist is very important in this scenario.[47]
Figure 1.
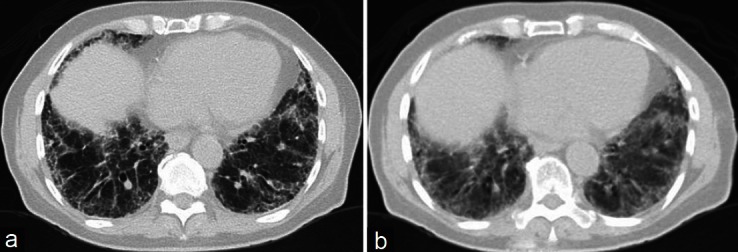
Panel (a) High resolution computerized tomogram image of a patient at the time of stable idiopathic pulmonary fibrosis. Panel (b) High resolution computerized tomogram image of the same patient in acute exacerbation showing extensive ground glass abnormalities against a background of basilar honeycombing consistent with acute exacerbation of idiopathic pulmonary fibrosis
Echocardiogram
Echocardiography is obtained to rule out congestive heart failure as a cause of AE-IPF.[5] Kubo et al. in a prospective study found that out of total 32 events of respiratory deterioration among patients with IPF, two events were secondary to heart failure.[47] Similarly in another study, Song et al., retrospectively analyzed 416 patients with IPF for a median follow-up period of 22.9 months. They showed that out of 163 episodes of AE-IPF, 5 (1.1%) were secondary to heart failure.[10]
Echocardiogram in patients with AE-IPF can reveal evidence of pulmonary hypertension. Prambil et al. studied seven patients with acute exacerbation of IPF who underwent surgical lung biopsy. Right ventricular enlargement and hypertrophy was present in all six patients in whom echocardiogram was performed, and right ventricular systolic function was impaired in five of these patients (83%). The mean calculated right ventricular systolic pressure (RVSP) was 65.2 mmHg, (range, 39 to 75 mmHg).[28] Similarly, Saydain et al. found evidence of pulmonary hypertension in 38 patients admitted to intensive care unit (ICU) with acute exacerbation. However, they noted that there were no significant differences in echocardiogram findings between survivors (mean pulmonary systolic artery pressure 58 mmHg) and non-survivors (mean pulmonary systolic artery pressure 59.3 mmHg).[48]
In another study Judge et al. found that pulmonary hypertension at baseline was associated with a significant risk of acute exacerbation (HR 2.217, 95% CI 1.005-4.889; p50.041). They found that 12 of the 17 patients who had pulmonary hypertension at baseline, subsequently experienced an acute exacerbation during follow-up period. Mean RVSP on echocardiogram increased significantly from 28.69 mmHg at baseline to 37.00 mmHg at the time of acute exacerbation (n = 27, P = 0.0025). They concluded that pulmonary hypertension was associated with the development of AE-IPF as well as poor survival.[49]
Bronchoscopic assessment
The role of BAL is pivotal in excluding infection as the etiology of AE-IPF; however, negative BAL culture still does not exclude the possibility of viral infections.[33] AE-IPF is morphologically characterized by BAL neutrophilia; however, lymphocytosis can be seen.[5] Kim et al. studied clinical, radiologic, and pathologic data of 11 patients with AE-IPF. At the time of AE-IPF, eight of eleven patients underwent BAL, three patients had only elevated neutrophils (5%) and five patients had increases in both neutrophils and lymphocytes (20%).[7]
Song and colleagues found that in the patients with AE-IPF the percentage of neutrophils in BAL fluid were significantly lower when compared with those with acute exacerbation secondary to infection, while the percentage of lymphocytes in BAL fluid were higher in the AE-IPF group. In the multivariate logistic analysis, the percentage of neutrophils in BAL fluid and fever were significant discriminating parameters between AE-IPF and infection.[10]
Lung biopsy
Surgical lung biopsy being potentially risky in a patient with AE-IPF is justifiable in certain cases but the decision should be based on scenario.[28] Histologic findings from lung biopsy in AE-IPF not only shows the typical UIP pattern but also shows diffuse alveolar damage with or without hyaline membranes, numerous fibroblastic foci, organizing pneumonia, and hemorrhage with capillaritis.[50]
Management
Presently AE-IPF lacks an effective treatment. There is a dearth of evidence-based management strategies available at this time. This is in part due to a lack of a consistent definition for describing AE-IPF and also due to the lack of randomized controlled trials evaluating different treatment approaches. In view of the unpredictable onset and rapid decline in patients with AE-IPF, it is extremely difficult to assess the true potential of any treatment. Modalities studied and used recently for AE-IPF have been extrapolated from those used to treat IPF and ARDS as AE-IPF combines the clinico-pathologic feature of these two diseases.
The available treatment includes pharmacological and non-pharmacological options. Frequently, aggressive therapy involving multiple modalities is used to deal with AE-IPF. Much research is currently focused on finding factors which predict the onset of this devastating complication as it might help in its prevention.[51] Patients developing this complication have complex management requirements which are usually best met in an ICU setting. There is a move worldwide to develop specialized centers where multidisciplinary coordinated care involving pulmonary, critical care, cardiothoracic surgery, and lung transplantation teams are readily available for managing IPF and AE-IPF.
Pharmacological therapy
Corticosteroids
Even though a Cochrane review found that there is no beneficial effect of corticosteroids in chronic non-exacerbated IPF,[51,52] studies were carried out to see the effect of steroids on AE-IPF. These studies were based on the fact that there is an increased inflammatory response in patients with AE-IPF, which might respond to steroid therapy. A study[4] carried out in the early 1990s showed some improvement with corticosteroid therapy, but subsequent studies have shown a high mortality rate despite their use.[7,42] Most investigators have used pulse corticosteroid therapy at a dose of 500 to 1,000 mg of methylprednisolone per day for 3 days (the same dose regimen used to treat idiopathic ARDS).[53,54] Recent studies[55,56] have used lower doses of steroids over a protracted period of time. The real potential of this approaches needs to be further analyzed in larger studies.
Immunosupressants
The role of Cyclosporine A used at a dose of 1.0 to 2.0 mg/kg/day in combination with corticosteroids has been investigated in a small, uncontrolled study to treat AE-IPF.[57] This study did show a trend towards prolonged survival in patients with AE-IPF. Data supporting use of Cyclophosphamide to treat AE-IPF is still lacking. Currently, there is no strong evidence favoring use of Cyclosporine A or Cyclophosphamide in patients with AE-IPF.
Pirfenidone
This novel antifibrotic compound is generating significant interest as a potential therapeutic agent for stable IPF. In a double-blinded, randomized controlled trial, patients who received Pirfenidone with IPF showed a reduced incidence of development of AE-IPF (placebo group, 13.9%; pirfenidone group, 0%).[58] Unfortunately, similar results were not reproduced in a recent study carried out by Taniguchi et al.[9] Thus the efficacy of Pirfenidone, both to prevent and treat AE-IPF, is still unknown.
Anticoagulation
Since AE-IPF causes alveolar injury that leads to a prothrombotic effect. anticoagulation can prevent escalation of vascular injury[59] Anticoagulating agent with prednisolone has been used as a treatment modality in a study on patients with IPF hospitalized for worsening disease compared with prednisolone alone with improved survival.[28] The mortality associated with acute exacerbations of IPF in the anticoagulant group was significantly reduced when compared with the non-anticoagulant group (18% vs. 71%, respectively; P = 0.008). This study was small, non-blinded, and lacked a placebo arm thereby decreasing the validity of the result.
Non-pharmacological therapy
Mechanical ventilation
Many patients with AE-IPF progress to respiratory failure. It is still controversial whether ventilatory support will improve long-term survival in patients suffering from AE-IPF. In a study carried out in patients with IPF admitted to ICU in a lung transplantation center, it was concluded that except for those deemed eligible for rapid lung transplantation, patients with IPF should not be intubated.[60]
Current knowledge about mechanical ventilation in patients with AE-IPF lacks consensus. There is a lack of systematic data analyzing different ventilator strategies and the effect of ventilator management on clinical outcomes. Most of the current knowledge is based on the experience gained by managing patients with diffuse alveolar damage as in ARDS.[7]
The major hurdle in providing effective ventilation in patients with AE-IPF is the inhomogeneous nature of the disease in the lung. Normal lung parenchyma is interspersed with areas of decreased compliance and extensive parenchymal alterations. This is unlike ARDS where the pattern of lung injury is more uniform. Many are also of the opinion that mechanical ventilation might act as a second insult to the lungs in patients with AE-IPF who are already ravaged by progressive parenchymal lung disease. This has been postulated to lead to further damage to the lungs resulting in death.[61]
Low tidal volumes (6 ml/kg) have been shown to reduce the sheer stress in patients with ARDS.[62] In patients with AE-IPF the same principles would hold true as there are areas of diffuse damage to the alveolar structures. Providing large tidal volumes (10 ml/kg) may lead to over inflation of the more compliant normal lung parenchyma with further respiratory compromise. The same reason restricts the amount of positive end expiratory pressure PEEP that can be given to the patients with AE-IPF. Detrimental effects of prolonged application of high PEEP have been studied in chronic IPF patients.[19] In the same context, there is little place for recruitment maneuvers or prone positioning.[63]
To maintain acceptable minute ventilation, as there is an increased amount of dead space in patients with AE-IPF, there is a potential need to increase the respiratory frequency to very high rates. This increased dead space can lead to hypercapnea and the increase required in respiratory rate can lead to patient discomfort. Permissive hypercapnea may be needed. Patient discomfort usually requires heavy sedation and possibly paralysis. Care should be taken to avoid development of auto-PEEP and the adverse effects of prolonged sedation.[64]
Non-invasive ventilation
The distinct advantage that non-invasive ventilation (NIV) can provide is avoidance of mechanical ventilation and its associated risks (aspiration, ventilator-associated pneumonia, and ventilator-associated injury). Unfortunately, the data is still lacking on short or long-term effects of NIV in patients with AE-IPF. The problem is that in most patients the excessive work of breathing associated with AE-IPF cannot be managed effectively by NIV for prolonged periods. Current data does not support use of NIV in patients who are suspected to have AE-IPF unless a rapidly reversible cause is found.
Extracorporeal membrane oxygenation
Extracorporeal membrane oxygenation can help circumvent the problem of hypoxia in patients with AE-IPF who are being mechanically ventilated. Limited availability, high cost, complicated technology, and increased rates of complications have been the most important factors limiting its use so far.[64,65] In the setting of IPF therapeutics, it has been used mainly as a bridge to transplantation.[66–68]
Lung transplantation
Lung transplantation is the only therapy which has been proven to increase the long-term survival of patients with AE-IPF.[14,69] Patients with AE-IPF who are transplant candidates should be considered for transfer to a transplant center to shorten the waiting time until transplantation, which can frequently be more than patient's life expectancy.
Conclusion
In conclusion AE-IPF is a potentially lethal complication of IPF. It is a disease of exclusion for which a diagnostic algorithm has been proposed. It is usually treated with corticosteroids with the possible addition of other immunosuppressive agents despite a lack of clear data to suggest any therapy is beneficial. Because mechanical ventilation when needed is often futile it should be used as a bridge to lung transplantation when AE-IPF is the sole cause of respiratory failure.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Flaherty KR, Travis WD, Colby TV, Toews GB, Kazerooni EA, Gross BH, et al. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med. 2001;164:1722–7. doi: 10.1164/ajrccm.164.9.2103074. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson AG, Colby TV, Du Bois RM, Hansell DM, Wells AU. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med. 2000;162:2213–7. doi: 10.1164/ajrccm.162.6.2003049. [DOI] [PubMed] [Google Scholar]
- 3.Carrington CB, Gaensler EA, Coutu RE, FitzGerald MX, Gupta RG. Natural history and treated course of usual and desquamative interstitial pneumonia. N Engl J Med. 1978;298:801–9. doi: 10.1056/NEJM197804132981501. [DOI] [PubMed] [Google Scholar]
- 4.Kondoh Y, Taniguchi H, Kawabata Y, Yokoi T, Suzuki K, Takagi K. Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest. 1993;103:1808–12. doi: 10.1378/chest.103.6.1808. [DOI] [PubMed] [Google Scholar]
- 5.Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE, Jr, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:636–43. doi: 10.1164/rccm.200703-463PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mason DP, Brizzio ME, Alster JM, McNeill AM, Murthy SC, Budev MM, et al. Lung transplantation for idiopathic pulmonary fibrosis. Ann Thorac Surg. 2007;84:1121–8. doi: 10.1016/j.athoracsur.2007.04.096. [DOI] [PubMed] [Google Scholar]
- 7.Kim DS, Park JH, Park BK, Lee JS, Nicholson AG, Colby T. Acute exacerbation of idiopathic pulmonary fibrosis: Frequency and clinical features. Eur Respir J. 2006;27:143–50. doi: 10.1183/09031936.06.00114004. [DOI] [PubMed] [Google Scholar]
- 8.Martinez FJ, Safrin S, Weycker D, Starko KM, Bradford WZ, King TE, Jr, et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med. 2005;142:963–7. doi: 10.7326/0003-4819-142-12_part_1-200506210-00005. [DOI] [PubMed] [Google Scholar]
- 9.Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–7. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 10.Song JW, Hong SB, Lim CM, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis: Incidence, risk factors and outcome. Eur Respir J. 2011;37:356–63. doi: 10.1183/09031936.00159709. [DOI] [PubMed] [Google Scholar]
- 11.Hiwatari N, Shimura S, Takishima T, Shirato K. Bronchoalveolar lavage as a possible cause of acute exacerbation in idiopathic pulmonary fibrosis patients. Tohoku J Exp Med. 1994;174:379–86. doi: 10.1620/tjem.174.379. [DOI] [PubMed] [Google Scholar]
- 12.Honoré I, Nunes H, Groussard O, Kambouchner M, Chambellan A, Aubier M, et al. Acute respiratory failure after interferon-gamma therapy of end-stage pulmonary fibrosis. Am J Respir Crit Care Med. 2003;167:953–7. doi: 10.1164/rccm.200208-818CR. [DOI] [PubMed] [Google Scholar]
- 13.Kondoh Y, Taniguchi H, Kitaichi M, Yokoi T, Johkoh T, Oishi T, et al. Acute exacerbation of interstitial pneumonia following surgical lung biopsy. Respir Med. 2006;100:1753–9. doi: 10.1016/j.rmed.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Kondoh Y, Taniguchi H, Katsuta T, Kataoka K, Kimura T, Nishiyama O, et al. Risk factors of acute exacerbation of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2010;27:103–10. [PubMed] [Google Scholar]
- 15.Sakamoto S, Homma S, Mun M, Fujii T, Kurosaki A, Yoshimura K. Acute exacerbation of idiopathic interstitial pneumonia following lung surgery in 3 of 68 consecutive patients: A retrospective study. Intern Med. 2011;50:77–85. doi: 10.2169/internalmedicine.50.3390. [DOI] [PubMed] [Google Scholar]
- 16.Lee JS, Song JW, Wolters PJ, Elicker BM, King TE, Jr, Kim DS, et al. Bronchoalveolar lavage pepsin in acute exacerbation of idiopathic pulmonary fibrosis. Eur Respir J. 2012;39:352–8. doi: 10.1183/09031936.00050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stern JB, Mal H, Groussard O, Brugière O, Marceau A, Jebrak G, et al. Prognosis of patients with advanced idiopathic pulmonary fibrosis requiring mechanical ventilation for acute respiratory failure. Chest. 2001;120:213–9. doi: 10.1378/chest.120.1.213. [DOI] [PubMed] [Google Scholar]
- 18.Al-Hameed FM, Sharma S. Outcome of patients admitted to the intensive care unit for acute exacerbation of idiopathic pulmonary fibrosis. Can Respir J. 2004;11:117–22. doi: 10.1155/2004/379723. [DOI] [PubMed] [Google Scholar]
- 19.Fernández-Pérez ER, Yilmaz M, Jenad H, Daniels CE, Ryu JH, Hubmayr RD, et al. Ventilator settings and outcome of respiratory failure in chronic interstitial lung disease. Chest. 2008;133:1113–9. doi: 10.1378/chest.07-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mollica C, Paone G, Conti V, Ceccarelli D, Schmid G, Mattia P, et al. Mechanical ventilation in patients with end-stage idiopathic pulmonary fibrosis. Respiration. 2010;79:209–15. doi: 10.1159/000225932. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto T, Ichiyasu H, Ichikado K, Muranaka H, Sato K, Okamoto S, et al. Clinical analysis of the acute exacerbation in patients with idiopathic pulmonary fibrosis. Nihon Kokyuki Gakkai Zasshi. 2006;44:359–67. [PubMed] [Google Scholar]
- 22.Noth I, Martinez FJ. Recent advances in idiopathic pulmonary fibrosis. Chest. 2007;132:637–50. doi: 10.1378/chest.06-1927. [DOI] [PubMed] [Google Scholar]
- 23.Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–7. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 24.Yüksel M, Ozyurtkan MO, Bostanci K, Ahiskali R, Kodalli N. Acute exacerbation of interstitial fibrosis after pulmonary resection. Ann Thorac Surg. 2006;82:336–8. doi: 10.1016/j.athoracsur.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 25.Sakamoto S, Homma S, Kawabata M, Kono T, Seki K, Nakata K, et al. Fatal acute exacerbation of idiopathic pulmonary fibrosis/ usual interstitial pneumonia initially in the right lung after surgery lobectomy for left lung cancer. Nihon Kokyuki Gakkai Zasshi. 2004;42:760–6. [PubMed] [Google Scholar]
- 26.Koizumi K, Hirata T, Hirai K, Mikami I, Okada D, Yamagishi S, et al. Surgical treatment of lung cancer combined with interstitial pneumonia: The effect of surgical approach on postoperative acute exacerbation. Ann Thorac Cardiovasc Surg. 2004;10:340–6. [PubMed] [Google Scholar]
- 27.Yokoyama A, Kohno N, Hamada H, Sakatani M, Ueda E, Kondo K, et al. Circulating KL-6 predicts the outcome of rapidly progressive idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;158:1680–4. doi: 10.1164/ajrccm.158.5.9803115. [DOI] [PubMed] [Google Scholar]
- 28.Kubo H, Nakayama K, Yanai M, Suzuki T, Yamaya M, Watanabe M, et al. Anticoagulant therapy for idiopathic pulmonary fibrosis. Chest. 2005;128:1475–82. doi: 10.1378/chest.128.3.1475. [DOI] [PubMed] [Google Scholar]
- 29.Fernández-Pérez ER, Yilmaz M, Jenad H, Daniels CE, Ryu JH, Hubmayr RD, et al. Ventilator settings and outcome of respiratory failure in chronic interstitial lung disease. Chest. 2008;133:1113–9. doi: 10.1378/chest.07-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akira M, Kozuka T, Yamamoto S, Sakatani M. Computed tomography findings in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:372–8. doi: 10.1164/rccm.200709-1365OC. [DOI] [PubMed] [Google Scholar]
- 31.Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–94. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 32.Fujimoto K, Taniguchi H, Johkoh T, Kondoh Y, Ichikado K, Sumikawa H, et al. Acute exacerbation of idiopathic pulmonary fibrosis: High-resolution CT scores predict mortality. Eur Radiol. 2012;22:83–92. doi: 10.1007/s00330-011-2211-6. [DOI] [PubMed] [Google Scholar]
- 33.Judge EP, Fabre A, Adamali HI, Egan JJ. Acute exacerbations and pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Eur Respir J. 2012;40:93–100. doi: 10.1183/09031936.00115511. [DOI] [PubMed] [Google Scholar]
- 34.Egan JJ, Stewart JP, Hasleton PS, Arrand JR, Carroll KB, Woodcock AA. Epstein-Barr virus replication within pulmonary epithelial cells in cryptogenic fibrosing alveolitis. Thorax. 1995;50:1234–9. doi: 10.1136/thx.50.12.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMillan TR, Moore BB, Weinberg JB, Vannella KM, Fields WB, Christensen PJ, et al. Exacerbation of established pulmonary fibrosis in a murine model by gamma herpes virus. Am J Respir Crit Care Med. 2008;177:771–80. doi: 10.1164/rccm.200708-1184OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wootton SC, Kim DS, Kondoh Y, Chen E, Lee JS, Song JW, et al. Viral infection in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:1698–702. doi: 10.1164/rccm.201010-1752OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huie TJ, Olson AL, Cosgrove GP, Janssen WJ, Lara AR, Lynch DA, et al. A detailed evaluation of acute respiratory decline in patients with fibrotic lung disease: Aetiology and outcomes. Respirology. 2010;15:909–17. doi: 10.1111/j.1440-1843.2010.01774.x. [DOI] [PubMed] [Google Scholar]
- 38.Konishi K, Gibson KF, Lindell KO, Richards TJ, Zhang Y, Dhir R, et al. Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;180:167–75. doi: 10.1164/rccm.200810-1596OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collard HR, Calfee CS, Wolters PJ, Song JW, Hong SB, Brady S, et al. Plasma biomarker profiles in acute exacerbation of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2010;299:L3–7. doi: 10.1152/ajplung.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ambrosini V, Cancellieri A, Chilosi M, Zompatori M, Trisolini R, Saragoni L, et al. Acute exacerbation of idiopathic pulmonary fibrosis: Report of a series. Eur Respir J. 2003;22:821–6. doi: 10.1183/09031936.03.00022703. [DOI] [PubMed] [Google Scholar]
- 41.Bonaccorsi A, Cancellieri A, Chilosi M, Trisolini R, Boaron M, Crimi N, et al. Acute interstitial pneumonia: Report of a series. Eur Respir J. 2003;21:187–91. doi: 10.1183/09031936.03.00297002. [DOI] [PubMed] [Google Scholar]
- 42.Tajima S, Oshikawa K, Tominaga S, Sugiyama Y. The increase in serum soluble ST2 protein upon acute exacerbation of idiopathic pulmonary fibrosis. Chest. 2003;124:1206–14. doi: 10.1378/chest.124.4.1206. [DOI] [PubMed] [Google Scholar]
- 43.Kurosu K, Takiguchi Y, Okada O, Yumoto N, Sakao S, Tada Y, et al. Identification of annexin 1 as a novel autoantigen in acute exacerbation of idiopathic pulmonary fibrosis. J Immunol. 2008;181:756–67. doi: 10.4049/jimmunol.181.1.756. [DOI] [PubMed] [Google Scholar]
- 44.Papiris SA, Manali ED, Kolilekas L, Kagouridis K, Triantafillidou C, Tsangaris I, et al. Clinical review: Idiopathic pulmonary fibrosis acute exacerbations – Unravelling Ariadne's thread. Crit Care. 2010;14:246. doi: 10.1186/cc9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silva CI, Müller NL, Fujimoto K, Kato S, Ichikado K, Taniguchi H, et al. Acute exacerbation of chronic interstitial pneumonia: High-resolution computed tomography and pathologic findings. J Thorac Imaging. 2007;22:221–9. doi: 10.1097/01.rti.0000213588.52343.13. [DOI] [PubMed] [Google Scholar]
- 46.Swigris JJ, Brown KK. Acute interstitial pneumonia and acute exacerbations of idiopathic pulmonary fibrosis. Semin Respir Crit Care Med. 2006;27:659–67. doi: 10.1055/s-2006-957337. [DOI] [PubMed] [Google Scholar]
- 47.Bradley B, Branley HM, Egan JJ, Greaves MS, Hansell DM, Harrison NK, et al. Interstitial lung disease guideline: The British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thora×. 2008;63:v1–58. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]
- 48.Parambil JG, Myers JL, Ryu JH. Histopathologic features and outcome of patients with acute exacerbation of idiopathic pulmonary fibrosis undergoing surgical lung biopsy. Chest. 2005;128:3310–5. doi: 10.1378/chest.128.5.3310. [DOI] [PubMed] [Google Scholar]
- 49.Saydain G, Islam A, Afessa B, Ryu JH, Scott JP, Peters SG. Outcome of patients with idiopathic pulmonary fibrosis admitted to the intensive care unit. Am J Respir Crit Care Med. 2002;166:839–42. doi: 10.1164/rccm.2104038. [DOI] [PubMed] [Google Scholar]
- 50.Wells AU. The clinical utility of bronchoalveolar lavage in diffuse parenchymal lung disease. Eur Respir Rev. 2010;19:237–41. doi: 10.1183/09059180.00005510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Churg A, Müller NL, Silva CI, Wright JL. Acute exacerbation (acute lung injury of unknown cause) in UIP and other forms of fibrotic interstitial pneumonias. Am J Surg Pathol. 2007;31:277–84. doi: 10.1097/01.pas.0000213341.70852.9d. [DOI] [PubMed] [Google Scholar]
- 52.Richeldi L, Davies HR, Ferrara G, Franco F. Corticosteroids for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev. 2003;3:CD002880. doi: 10.1002/14651858.CD002880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davies HR, Richeldi L, Walters EH. Immunomodulatory agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev. 2003;3:CD003134. doi: 10.1002/14651858.CD003134. [DOI] [PubMed] [Google Scholar]
- 54.Rice AJ, Wells AU, Bouros D, Du Bois RM, Hansell DM, Polychronopoulos V, et al. Terminal diffuse alveolar damage in relation to interstitial pneumonias. An autopsy study. Am J Clin Pathol. 2003;119:709–14. doi: 10.1309/UVAR-MDY8-FE9F-JDKU. [DOI] [PubMed] [Google Scholar]
- 55.Saydain G, Islam A, Afessa B, Ryu JH, Scott JP, Peters SG. Outcome of patients with idiopathic pulmonary fibrosis admitted to the intensive care unit. Am J Respir Crit Care Med. 2002;166:839–42. doi: 10.1164/rccm.2104038. [DOI] [PubMed] [Google Scholar]
- 56.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–84. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 57.Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, et al. Methylprednisolone infusion in early severe ARDS: Results of a randomized controlled trial. Chest. 2007;131:954–63. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 58.Inase N, Sawada M, Ohtani Y, Miyake S, Isogai S, Sakashita H, et al. Cyclosporin A followed by the treatment of acute exacerbation of idiopathic pulmonary fibrosis with corticosteroid. Intern Med. 2003;42:565–70. doi: 10.2169/internalmedicine.42.565. [DOI] [PubMed] [Google Scholar]
- 59.Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–9. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 60.Magro CM, Allen J, Pope-Harman A, Waldman WJ, Moh P, Rothrauff S, et al. The role of microvascular injury in the evolution of idiopathic pulmonary fibrosis. Am J Clin Pathol. 2003;119:556–67. doi: 10.1309/0B06-Y93E-GE6T-Q36Y. [DOI] [PubMed] [Google Scholar]
- 61.Bates JH, Davis GS, Majumdar A, Butnor KJ, Suki B. Linking parenchymal disease progression to changes in lung mechanical function by percolation. Am J Respir Crit Care Med. 2007;176:617–23. doi: 10.1164/rccm.200611-1739OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 63.Patroniti N, Pesenti A. Low tidal volume, high respiratory rate and auto-PEEP: The importance of the basics. Crit Care. 2003;7:105–6. doi: 10.1186/cc1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakos G, Tsangaris I, Kostanti E, Nathanail C, Lachana A, Koulouras V, et al. Effect of the prone position on patients with hydrostatic pulmonary edema compared with patients with acute respiratory distress syndrome and pulmonary fibrosis. Am J Respir Crit Care Med. 2000;161:360–8. doi: 10.1164/ajrccm.161.2.9810037. [DOI] [PubMed] [Google Scholar]
- 65.Lewandowski K. Extracorporeal membrane oxygenation for severe acute respiratory failure. Crit Care. 2000;4:156–68. doi: 10.1186/cc689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet. 2009;374:1351–63. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 67.Zimmermann M, Bein T, Arlt M, Philipp A, Rupprecht L, Mueller T, et al. Pumpless extracorporeal interventional lung assist in patients with acute respiratory distress syndrome: A prospective pilot study. Crit Care. 2009;13:R10. doi: 10.1186/cc7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Santambrogio L, Nosotti M, Palleschi A, Tosi D, Mendogni P, Lissoni A, et al. Use of venovenous extracorporeal membrane oxygenation as a bridge to urgent lung transplantation in a case of acute respiratory failure. Transplant Proc. 2009;41:1345–6. doi: 10.1016/j.transproceed.2009.02.065. [DOI] [PubMed] [Google Scholar]
- 69.Orens JB, Estenne M, Arcasoy S, Conte JV, Corris P, Egan JJ, et al. International guidelines for the selection of lung transplant candidates: 2006 update – A consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:745–55. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]


