Abstract
High resolution microscope systems with optical traps allow for precise manipulation of various refractive objects, such as dielectric beads 1 or cellular organelles 2,3, as well as for high spatial and temporal resolution readout of their position relative to the center of the trap. The system described herein has one such "traditional" trap operating at 980 nm. It additionally provides a second optical trapping system that uses a commercially available holographic package to simultaneously create and manipulate complex trapping patterns in the field of view of the microscope 4,5 at a wavelength of 1,064 nm. The combination of the two systems allows for the manipulation of multiple refractive objects at the same time while simultaneously conducting high speed and high resolution measurements of motion and force production at nanometer and piconewton scale.
Keywords: Physics, Issue 74, Molecular Biology, Optics, refraction (optics), optical traps, Molecular motors, microtubules, motility, holographic mirror, wavelength, dual traps, microscopy, imaging
Introduction
Optical trapping is one of the key techniques in biophysics 6. A crucial advancement in optical trapping has been the development of holographic traps which allow for the creation of three-dimensional trapping patterns rather than conventional point traps 7. Such holographic traps possess the advantage of versatility in positioning of refractive objects. However conventional traps can be easily aligned to be more symmetric than commercially available holographic kits. They also allow for fast precise tracking of the trapped objects. Here we describe a system (Figure 1) which combines the two trapping approaches in one instrument and allows the user to exploit the benefits of both as appropriate.
The general considerations of constructing optical traps (based on single or multiple laser beams) are discussed in detail elsewhere 8-10. Here, we outline the considerations specific to our setup and provide detail of our alignment procedure. For instance, systems with two optical trapping beams have been described before (e.g. ref. 11), typically using one laser beam for trapping a refractive object and using the other (intentionally low power beam) for decoupled readout of the position of the trapped object. Here however, both laser beams need to be high powered (300 mW or higher) because both are to be used for trapping. For measurements of biological systems, the lasers used for trapping should optimally fall within a specific NIR window of wavelength to minimize light induced protein degradation 1. Here we have chosen to use 980 nm diode and 1,064 nm DPSS lasers because of their low cost, high availability and ease of operation.
We have also chosen to use a spatial light modulator (SLM) to create and manipulate multiple traps simultaneously in real time 4,5. These devices are commercially available however their integration into a complete setup presents unique challenges. Here we describe a practical approach which addresses these potential difficulties and provides a highly versatile instrument. We provide an explicit example for the specific setup described which can be used as a guide for modified designs.
Protocol
1. Installation of 980 nm Wavelength Single Optical Trap
Optical trapping at 980 nm wavelength is often optimal for biophysics experiments and inexpensive laser diodes are readily available with power output as high as 300 mW. It is preferable for a diode laser to be pigtailed with polarization-preserving single mode fiber with a known mode field diameter. The fiber needs to be sufficiently long to act as a mode filter and is typically terminated with either a FC/PC or FC/APC connector. Of these, FC/APC is preferable to minimize back reflection of light and potential feedback instabilities.
Secure the 980 nm laser diode in a mount which allows for power and temperature control. It is best to fix the mount to an optical table directly to maximize passive heat sinking and thereby minimize the danger of diode failure due to a malfunction of temperature controller.
Mount the PC/APC fiber connector to the beam collimating optics. It is critical to assure that the collimated beam have minimal divergence so adjustable fiber ports are most useful. Make sure that the chosen fiber port matches the mode field diameter of the diode pigtail fiber. If the beam is to be rastered using acousto-optic deflectors (AOD) or electro-optic deflectors (EOD) then the collimated laser beam waist must also be slightly less than the size of the deflector aperture.
Secure the collimating adapter to the optical table at sufficient distance from the microscope to allow for beam routing, expansion, and placement of other desired components. Adjust the fiber port to ensure consistent beam waist over distances comparable to overall beam path to the microscope.
Install mirrors indicated in Figure 1. Remove the objective from the microscope and use the mirrors to route the beam through the aperture in the objective mounting stage. If preferred, placement of the dichroic mirrors DM1 and DM3 can be omitted until later. DM2 and DM3 are both shortpass and transmit visible light while reflecting near IR and above.
It is helpful to temporarily mount a red laser pointer in place of the objective, aligned on the optical axis of the microscope. A custom mechanical adapter is necessary to assure centration of the laser pointer. Visible beam from the laser pointer can then be routed back to the center of the aperture of the fiber port and can then be used to install the lenses (see below).
Install the 980 nm beam expander (L8 and L9) at a proper distance from the fiber port to allow future insertion of steering components (AOD or EOD) as necessary 1. The expanded beam must slightly overfill the back focal aperture of the objective. (Here, lenses with focal lengths of 125 mm and 60 mm are in a Keplerian arrangement to approximately double the beam waist). Use visible laser pointer beam (see section 1.6) to ensure proper lens placement and rough alignment.
Install the 980 nm steering lenses (L2 and L3) in a telescope arrangement as indicated (here both have 60 mm focal length) 1. L3 is mounted in a plane conjugate to the back-focal plane of the objective. Mount L3 on a precision XYZ positioning stage to allow for beam steering. It is helpful for the XYZ stage to have digital indicators for its micrometers, allowing for repeatable positioning and repositioning of the trap. The 0.5" range of travel is usually sufficient, however longer travel for L3 positioning along the optical axis may be helpful. Use visible laser pointer beam (see section 1.6) to ensure proper lens placement and rough alignment.
2. Installation of Laser Detector
Install the dichroic mirror DM3 above the condenser as shown in Figure 1. A custom mount is typically required. Secure the quad photo diode (QPD) or a position sensitive detector (PSD) 8 to the side of the condenser assembly and ensure that the 980 nm laser beam reflected by DM3 is hitting it roughly on center. When using QPD, ensure that it is mounted on a small XY stage to allow for precise centering of the sensor on the laser beam.
Install L1 (typically a 30 mm lens) between DM3 and the sensor. Position L1 so as to focus the beam to a single spot on the sensor.
Install the notch filter just before L1 to block the 1,064 nm beam as well as any stray visible light reflections from the microscope illuminator and ambient lighting.
3. Installation of 1,064 nm Wavelength Holographic Trap
The holographic part of the setup is built around a commercially available hardware/software package The holographic mirrors used in this package are rated to a maximum incident power of 5 or 10 W/cm2. Single mode TEM00 beams in this power range can be easily sourced from a DPSS laser at 1,064 nm wavelength.
Mount the 1,064 nm laser on an elevated platform to roughly match the height of the beam path for the 980 line (see section 1).
If not directly controllable, laser power can be manually adjusted by installing a half wave plate (HWP) and a polarizer (PBS) right after laser output aperture. It is helpful to mount the polarizer in a rotary stage to be able to match holographic mirror requirement for beam polarization.
Install the 1,064 nm beam expander (L6 and L7). The laser beam waist must be expanded to match the diagonal size of the holographic mirror. For large expansion ratios (above 10X) it may be a concern to keep the size of the expander small. Thus it may be desirable to use lenses with unusually small focal length (here: 16 mm and 175 mm).
- Install the other mirrors as indicated to direct the 1,064 nm beam through the objective.
- Secure DM1 dichroic (45° angle of incidence) in a kinematic mount and place the assembly in the 980 nm beam path so that it allows for undiminished transmission of that beam.
- Activate laser pointer light. The DM1 mirror should reflect sufficient amount of visible light to properly position the spatial light modulator (SLM) in the path of this beam. The SLM also needs to be angled so that the incoming and outgoing laser beams are as close as possible to normal incidence. However the angle of incidence must be sufficiently large to ensure that the laser beam is not clipped by lens mounts and other optical components. A 5° angle should be easily achievable and is sufficiently small. Finally the distance from DM1 to the SLM must be accurately measured so that the insertion of lenses L4 and L5 (see 3.6 below) can conjugate the SLM mirror plane and the back-focal plane of the objective.
- Install a mirror to direct the light from 1,064 nm beam expander to the SLM. Ensure that the laser pointer light hits the beam expander aperture on center.
Install lenses L4 and L5 (here: lenses with 125 mm and 200 mm respectively). This telescope pair conjugates the SLM mirror plane to the back focal plane of the objective and also reduces the beam waist to only slightly overfill the back aperture of the objective. We chose lenses with long focal lengths to space the SLM away from DM1. This not only clears room for the second laser line but also tends to make alignment easier.
Remove the laser pointer. Leave the mounting adapter to serve as coarse alignment aperture.
4. System Installation and Alignment Notes
Lens L3 and SLM must be positioned so as to be optically conjugated to the back focal plane of the objective. The common focal point of L4 and L5 is optically conjugated to the sample plane if the optical trapping beams are injected into the infinity space of the microscope.
sing IR card viewer align the 980 nm beam to go along the center axis of the aperture in the laser pointer adapter.
Use IR card to ensure that the 1,064 nm beam hits the same spot as the 980 nm beam on DM1, L2, and L3 and that the 1,064 nm beam goes along the center axis of the aperture in the laser pointer adapter.
Replace the laser pointer mounting adapter with an objective. High numerical aperture oil or water objective is typical.
Align the 980 nm trap as described in 9 by "walking" the laser beam until a radially symmetric interference pattern is seen on the camera.
With the holographic mirror off (i.e. acting as a passive mirror) use SLM and DM1 to "walk" the undiffracted 1,064 nm beam to align the 1,064 nm trap.
The SLM produces a significant undiffracted beam which results in a strong unmovable laser trap in the field of view. This is useful for alignment but may be undesirable for experiments. To block this trap one can insert a small opaque object in the path of undiffracted light at the location conjugate to the sample plane (e.g. the common focal point of L4 and L5). The size of this central spot blocker needs to be somewhat larger than the diameter of the Airy disk for the focused light (a blocker with 100-300 μm diameter for the system described).
djust 1,064 nm beam polarization using the polarizer to match SLM orientation. Rotate the half-wave plate to set the output power of the beam as desired.
If desired, insert AOD or EOD beam steering elements into the 980 nm laser line. Ensure proper conjugation of these elements to the back-focal plane of the objective and re-align the trap. It is helpful to mount the steering elements on a goniometric stage.
Representative Results
The assembled setup allows the operator to trap multiple refractive objects in real time and position them in all three dimensions within the field of view. We illustrate the holographic capabilities of the instrument by trapping 11 microspheres (Figure 2). The trap confining each object is manually re-positioned upon trapping so that the final arrangement depicts the logo of the University of Utah where this experiment was performed. A combined function of holographic and conventional trap is shown in Figure 3. The conventional trap moves central bead progressively faster (trap speeds of 1.3, 10 and 82 μm/sec are shown), while holographically defined traps remain stationary. At the highest speed, the entire motion of the bead occurs during the recording of one frame of video and thus appears as extreme motion blur. It is possible to move the conventional trap fast enough that beads are forced from the trapping potential by hydrodynamic drag (not shown).
Note that the assembly of complex shapes utilizing multiple microspheres may lead to a case where the number of microspheres in the field of view is insufficient for full assembly (as is evident in Figure 2). In such cases, the operator needs to physically move the field of view relative to the sample (i.e. reposition the sample stage in the microscope) to expose additional microspheres while retaining the objects already trapped.
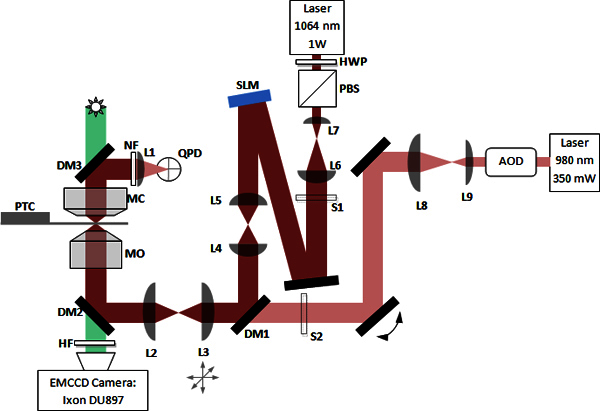 Figure 1. Schematic of the high resolution microscope system with two trapping beams. Components labeled L1-L9 are basic lenses. Components labeled DM1-DM3 are dichroic mirrors. Lenses L2 and L3 are used for steering. Lenses L4 and L5 act as a beam reducer and spacer. Lenses L6/L7 and L8/L9 are beam expander pairs for their respective laser beams. Unlabeled components depicted as solid black rectangles are basic mirrors. Components labeled MC and MO are the microscope condenser and object, respectively. Other components are a quad photo diode (QPD), notch filter (NF), Peltier temperature controller stage (PTC), hot filter (HF), spatial light modulator (SLM), acousto-optic deflector (AOD), shutters (S1 and S2), half-wave plate (HWP) and polarizing beam splitter (PBS).
Figure 1. Schematic of the high resolution microscope system with two trapping beams. Components labeled L1-L9 are basic lenses. Components labeled DM1-DM3 are dichroic mirrors. Lenses L2 and L3 are used for steering. Lenses L4 and L5 act as a beam reducer and spacer. Lenses L6/L7 and L8/L9 are beam expander pairs for their respective laser beams. Unlabeled components depicted as solid black rectangles are basic mirrors. Components labeled MC and MO are the microscope condenser and object, respectively. Other components are a quad photo diode (QPD), notch filter (NF), Peltier temperature controller stage (PTC), hot filter (HF), spatial light modulator (SLM), acousto-optic deflector (AOD), shutters (S1 and S2), half-wave plate (HWP) and polarizing beam splitter (PBS).
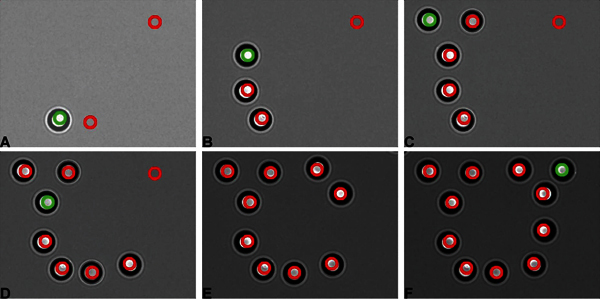 Figure 2. A spatial arrangement representing a University of Utah logo is made using 11 operator defined and controlled holographic traps. The objects trapped are refractive beads (see Table of Materials for more details) suspended in de-ionized water. Red and green circles show trap positions. Frames (a)-(f) represent successive stages in logo construction.
Figure 2. A spatial arrangement representing a University of Utah logo is made using 11 operator defined and controlled holographic traps. The objects trapped are refractive beads (see Table of Materials for more details) suspended in de-ionized water. Red and green circles show trap positions. Frames (a)-(f) represent successive stages in logo construction.
 Figure 3. Two rows of traps are made using 6 operator defined and controlled holographic traps. An additional conventional trap is defined between the two rows and its position is adjusted at various speeds as indicated. The bead is moved to a maximal spatial displacement of 4.1 μm and then back to the original location. A video of bead motion is recorded at 47 fps. As trap repositioning speed is increased, progressively larger motion blur is observed in the video. The objects trapped are refractive beads (see Table of Materials for more details) suspended in de-ionized water. Frame timings are shown in red. Trap repositioning speed is shown for each row. Green scale bars correspond to 5 μm in each direction.
Figure 3. Two rows of traps are made using 6 operator defined and controlled holographic traps. An additional conventional trap is defined between the two rows and its position is adjusted at various speeds as indicated. The bead is moved to a maximal spatial displacement of 4.1 μm and then back to the original location. A video of bead motion is recorded at 47 fps. As trap repositioning speed is increased, progressively larger motion blur is observed in the video. The objects trapped are refractive beads (see Table of Materials for more details) suspended in de-ionized water. Frame timings are shown in red. Trap repositioning speed is shown for each row. Green scale bars correspond to 5 μm in each direction.
Discussion
We have constructed an instrument which combines two optical traps of different types (Figure 1) to provide separate trapping facilities for object manipulation and measurement. The "conventional" optical trap is built around a 980 nm diode laser. This beam is expanded, steered and then injected into our inverted microscope ("light red" beam in Figure 1). The holographic optical trap is built around a 1,064 nm DPSS laser. The beam is expanded to fit the size of the spatial light modulator (SLM), reflected off the SLM at low angle of incidence, reduced to slightly overfill the back focal aperture of the objective, combined with the "conventional" trapping line using a dichroic mirror, and finally injected into the our microscope ("dark red" beam in Figure 1). Note that the SLM must be placed in a plane which is optically conjugate to the back focal plane of the objective.
In the protocol section, we describe the design and alignment considerations which allow us to minimize spatial footprint of the setup and still enable relatively easy construction. We also describe the blocking of the undiffracted component produced by the SLM, which may be necessary for a commercial package like the system used here but is somewhat challenging and to date poorly documented.
The design described here is highly customizable. We have included brief mentions of several popular high level customizations for optical traps and how one would integrate those into our design. For instance, a single trap can be steered in multiple ways, including acousto-optic deflectors (AOD), electro-optic deflectors (EOD)12, movable or deformable reflectors or simply rastering the steering lens (L3 in our setup) 1. Similarly, the position of a trapped object can be determined using many schemes and sensors. In such cases, the typical placement and alignment of relevant components is briefly described. We expect that this work may provide a template for more complex designs in the future.
Several practical considerations and usage limitations are of note. First, the optical traps should not be positioned too close to each other so as not to interfere with their attractive potentials near trap center. If close positioning of two traps is needed, then it is possible to define a line trap connecting the two points so that the attractive potential of the trap extends along the entire line. Another practical issue is that the trapped objects cannot be moved so fast that they experience excessive hydrodynamic drag (the exact threshold depends on trap strength) otherwise the drag can push the objects out of the trap.
Disclosures
The authors have nothing to disclose.
Acknowledgments
Funding was provided by the University of Utah. We would like to thank Dr. J. Xu (UC Merced) and Dr. B.J.N Reddy (UC Irvine) for useful discussions.
References
- Svoboda K, Block SM. Biological applications of optical forces. Annu. Rev. Biophys. Biomol. Struct. 1994;23:247–285. doi: 10.1146/annurev.bb.23.060194.001335. [DOI] [PubMed] [Google Scholar]
- Ashkin A, Schutze K, Dziedzic JM, Euteneuer U, Schliwa M. Force generation of organelle transport measured in vivo by an infrared laser trap. Nature. 1990;348:346–348. doi: 10.1038/348346a0. [DOI] [PubMed] [Google Scholar]
- Shubeita GT, Tran SL, et al. Consequences of motor copy number on the intracellular transport of kinesin-1-driven lipid droplets. Cell. 2008;135:1098–1107. doi: 10.1016/j.cell.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polin M, Ladavac K, Lee SH, Roichman Y, Grier D. Optimized holographic optical traps. Opt Express. 2005;13:5831–5845. doi: 10.1364/opex.13.005831. [DOI] [PubMed] [Google Scholar]
- Sun B, Roichman Y, Grier DG. Theory of holographic optical trapping. Opt. Express. 2008;16:15765–15776. doi: 10.1364/oe.16.015765. [DOI] [PubMed] [Google Scholar]
- Moffitt JR, Chemla YR, Smith SB, Bustamante C. Recent advances in optical tweezers. Annu. Rev. Biochem. 2008;77:205–228. doi: 10.1146/annurev.biochem.77.043007.090225. [DOI] [PubMed] [Google Scholar]
- Grier DG. A revolution in optical manipulation. Nature. 2003;424:810–816. doi: 10.1038/nature01935. [DOI] [PubMed] [Google Scholar]
- Neuman KC, Block SM. Optical trapping. Rev. Sci. Instrum. 2004;75:2787–2809. doi: 10.1063/1.1785844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz MP. Laser tweezers in cell biology. Introduction. Methods Cell Biol. 1998;55:xi–xii. [PubMed] [Google Scholar]
- Spudich JA, Rice SE, Rock RS, Purcell TJ, Warrick HM. Optical traps to study properties of molecular motors. Cold Spring Harb. Protoc. 2011;2011:1305–1318. doi: 10.1101/pdb.top066662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher K, Gross SP, Block SM. Construction of multiple-beam optical traps with nanometer-resolution position sensing. Selected Topics in Quantum Electronics. IEEE Journal of. 1996;2:1066–1076. [Google Scholar]
- Valentine MT, Guydosh NR, et al. Precision steering of an optical trap by electro-optic deflection. Opt Lett. 2008;33:599–601. doi: 10.1364/ol.33.000599. [DOI] [PMC free article] [PubMed] [Google Scholar]


