Abstract
CONTEXT:
Bronchoscopic intervention can provide immediate relief from suffocation and an opportunity for additional treatment in patients with malignant airway obstruction. However, few studies have specifically identified prognostic factors affecting the survival of advanced lung or esophageal cancer patients receiving bronchoscopic intervention.
AIMS:
We aimed to investigate prognostic factors influencing survival in these patients.
STUDY DESIGN:
We conducted retrospective study.
METHODS:
The clinical parameters were retrospectively reviewed in 51 patients (lung cancer: n = 35; esophageal cancer: n = 16) who underwent palliative bronchoscopic interventions due to malignant airway.
RESULTS:
Bronchoscopic interventions, such as mechanical removal (n = 26), stenting (n = 31), laser cauterization (n = 19), and ballooning (n = 16), were performed on intraluminal (n = 21, 41%), extrinsic (n = 8, 16%), and combined lesions (n = 22, 43%). Tracheal invasion was found in 24 patients (47%). Successful palliation was achieved in 49 patients (96%). After the intervention, additional anti-cancer treatment was followed in 24 patients (47%). The median survival time and overall survival rate were 3.4 months and 4%. Survival was increased with selected conditions, including a treatment-naïve status (hazard ratio [HR], 0.359; confidence interval [CI], 0.158-0.815; P = 0.01), an intact proximal airway (HR, 0.265; CI, 0.095-0.738; P = 0.01), and post-procedural additional treatment (HR, 0.330; CI, 0.166-0.657; P < 0.01).
CONCLUSIONS:
Brochoscopic intervention could provide immediate relief and survival improvement in advanced lung or esophageal cancer patients with selected conditions such as a treatment-naïve status, an intact proximal airway, and available post-procedural additional treatment.
Keywords: Airway obstruction, bronchoscopy, esophageal cancer, lung cancer, survival
Introduction
The most common etiologies of malignant airway obstruction (MAO) are lung and esophageal cancer,[1] and approximately 30% of patients with lung cancer will develop central airway obstruction.[2] Although, stenosis of the central airway can deteriorate the functional and clinical statuses of patients, and lead to severe morbidity with impending respiratory failure,[3,4] the available definite therapeutic options are limited. Many lung or esophageal cancer patients with airway involvement are poor surgical candidates based on physiologic or oncologic criteria, although, surgical resection and reconstruction are the gold-standard treatment for MAO.[1,3] Radiation is also an another treatment of choice if time permits.[5] However, its use in tumor recurrence is limited due to toxicity to surrounding normal tissues.
In this situation, bronchoscopic intervention provides the most appropriate method of achieving immediate airway relief and maintaining airway patency, resulting in an improved quality of life and functional status.[1,6] Additionally, bronchoscopic intervention offers the opportunity for additional therapeutic modalities in inoperable patients, leading to longer survival.[7] In selected patients with locally-advanced lung cancer, preliminary bronchoscopic palliation could improve tumor staging, make surgical resection feasible, and improve the surgical results.[8]
However, there are few in-depth studies specifically identifying prognostic factors affecting the survival of advanced lung cancer or esophageal cancer patients receiving palliative bronchoscopic intervention. Therefore, the aim of the study was to investigate the clinical outcome and prognostic factors in advanced cancer patients with MAO caused by extension from primary lung cancer to esophageal cancer after bronchoscopic relief from suffocation.
Methods
Patient selection and recorded parameters
Patients who received palliative bronchoscopic interventions due to MAO at the Samsung Medical Center (a 1,250-bed university-affiliated, tertiary referral hospital in Seoul, South Korea) between January 2004 and December 2009 were considered for inclusion. Inoperable cancer patients with airway obstruction caused by a primary tumor of the lung or esophagus were included. Patients were excluded if other medical conditions could be responsible for the symptoms of dyspnea, hemoptysis, or chronic cough; if irreversible bleeding diathesis was present; or if the assessing interventional pulmonologist determined the patient to be in severe cardiopulmonary compromise, and hence unable to tolerate bronchoscopy.[6]
Medical records and procedure notes were examined to determine demographic characteristics, underlying malignancies, previous therapeutic modalities, location and type or site of airway obstruction, bronchoscopic intervention modalities, and immediate successful palliation. The occurrence of procedure-related complications was also reviewed. Additionally, any anti-cancer treatment after intervention and survival were investigated. The stage of the cancer at the first intervention was also recorded.
The current study was approved by the Institutional Review Board of the Samsung Medical Center, which waived the requirements for informed consent of the individual patients given the retrospective nature of the study.
Rigid bronchoscopic intervention
Rigid bronchoscopic intervention was performed in an operating room under general anesthesia using intravenous propofol. Patients were intubated with a rigid bronchoscope tube (Hopkins, Karl-Storz, Tuttlingen, Germany). A flexible bronchoscope (EVIS BF 1T240; Olympus, Tokyo, Japan) was introduced through the rigid bronchoscope tube to optimize visualization. In each procedure, the obstructed airway was dilated gently using rigid bronchoscope tubes of progressively increasing diameter until adequate airway patency was established. In certain patients, a controlled radial expansion balloon (Boston Scientific, Boston, MA) was used to enlarge the airway sufficiently to allow bronchoscopic dilation.
Most of the intraluminal mass was removed mechanically with the tip or forceps of a rigid bronchoscope or a snare. Frequently, a neodymium-yttrium aluminum garnet laser (20 W; LaserSonics, Milpitas, CA) was used to ablate the residual endobronchial tumor or to cauterize the tumor bed after excision of the tumor. After mechanical dilation, a silicone stent (Dumon-style stent; Bryan Corp., Woburn, MA; Natural stent; TNO Corp., Seoul, Korea) was used for extrinsic compression, malacia, and combined extrinsic compression and intrinsic lesions. The stents were inserted through the rigid bronchoscope using a previously-described technique.[9,10] Stent insertion was also performed in patients with intrinsic lesions when significant airway obstruction (more than 50% of the lumen) was present even after intervention.[11] All palliative types of therapy were performed by the same attending physician (H. Kim). Subsequent treatment was performed where indicated.
Definitions
Successful palliation was assessed by the patients’ improved respiratory status according to three criteria: Patient subjective response (improved breathlessness and exercise tolerance evaluated by the presence of “improvement of dyspnea” on chart record and improvement of Eastern Cooperative Oncology Group performance status) objective clinical response (diminished need for supplemental oxygen, improved breath sounds estimated patency of lumen increased by more than 50% after intervention), and radiographic improvement by computed tomography or chest radiography (resolution of mediastinal shift and pulmonary infiltrates/atelectasis).[6,12] However, it was not possible to judge the response with objective parameters for lung function, because the poor condition of the patient prevented assessment in most cases. Palliation of airway obstruction was considered to be successful if improvement was found in at least two of the three categories. The type of airway obstruction was classified as extrinsic, intrinsic (endoluminal and/or submucosal), or combined.[11] The duration of survival was calculated to be the time interval between the date of bronchoscopic intervention and death due to any cause or last clinical contact. Follow up was completed by July 31, 2010. Death was confirmed from medical records or by contact with the patient's family.
Statistical analysis
Data are presented as medians and interquartile ranges (IQRs; 25th and 75th percentiles) for continuous variables and as numbers and percentages for categorical variables. Data were compared using the Mann-Whitney U-test for continuous variables and Chi-squared or Fisher's exact test for categorical variables. Time zero for the survival analysis was defined as the date of the rigid bronchoscopy. Patients were followed until death or the last documented follow-up, if patients were still alive. The duration of overall survival was analyzed using the Kaplan–Meier method. Differences in overall survival were assessed using the log-rank test. Thereafter, multivariate analysis was performed using the Cox proportional hazards model to identify independent prognostic factors for overall survival after bronchoscopic intervention, as measured by the estimated hazard ratio (HR) with a 95% confidence interval (CI). The variables with a P value less than 0.20 in the univariabe analysis were entered into a Cox regression model. All tests were two-sided, and a P value less than 0.05 was deemed to indicate statistical significance. Data were analyzed using IBM SPSS Statistics 19.0 (IBM, Armonk, NY).
Results
Baseline characteristics
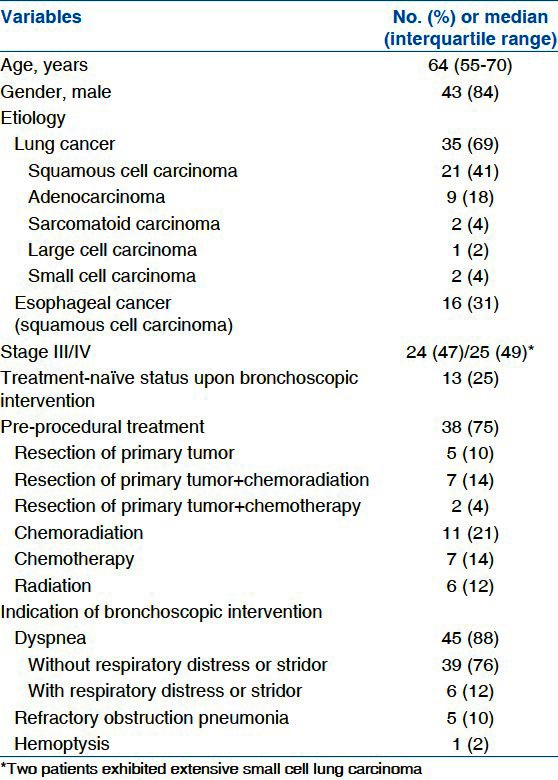
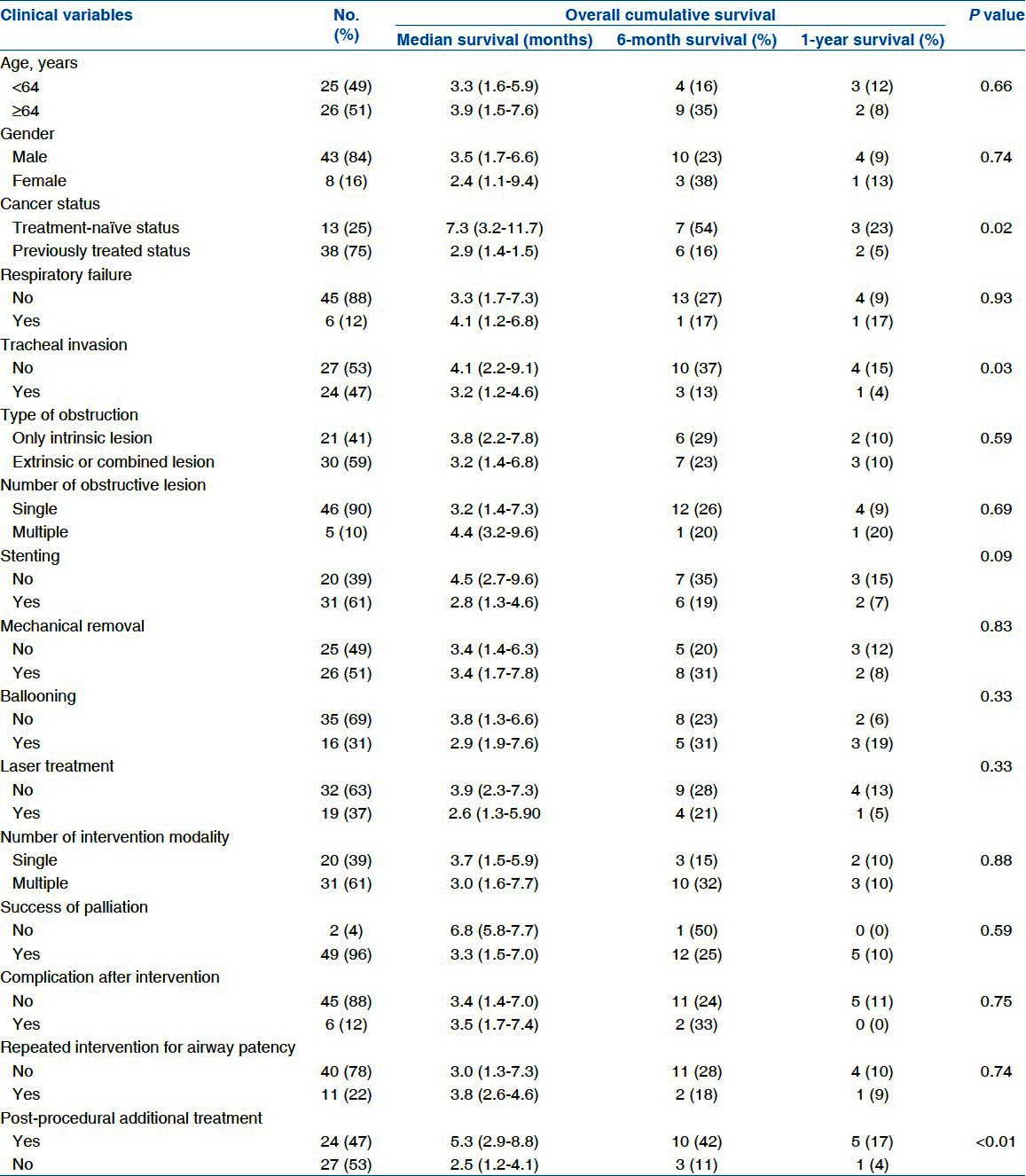
The baseline clinical characteristics of the enrolled 51 patients are summarized in Table 1. Among the 51 patients, 43 were male with a median age of 64 years (55-70 years) and 35 (69%) had airway obstruction caused by extension from lung cancer. The main causes of airway obstruction by primary lung cancer were squamous cell carcinoma in 21 patients and adenocarcinoma in nine patients. Overall, 13 treatment-naïve patients demonstrated a first presentation of MAO; 38 patients (75%) had previously undergone various therapeutic modalities. Only chemotherapy or radiation was administered in 7 (14%) and 6 (12%) patients, respectively, and chemoradiation was administered in 11 patients (21%). Five patients (10%) underwent only an operation for the primary tumor. The remaining patients received a combination treatment of chemotherapy (n = 2, 4%), and chemoradiation (n = 7, 14%) followed by surgical resection of the primary tumor. All patients were symptomatic. Most patients experienced dyspnea (n = 45, 88%). Of these 45 patients, 6 had accurate respiratory distress or stridor. Refractory obstructive pneumonia was found in five patients (10%), and hemoptysis in one patient (2%)
Table 1.
Baseline characteristics of enrolled patients (n=51)
Bronchoscopic findings, intervention modalities, and outcomes
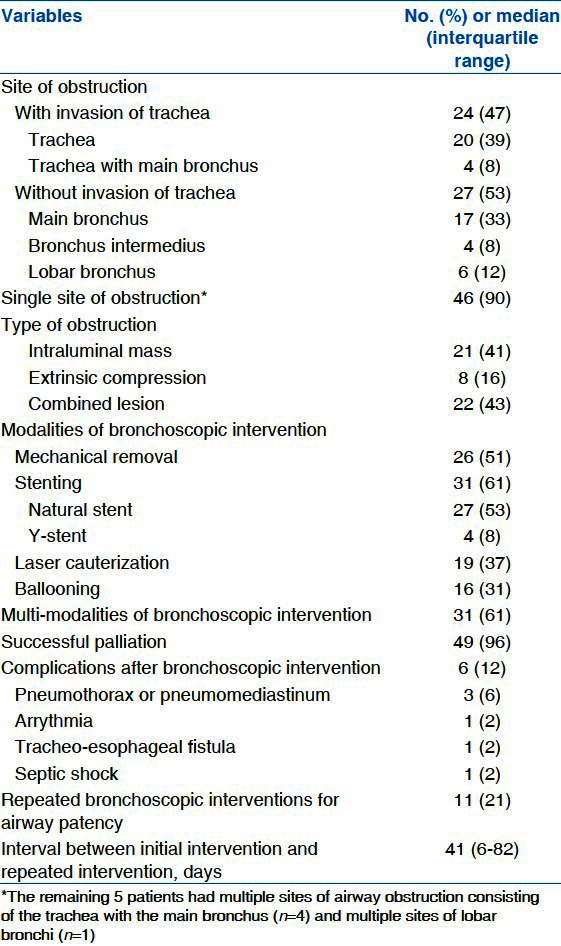
Tracheal obstruction was found in 24 patients (47%), and 17 patients had only an obstructive lesion of main bronchus. The distal airway such as bronchus intermedius or lobar bronchus was involved in 10 patients (20%). These patients had inoperable cancer stage because of advanced nodal stage (n = 2), malignant pleural effusion (n = 2), and distant metastasis (n = 5) despite involvement of the distal airway. Additionally, one patient had small cell carcinoma with an extensive stage.
Mechanical removal, laser cauterization or both were performed in 21 patients with airway obstruction caused by an intraluminal mass. Pure extrinsic compression of the airway requiring dilatation and stenting was found in eight patients (16%). Both extrinsic compression and an intraluminal mass requiring mechanical resection and stenting were found in 22 patients (43%).
Successful palliation was achieved in 49 patients (96%). However, it was impossible to improve the airway obstruction in two patients: One had a procedure-related persistent pneumothorax; the other had extensive tumor involvement into the segmental bronchus and the orifice of the occluded bronchial segment could not be located or catheterized. After an immediate successful palliation, repeated bronchoscopic interventions were required to maintain airway patency in 11 patients (21%). Complications relating to the procedure occurred in six patients (12%), including a pneumothorax or pneumomediastinum (n = 3), arrhythmia (n = 1), a tracheo-esophageal fistula (n = 1), and septic shock (n = 1) [Table 2].
Table 2.
Modalities and outcomes of bronchoscopic intervention
Post-procedural adjuvant treatment and survival
Additional treatments were initiated on average 11 days (4-15 days) after the bronchoscopic intervention in 24 patients (47%). Additional treatment included radiation (n = 17, 33%), chemotherapy (n = 3, 6%), and chemo-radiation (n = 3, 6%). Only one patient received surgical resection of the primary tumor, tracheal sleeve pneumonectomy. Additional treatment could not be applied to the remaining 27 patients (53%) due to exhaustion from radiation or chemotherapy (n = 17, 33%), infection (n = 4, 8%), or patient refusal of treatment (n = 6, 12%).
The overall survival after bronchoscopic intervention ranged from 15 days to 43.4 months, with a median of 3.4 months (IQR, 1.6-7.3 months). Although, no intra-procedural death was observed in the present study, six patients died within 30 days after the procedure: One from septic shock, three from disease progression, and two from unknown causes. The latter two patients received no additional treatment and were referred to another hospital for supportive care, limiting the information.
Longer survival was observed in patients who received additional treatment after the airway intervention, compared with those who did not (5.3 months; IQR, 2.9-8.8 months vs. 2.5 months; IQR, 1.1-4.1 months; P < 0.01). Additionally, longer survival was observed for treatment-naïve patients than for those who had undergone previous therapy (7.3 months; IQR, 3.2-11.7 months vs. 2.9 months; IQR, 1.4-4.5 months P = 0.02) [Table 3]. Survival curves for these parameters are demonstrated in Figure 1.
Table 3.
Post-procedural additional treatment and overall survival (n=24)
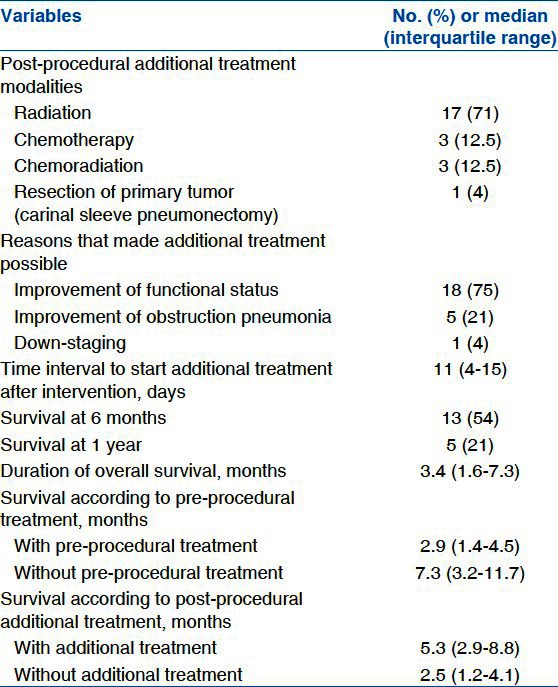
Figure 1.
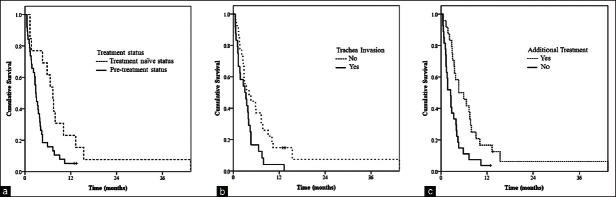
Overall survival curves using Kaplan–Meier estimates according to clinical variables after bronchoscopic intervention. (a) Survival curve based on pre-bronchoscopic treatment status (P= 0.02), (b) Survival curve based on tracheal invasion of tumor (P= 0.03), (c) Survival curve based on the post-bronchoscopic additional traetment (P< 0.01)
Prognostic factors associated with overall survival
Factors significantly influencing survival using Kaplan–Meier log-rank analysis were a history of previous treatment before bronchoscopic intervention, the site of obstruction and availability of post-procedural adjuvant treatment [Table 4].
Table 4.
Overall survival according to clinical variables
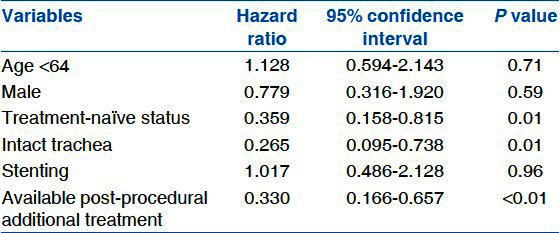
In multivariable analysis using a Cox proportional hazards model, longer survival was observed with selected conditions; a treatment-naïve status (HR, 0.359; CI, 0.158-0.815; P = 0.01), an intact proximal airway (HR, 0.265; CI, 0.095-0.738; P = 0.01), and post-procedural additional treatment (HR, 0.330; CI, 0.166-0.657; P < 0.01), among the statistically significant variables from Kaplan–Meier log-rank analysis [Table 5].
Table 5.
Cox proportional hazards model for overall survival in patients with malignant airway obstruction
Discussion
The present study revealed that palliative bronchoscopic intervention is effective in the immediate stabilization of MAO, and survival is better in patients a treatment-naïve status before intervention, intact trachea, and available additional post-procedural treatment even after adjusting other factors.
Bronchoscopic intervention followed by adjuvant treatment is well known to be associated with longer survival[3,4,9,13–15] rather than intervention itself.[9,14,16,17] Our results consistently demonstrated that adjuvant treatment after bronchoscopic intervention significantly increased the median survival (2.5 months vs. 5.3 months) and 1-year survival rate (4% vs. 17%). Our study yielded several strengths when approaching this conclusion compared with previous studies. First, we analyzed many factors that could occur in patients and adjusted these factors to identify significant prognostic factors, whereas previous studies focused on only a single modality of bronchoscopic intervention such as stenting or laser therapy before adjuvant treatment to observe survival benefits.[16,17] Because bronchoscopic modalities have been diversely used according to the patients’ lesion site and state, our study, which considered all possible modalities, could be more relevant clinically. Secondly, while our study was limited to lung cancer or esophageal cancer, previous studies – Reporting a survival benefit of additional therapy – included primary airway tumors,[9,16–18] which accounted for 20% of the enrolled patients in two studies.[9,18] Given that a patient suffering from a primary airway tumor has a better prognosis than other solid malignancies,[19,20] primary airway tumors itself could be a confounding factor for results of longer survival in these studies. Thus, our results might provide more clinically-relevant information for clinicians to decide additional post-broncho procedural treatment in advanced lung or esophageal cancer patients with MAO.
Confirming a previous report,[11] our study also showed that the site of obstruction was an independent predictor of overall survival. Particularly, patients with tracheal obstruction had a bad prognosis, which could be because of a more severe disease, compared with the disease being restricted to the lung or esophagus. Regarding bronchoscopic modalities for tracheal obstruction, stenting was performed in 21 of 24 (88%) patients with tracheal obstruction, which was consistent with a recent report showing that stenting was most commonly used for tracheal obstruction.[11] However, our study found no significant association between survival and type of tracheal obstruction such as an extrinsic compression or intraluminal mass, in contrast to a previous study.[11]
As suggested by previous reports,[7,8] our study documented that patients who are treatment naïve upon admission had longer survival than those with any cancer treatment before bronchoscopic intervention. The patients with pre-bronchoscopic procedure treatment could have obtained a poorer performance status after cancer treatment and could have already had a refractory or relapse status, resulting in a poorer additional treatment response. Based on previous data, only about 40-70% of the patients were eligible to receive post-procedural adjuvant treatment.[16] In our study, 47% of all patients received additional cancer therapy after bronchoscopy and survival rate in those patients was significantly higher that patients who are not eligible to additional therapy.
Among them, treatment-naïve patients upon admission obtained more additional treatment and survived longer than those with cancer treatment before the bronchoscopic procedure (54% vs. 45%; P < 0.01, 7.1 [3.2-11.7] months vs. 2.8 [1.4-4.5] months; P < 0.01, respectively). Thus, our study revealed that patients with a treatment-naïve status upon admission acquired more benefit from adjuvant treatment after bronchoscopy. In our study, the median survival time and 1-year survival rate after bronchoscopic intervention were 3.4 months and 10%, during the median follow-up period of 45.5 (IQR, 22.2-61.1) months. Our 1-year survival rate was lower than that reported by previous studies (14-15%),[16,21] although, the median survival time was similar to those studies (3.1-3.4 months).[16,21] This might be due to the fact that our study included more patients with advanced esophageal cancer, compared with the two studies. As demonstrated in our study, patients with airway obstruction from esophageal cancer (median, 2.8 months; IQR, 1.3-4.0 months) had a worse prognosis compared with those with lung cancer (median, 4.0 months; IQR, 1.7-7.7 months) in the Kaplan–Meier log-rank analysis (P = 0.04). The cause for this may be the more extensive degree of obstruction from esophageal cancer abutting to the airway, compared with lung cancer.
The current study has several limitations. One major limitation is the potential selection bias. This was a retrospective study performed on a small number of patients in a single referral center, limiting its statistical significance. These factors may limit the generalization of the findings. However, controlled, prospective studies comparing various therapies using interventional bronchoscopy have been problematic because of the often critical nature of airway obstruction that requires urgent therapy. Secondly, the clinical outcome after bronchoscopic intervention was evaluated according to the patients’ symptoms, radiographic findings, and patency of the airway lumen. We could not provide more objective measurements of clinical improvement after intervention. Spirometric data for patients before and after treatment would have enhanced the study, but pre-treatment lung function tests could not be performed because of the extreme breathlessness and discomfort that enrolled patients were experiencing. Although, the conclusions from our study are limited, our study is unique in that we determined the factors influencing survival in patients with MAO caused by advanced lung cancer or esophageal cancer. Our findings suggested that good results from bronchoscopic intervention largely depend on patient selection, and that availability of post-procedural additional treatment is necessary for longer survival, as confirmed by Cox regression analysis.
Conclusion
In this retrospective study confirmed that bronchoscopic intervention could achieve immediate airway relief from MAO, but also improve survival in advanced lung or esophageal cancer patients with selected conditions such as a treatment-naïve status, an intact proximal airway, and available post-procedural additional treatment. However, this observation needs to be further evaluated by multicenter-large sample sized study.
Footnotes
Source of Support: Nill,
Conflict of Interest: None declared.
References
- 1.Wood DE. Management of malignant tracheobronchial obstruction. Surg Clin North Am. 2002;82:621–42. doi: 10.1016/s0039-6109(02)00025-7. [DOI] [PubMed] [Google Scholar]
- 2.Stöhr S, Bolliger CT. Stents in the management of malignant airway obstruction. Monaldi Arch Chest Dis. 1999;54:264–8. [PubMed] [Google Scholar]
- 3.Petrou M, Goldstraw P. The management of tracheobronchial obstruction: A review of endoscopic techniques. Eur J Cardiothorac Surg. 1994;8:436–41. doi: 10.1016/1010-7940(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 4.Stanopoulos IT, Beamis JF, Jr, Martinez FJ, Vergos K, Shapshay SM. Laser bronchoscopy in respiratory failure from malignant airway obstruction. Crit Care Med. 1993;21:386–91. doi: 10.1097/00003246-199303000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Rostom AY, Morgan RL. Results of treating primary tumours of the trachea by irradiation. Thorax. 1978;33:387–93. doi: 10.1136/thx.33.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amjadi K, Voduc N, Cruysberghs Y, Lemmens R, Fergusson DA, Doucette S, et al. Impact of interventional bronchoscopy on quality of life in malignant airway obstruction. Respiration. 2008;76:421–8. doi: 10.1159/000152832. [DOI] [PubMed] [Google Scholar]
- 7.Venuta F, Rendina EA, De Giacomo T, Mercadante E, Ciccone AM, Aratari MT, et al. Endoscopic treatment of lung cancer invading the airway before induction chemotherapy and surgical resection. Eur J Cardiothorac Surg. 2001;20:464–7. doi: 10.1016/s1010-7940(01)00742-4. [DOI] [PubMed] [Google Scholar]
- 8.Daddi G, Puma F, Avenia N, Santoprete S, Casadei S, Urbani M. Resection with curative intent after endoscopic treatment of airway obstruction. Ann Thorac Surg. 1998;65:203–7. doi: 10.1016/s0003-4975(97)01124-7. [DOI] [PubMed] [Google Scholar]
- 9.Jeon K, Kim H, Yu CM, Koh WJ, Suh GY, Chung MP, et al. Rigid bronchoscopic intervention in patients with respiratory failure caused by malignant central airway obstruction. J Thorac Oncol. 2006;1:319–23. [PubMed] [Google Scholar]
- 10.Wood DE. Airway stenting. Chest Surg Clin N Am. 2001;11:841–60. [PubMed] [Google Scholar]
- 11.Chhajed PN, Somandin S, Baty F, Mehta AJ, Azzola A, Leuppi J, et al. Therapeutic bronchoscopy for malignant airway stenoses: Choice of modality and survival. J Cancer Res Ther. 2010;6:204–9. doi: 10.4103/0973-1482.65250. [DOI] [PubMed] [Google Scholar]
- 12.Walser EM, Robinson B, Raza SA, Ozkan OS, Ustuner E, Zwischenberger J. Clinical outcomes with airway stents for proximal versus distal malignant tracheobronchial obstructions. J Vasc Interv Radiol. 2004;15:471–7. doi: 10.1097/01.rvi.0000124944.58200.d9. [DOI] [PubMed] [Google Scholar]
- 13.Cavaliere S, Venuta F, Foccoli P, Toninelli C, La Face B. Endoscopic treatment of malignant airway obstructions in 2,008 patients. Chest. 1996;110:1536–42. doi: 10.1378/chest.110.6.1536. [DOI] [PubMed] [Google Scholar]
- 14.Saji H, Furukawa K, Tsutsui H, Tsuboi M, Ichinose S, Usuda J, et al. Outcomes of airway stenting for advanced lung cancer with central airway obstruction. Interact Cardiovasc Thorac Surg. 2010;11:425–8. doi: 10.1510/icvts.2010.238196. [DOI] [PubMed] [Google Scholar]
- 15.Stephens KE, Jr, Wood DE. Bronchoscopic management of central airway obstruction. J Thorac Cardiovasc Surg. 2000;119:289–96. doi: 10.1016/S0022-5223(00)70184-X. [DOI] [PubMed] [Google Scholar]
- 16.Furukawa K, Ishida J, Yamaguchi G, Usuda J, Tsutsui H, Saito M, et al. The role of airway stent placement in the management of tracheobronchial stenosis caused by inoperable advanced lung cancer. Surg Today. 2010;40:315–20. doi: 10.1007/s00595-008-4058-2. [DOI] [PubMed] [Google Scholar]
- 17.Han CC, Prasetyo D, Wright GM. Endobronchial palliation using Nd: YAG laser is associated with improved survival when combined with multimodal adjuvant treatments. J Thorac Oncol. 2007;2:59–64. doi: 10.1097/JTO.0b013e31802bff2d. [DOI] [PubMed] [Google Scholar]
- 18.Mathisen DJ, Grillo HC. Endoscopic relief of malignant airway obstruction. Ann Thorac Surg. 1989;48:469–73. doi: 10.1016/s0003-4975(10)66842-7. [DOI] [PubMed] [Google Scholar]
- 19.Ahn Y, Chang H, Lim YS, Hah JH, Kwon TK, Sung MW, et al. Primary tracheal tumors: Review of 37 cases. J Thorac Oncol. 2009;4:635–8. doi: 10.1097/JTO.0b013e31819d18f9. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Jung EJ, Jeon K, Koh WJ, Suh GY, Chung MP, et al. Treatment outcomes of patients with adenoid cystic carcinoma of the airway. Lung Cancer. 2011;72:244–9. doi: 10.1016/j.lungcan.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Lemaire A, Burfeind WR, Toloza E, Balderson S, Petersen RP, Harpole DH, Jr, et al. Outcomes of tracheobronchial stents in patients with malignant airway disease. Ann Thorac Surg. 2005;80:434–7. doi: 10.1016/j.athoracsur.2005.02.071. [DOI] [PubMed] [Google Scholar]


