Abstract
Purpose:
To investigate the effect of 2-deoxy–D-glucose (2-DG), an inhibitor of glucose transport and glycolysis, on glioblastoma and the normal brain tissue during combined treatment with hypofractionated radiotherapy.
Materials and Methods:
Twenty patients with malignant gliomas (18 Glioblastoma Multiformae, 2 Anasplastic Astrocytoma grade III) following surgery were treated weekly (once) with 2-DG, (250 mg/kg body weight), followed by 5 Gy of radiation to the tumor bed per fraction for 7 weeks. Clinical evaluation, complete hemogram, and random blood sugar levels were carried out in each cycle. Follow-up computed tomography (CT)/magnetic resonance imaging (MRI) was done to evaluate radiation-induced changes. Kernofsky Performance scale (KPS) was recorded preoperatively; postoperatively, and post-therapy till the last follow-up.
Results:
Twenty patients were recruited for this trail; 19 of them completed the treatment and 1 discontinued. The survival period ranged between 6 and 36 months after the treatment, with a median survival of 14 months. CT and MRI revealed significant tumor necrosis. Histological evidence from the tissue during reexploration confirms the hypothesis of protective effect of 2-DG on normal brain. KPS was above 80% in majority of the patients, 6 months after the surgery.
Conclusion:
Radiotherapy coupled with 2-DG enhances tumor necrosis selectively and significantly while the normal brain gets relatively protected. This has been reflected in our study both clinically by preservation of quality-of-life and pathologically by retaining the integrity of normal brain architecture.
Keywords: 2-deoxy-D-glucose, malignant gliomas, neural protection, radionecrosis, radiotherapy, quality-of-life
Introduction
The prognosis of patients suffering from malignant cerebral gliomas has remained dismal, despite utilization of multimodal therapies and many advances in radiation technology and delivery (Alexander and Kaplan).[1,2] Radiation therapy for malignant astrocytomas has generally increased the median survival from 4 to 10 months only.
The failure of radiotherapy (RT) in cerebral gliomas is primarily due to the presence of hypoxic, repair-proficient, and radioresistant subpopulation of cells in the tumor.[3,4] Amplification in the expression of cellular oncogenes like Raf, Myc, and N-ras is known to cause radioresistance in gliomas.[5,6] An analysis of the pattern of failures after conventional therapy, i.e., surgery, RT, and chemotherapy, indicates local regrowth of the tumor, implying that the conventional treatment (1.8-2.0 Gy/fraction, 5 fractions per week, a total of 30-35 fractions) is not as effective.[1] On the other hand, delivery of high doses of radiation has limitation of damaging the surrounding normal brain.[7] Strategies directed toward differentially enhancing radiation damage in tumor cells and reducing the damage to normal brain tissue could significantly improve the treatment efficacy of RT.[7]
Glucose usage is significantly increased in tumor cells, and these cells derive a large part of their metabolic energy (ATP) from glycolytic pathway.[8] Therefore, it has been postulated that inhibitors of glucose transport and glycolysis could differentially inhibit repair process in these cells, leading to an enhancement of the radiation damage.[9] A number of studies have indeed demonstrated that presence of 2-deoxy-D-glucose (2-DG), an inhibitor of glucose transport and glycolysis, prior to radiation could inhibit the repair of DNA lesions, thereby enhancing the radiation damage in various cellular systems with high rates of glycolysis like cancer cells under euoxic as well as hypoxic conditions.[2] Interestingly, under similar conditions, minimal radiation damage is observed in normal cells[2] Therefore, combination of ionizing radiation with 2-DG provides an unique opportunity to selectively destroy tumors by differentially enhancing the radiation damage in cancer cells and preventing radiation damage to normal tissue at the same time.[2] Positron Emitted Tomography studies have shown that 2-DG accumulation in most of the cerebral gliomas correlates with degree of malignancy[10] Glioma cells in vitro have also been observed to manifest high rates of glucose usage and glycolysis. It is expected that combining 2-DG with radiation would significantly enhance the efficacy of RT in cerebral gliomas.
In our study, we have recruited 20 patients of malignant cerebral gliomas, with age ranging from 27 to 67 years. All of them received 2-DG orally, 25 min prior to irradiation. Tolerance to the treatment was good with minimal, clinically insignificant side effects. During the follow-up, 13 of them developed symptoms of recurrence requiring re -exploration. They had mass effect on imaging and symptoms of raised intracranial pressure (ICP). Initially, steroids and mannitol were administered. Reexploration and debulking was planned as symptoms remained unabated. At surgery, the tissue was looking pale, avascular, soft, and suckable. As the decompression progressed peripherally, firm margins were encountered adjacent to normal brain. Abnormal looking necrotic tissue was completely removed till normal brain tissue was seen all around. Histopathology revealed extensive tumor necrosis, relatively preserved surrounding normal brain structure, giant cells, and gliomesenchymal scar delineating tumor necrosis from the surrounding brain.
Materials and Methods
The present study was conducted after obtaining clearance from the Institutional Ethics Committee of Manipal Hospital Bangalore and approval from the Drug Controller General, Ministry of Health and Family Welfare, India.
Twenty patients (13 males and 7 females) in the age group of 27-67 years with malignant gliomas were studied. Pre-operative Kernofsky performance scale (KPS) was more than 70. After scrutinizing the inclusion criteria, informed consent was obtained and treatment was provided between December 2001 and March 2004. Hematological investigations, liver function test, renal function test, and contrast computed tomography (CT) scans were performed preoperatively, postoperatively, post-RT, and during follow-up on every 3 months. Macroscopic near-total removal was done in all the patients, and only diagnostic biopsy was done in two deep-seated lesions.
After an overnight fasting, aqueous solution (100 ml) of 2-DG, at a dose of 250 mg/kg, was administered orally, 25 min before focal irradiation of the tumor with linear accelerator. The radiation dose was 5 Gy per fraction by two parallel opposing lateral portals. Radiation was given to the tumor bed and 3 cm surrounding the tumor margins. This schedule was given once a week for 7 weeks, aiming at a total radiation of 35 Gy. The Biologically equivalent dose (BED) was G2 Gy in comparison to the conventional protocol of 62.5 Gy/fraction.
Tolerance to the treatment was assessed by vital parameters such as blood pressure, pulse rate, and respiratory rate, monitored hourly on treatment days. Symptoms like chills, nausea, and headache, which were transient, were recorded. KPS was measured throughout the treatment and follow-up period.
Results
Among the 20 who fulfilled the inclusion criteria, 13 were males and 7 were females between 27 and 67 years, 18 had Glioblastoma Multiformae, and 2 had astrocytoma grade III. Individual survival periods are shown in Table 1, and Table 2 shows the KPS measured at preoperative, postoperative, post-radiation therapy, and during follow-up. Improvement in KPS suggests preserved or enhanced quality of life (QOL)/general condition in this trail group.
Table 1.
List of patients with site of the lesion and their survival period

Table 2.
Quality of life
Only few patients experienced mild, clinically insignificant side effects like nausea, chills, and headache. Blood sugars showed an increase after 4 h of treatment and gradually reduced to normal level at the end of 8 h, as reported in previous studies. However, vital parameters and hematological investigations remained normal. None of them experienced any neurological worsening during the entire period of therapy.
Thirteen of them developed symptoms of raised ICP at an interval ranging from 3 to 9 months. Initially, they were treated with steroids and mannitol. Imaging had shown mass effect surrounding edema with operative area filled with necrotic/tumor tissue. CT/magnetic resonance imaging (MRI) of these patients, done after radiation, showed hyperdensity around the tumor bed, indicating tumor necrosis [Figures 1a & b and 2a–d]. Clinically, possibilities of tumor necrosis versus tumor recurrence were considered.
Figure 1.

(a) Pre-operative image; (b) Post-operative image
Figure 2.

(a) Pre-operative image; (b) Post-operative image; (c) Radiotherapy planning {Tumor and 3 cm around the tumor}; (d) Follow up after 3 months
Despite the medical treatment, raised ICP features persisted in 13 patients. Hence, reexploration was performed. Intraoperatively, the tumor was soft, highly necrotic, avascular, and suckable. This confirmed the clinical diagnosis as tumor necrosis which was ratified further by histopathologic evidence. Postoperatively, all of them had clinical improvement. There were no fresh neurological deficits, and the postoperative period QOL score also improved.
Histology
Administration of 2-DG has dual and differential effect on tumor cells and normal brain cells. Post-radiation changes were seen only at the tumor bed and did not affect the normal brain structures. Histopathology from reexploration revealed severe tumor necrosis, and hyalinization of blood vessels with gliomesenchymal scar at the junction of the tumor and brain, with adjacent totally preserved choroid plexus. The combined treatment (2-DG + RT) induced sheets of necrosis in the tumor, which was classical [Figures 3a & b, and 4–7]. We did find significant number of giant cells, the significance of which is not clearly understood at present.
Figure 3.

(a) Severe tumor necrosis with 2-DG+RT (H & E); (b) Tumor necrosis with conventional RT and with 2-DG+RT
Figure 4.

Thickened blood vessel and hyaline formation with 2-DG+RT
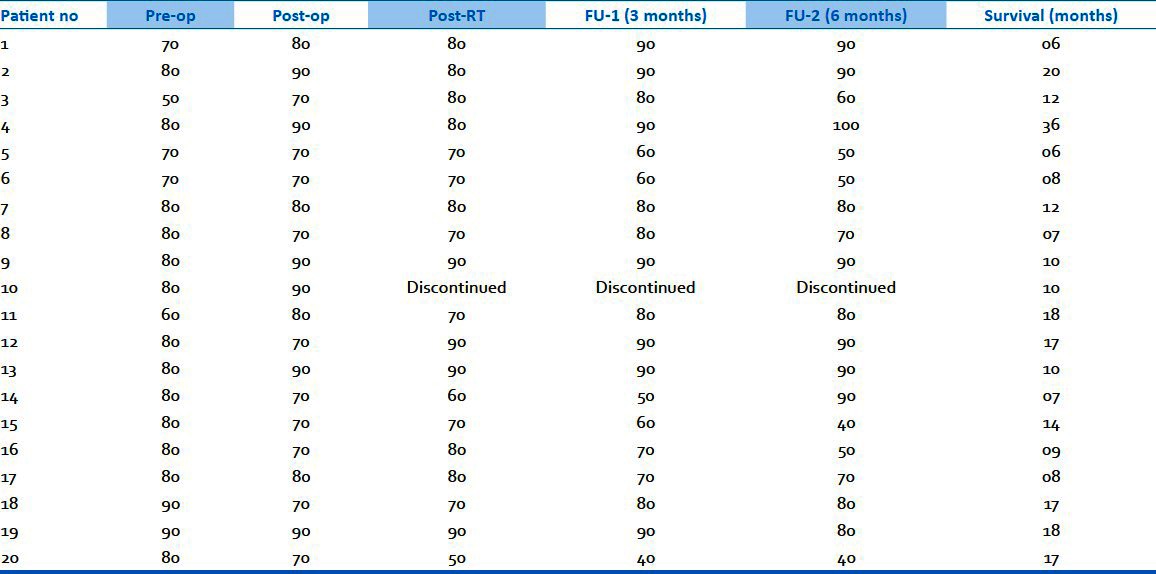
Figure 7.
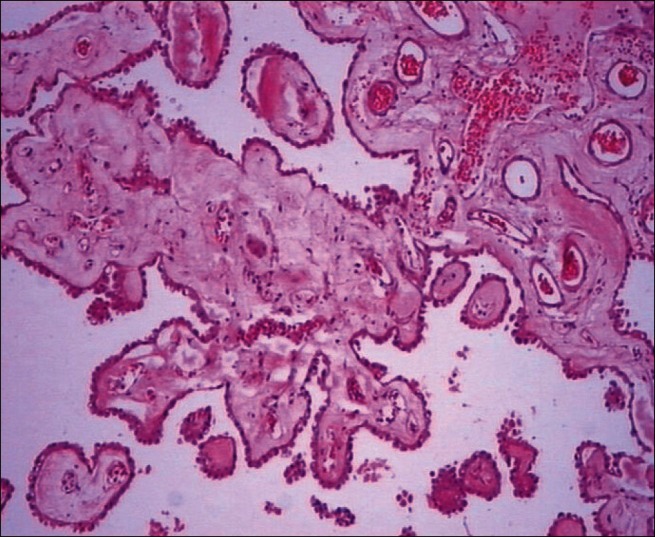
Preserved choroid plexus in 2-DG+RT
Figure 5.
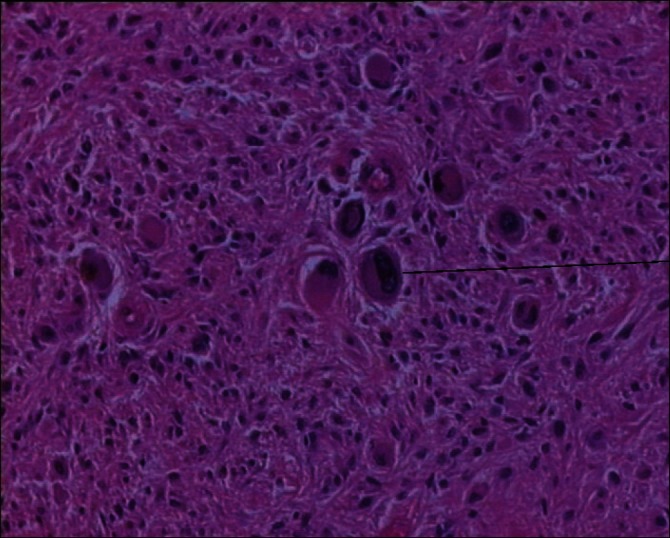
Presence of giant cells following 2-DG+RT therapy
Figure 6.

Gliomesenchymal scar demarking the parenchyma after 2-DG+RT
However, we feel it could be a reaction to the necrotic tissue. There seems to be a clear mesenchymal scar separating this from the surrounding brain. In a few specimens, we had the opportunity to see the surrounding brain and choroid plexus adjacent to the tumor, well within the field of radiation. This clearly demonstrates the total preservation of architecture and mucosa of the choroid plexus, suggesting and confirming the relative protective effect of 2-DG on the normal brain tissue.
Discussion
Inability of currently available therapies to achieve sufficient local tumor control, leading to the regrowth of tumor within the cranium has been suggested to be primarily responsible for the failure of curative therapy in glioblastoma multiforme, resulting in only marginal survival benefits. Further, radiation-induced necrosis of the adjoining normal brain tissue leads to neurological deficits and functional disturbances, resulting in deterioration of QOL following RT. Therefore, it is imperative to develop newer approaches for improving the therapy of CNS neoplasms, particularly the malignant gliomas. The present study demonstrated that combination of 2-DG with hypofractionated regimen of RT has the potential to induce extensive tumor necrosis (contributing to local tumor control) and also protect the normal brain tissue, contributing to the maintenance or improvement in the QOL.
Earlier studies have also indicated a reduction in the damage to the normal brain tissue observed by the changes in periventricular hyperintensity in the MRI analysis.[11,12] Reexploration in the present study provided an opportunity to carry out detailed histological examination and provided evidence for extensive tumor necrosis, as well as for relative preservation of choroid plexus and the surrounding brain [Figures 3a & b, and 4–7]. Therefore, this perhaps is the first study providing direct histological evidence for brain protection by 2-DG. Earlier, in vitro studies in human Peripheral blood lymphocytes (Kalia et al., 1982), ex vivo studies with mouse splenocytes and thymocytes, as well as in vivo studies in whole body irradiated mice have shown protection against radiation-induced cytogenetic damage and apoptosis by 2-DG.[13,14]
The reasons for well-preserved normal tissue observed here and reported earlier with MRI (Mohanti et al., 1996 and Singh et al., 2005) could be multifold.[11,12] Firstly, it could be due to the lower physical dose of 35 Gy used (7 × 5 Gy/fraction), although it is biologically equivalent (BED) to a dose of 62 Gy (with α/β value of 3 for normal brain and 10 for the tumor), as against the conventional fractionation where 5 fractions per week of 2 Gy/fraction is administered for 30 fractions. Alternatively, it could also be due to reductions in the manifestations of radiation-induced primary lesions and metabolic oxidative stress that lead to delayed tissue damage. At therapeutically relevant and related doses, radiation mainly induces mitosis with minimal apoptotic death leading to secondary necrosis in proliferating epithelial and epithelial tumor cells.[15] Although the late radiation-induced necrosis of the normal brain tissue is not completely understood, it appears to be related to inflammatory response involving aberrant cytokine secretion.[16] Consequently, in non-proliferating cells of the normal brain tissue, apoptosis is likely to be a major contributor to the treatment-induced tissue damage. Therefore, it appears that inhibition of radiation-induced apoptosis by 2-DG in the normal brain tissue and restoration of normal cytokine profile are the contributing factors for the well-preserved normal tissue architecture observed in these studies. Recent studies in human glioma cells (U-87) have shown 2-DG induced changes in the expression of certain genes related to apoptosis and alterations in the immune status including cytokine secretion in tumor-bearing mice.[17,18] Further studies are required to understand the mechanisms underlying protection of normal tissue damage, as it would allow the use of higher radiation doses for achieving better therapeutic efficacy. Most recently, gluco-deprivation induced either by glucose withdrawal or by addition of 2-DG has been found to enhance apoptosis in glioblastoma cells, but not in normal astrocytes, which could be linked to maintenance of ATP levels and redox status in astrocytes.[18,19]
Although the late radiation-induced necrosis of the normal brain tissue is not completely understood, it appears to be related to inflammatory response involving aberrant cytokine secretion[18] In late cerebral radionecrosis, there are typical coagulation necrosis areas containing fibrinoid necrosis with occlusion of the lumina and poorly active inflammatory areas with many inflammatory ghost cells, focal perivascular lymphocytes, hyalinized vessels, and telangiectatic vascularization. It is difficult to believe that coagulation necrosis occurs without first disordering the vascular endothelial cells because fibrinoid necrosis is the main feature in late cerebral radionecrosis. Because various histological findings do develop, progress, and extend sporadically at different areas and times in the irradiated field of the brain for a long time after radiation, uncontrolled chronic inflammation containing various cytokine secretions may also play a key role in the progression of this radionecrosis.[16]
We would like to emphasize the fact that the quality of survival in this group of patients was much better preserved in comparison to the conventional method of treatment for malignant cerebral gliomas. It proves the fact of comparable efficacy and safety. Though few of them had relatively longer period of survival in this study, it does not stand the statistical significance at present. However, larger group of patients with longer follow-up is required to ratify this. Preserved QOL is an important quality indicator in proving the safety and efficacy of the treatment regimen, particularly in malignant gliomas.
Conclusion
2-DG with RT enhances tumor necrosis significantly and relatively protects the surrounding normal brain. This has been reflected in our study clinically as preservation of QOL and pathologically as preserved normal brain architecture. Evaluation of the mechanisms of the development or aggravation of late cerebral radionecrosis requires a further study for abnormal cytokine secretions and aberrant inflammatory reactions. Preserved QOL post-RT is an important quality indicator of the therapeutic regimen adopted in malignant gliomas.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Alexander E., III Glioblastoma revisited. Do clinical observation match basic science theory. J Neurooncol. 1993;17:169–73. doi: 10.1007/BF01050220. [DOI] [PubMed] [Google Scholar]
- 2.Jain VK, Kalia VK, Gopinath PM, Narqui S, Kucheria K. Optimization of cancer therapy: Part Ш-Effects of combining 2 Deoxy-D-Glucose treatment with Gama radiation in normal mice. Indian J Exp Biol. 1979;17:1320–5. [PubMed] [Google Scholar]
- 3.Suit HD, Baumann M, Shates S, Convery K. clinical interest in determination of cellular radiation sensitivity. Int J Radiat Biol. 1989;56:725–37. doi: 10.1080/09553008914551971. [DOI] [PubMed] [Google Scholar]
- 4.Yang S, Darlilng JL, Mc Millian TJ, Peacock JH, Steel G. Radio sensitivity, recovery and dose rate effect in three glioma cell lines. Radiother Oncol. 1990;19:49–56. doi: 10.1016/0167-8140(90)90165-s. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan RS. Supratentorial malignant gliomas: Risk patterns and therapy. J Natl Cancer Inst. 1993;85:690–1. doi: 10.1093/jnci/85.9.690. [DOI] [PubMed] [Google Scholar]
- 6.Rossw GM, Mcllwrath A, Brown R. Mollecular determinants of radio resistance in human glioma. J Neurooncol. 1994;21:46. [Google Scholar]
- 7.Salazar OM, Rubin P, Fieldstien ML, Pizzutiello R. High dose radiation therapy in malingnant gliomas;final report. Int J Radiat Oncol Biol Phys. 1979;5:1733–40. doi: 10.1016/0360-3016(79)90554-6. [DOI] [PubMed] [Google Scholar]
- 8.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 9.Jain VK, Pohlit W. Influence of energy metabolism on the repair of X-Ray damage in living cells. III Effect of 2-Deoxy–D-Glucose on liquid holding reactivation in yeast. Biophysik. 1973;10:137–42. doi: 10.1007/BF01191239. [DOI] [PubMed] [Google Scholar]
- 10.Dichiro G, Brooks RA, Patronas NT, Bairamain D, Kornblith PL, Smith BH, et al. Issues in the in vivo measurement of glucose metabolism of human central nervous system tumors. Ann Neurol. 1984;15:S138–46. doi: 10.1002/ana.410150727. [DOI] [PubMed] [Google Scholar]
- 11.Mohanti BK, Rath GK, Anantha N, Kannan V, Das BS, Chandramouli BA, et al. Improving cancer radiotherapy with 2-deoxy-D-glucose: Phase I/II clinical trials on human cerebral gliomas. Int J Radiat Oncol Biol Phys. 1996;35:103–11. doi: 10.1016/s0360-3016(96)85017-6. [DOI] [PubMed] [Google Scholar]
- 12.Singh D, Banerji AK, Dwarakanath BS, Tripathi RP, Gupta JP, Mathew TL, et al. Optimizing cancer radiotherapy with 2-deosxy-D-glucose: Dose escalation studies in patients with glioblastoma multiforme. Strahlenther Onkol. 2005;181:507–14. doi: 10.1007/s00066-005-1320-z. [DOI] [PubMed] [Google Scholar]
- 13.Swamy RK, Manickam J, Adhikari JS, Dwarakanath BS. The glycolytic inhibitor 2-deoxy-D-glucose does not enhance radiation-induced apoptosis in mouse splenocytes and thymocytes in vitro. Indian J Exp Biol. 2005;43:686–92. [PubMed] [Google Scholar]
- 14.Jain V. Modifications of radiation responses by 2-deoxy-D-glucose in normal and cancer cells. Indian J Nucl Med. 1996;11:8–17. [Google Scholar]
- 15.Hatfield P, Merrick A, Harrington K, Vile R, Bateman A, Selby P, et al. Radiation-induced cell death and dendritic cells: Potential for cancer immunotherapy? Clin Oncol. 2005;17:1–11. doi: 10.1016/j.clon.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Yoshii Y. Pathological review of late cerebral radionecrosis. Brain Tumor Pathol. 2008;25:51–8. doi: 10.1007/s10014-008-0233-9. [DOI] [PubMed] [Google Scholar]
- 17.Farooque A, Singh S, Adhikari JS, Dwarakanath BS. “Cytometry in the age of systems biology”. Budapest, Hungr: XXIV International congress; 2008. Role of T-regulatory cells (CD4+CD25highFoxP3+), Th1, Th2 and Th3 cytokines in the radiosensization of Ehrlich ascites tumor by the glycolytic inhibitor 2deoxy-D- glucose (2-DG) pp. 17–21. [Google Scholar]
- 18.Heminger K, Jain V, Kadakia M, Dwarakanath B, Berberich SJ. Altered gene expression induced by ionizing radiation and glycolytic inhibitor 2-deoxy-glucose in a human glioma cell line: Implications for radiosensitization. Cancer Biol Ther. 2006;5:815–23. doi: 10.4161/cbt.5.7.2812. [DOI] [PubMed] [Google Scholar]
- 19.Jelluma N, Yang N, Stokoe D, Evan GI, Dansen TB, Haas-Kogan DA. Glucose Withdrawal Induces Oxidative Stress followed by Apoptosis in Glioblastoma Cells but not in Normal Human Astrocytes. Mol Cancer Res. 2006;4:319–30. doi: 10.1158/1541-7786.MCR-05-0061. [DOI] [PubMed] [Google Scholar]


