Abstract
Antitumor necrosis factor α (anti-TNF) agents have dramatically influenced management of refractory inflammatory bowel disease (IBD). However, not all patients respond to treatment and some lose response or become intolerant over time. Immunogenicity, a well established phenomenon with anti-TNF agents, may have important clinical implications in patients with IBD. A comprehensive review of available evidence demonstrating how drug concentrations, immunogenicity, and other factors influence outcomes with anti-TNF agents was performed. PubMed, EMBASE, Biosis, Dialog, and Conference Papers Index were searched from 1 January 1995 to 7 April 2012 to identify clinical trials in adult and pediatric patients with IBD treated with anti-TNF agents for Crohn’s disease or ulcerative colitis. Data on serum drug levels and immunogenicity and their relationship with clinical efficacy and safety outcomes were extracted and examined. Serum infliximab concentrations correlated with clinical efficacy and treatment outcomes in patients with IBD; this relationship is less well characterized with adalimumab and certolizumab pegol concentrations. In multiple studies, the presence and level of antibodies to infliximab correlated with loss of clinical efficacy and increased risk of infusion reactions. The incidence and clinical impact of antibody formation with adalimumab or certolizumab in IBD is becoming evident as more data become available. Current, enzyme-linked immunosorbent assay based anti-TNF antibody assays are suboptimal in that results are often inconclusive and comparisons between agents cannot be made. Measurement of anti-TNF agent drug concentrations and assessment of immunogenicity has the potential to positively impact clinical decision making during anti-TNF therapy for IBD. As assays are optimized, it is expected that the clinical impact of these determinations will be better characterized.
Keywords: adalimumab, antitumor necrosis factor α, certolizumab, Crohn’s disease, enzyme-linked immunosorbent assay, immunogenicity, inflammatory bowel disease, infliximab, ulcerative colitis
Introduction
Inflammatory bowel disease (IBD), specifically Crohn’s disease (CD) and ulcerative colitis (UC), affect approximately 1.4 million Americans, and nearly 30,000 new cases are identified annually [Crohn’s & Colitis Foundation of America, 2007]. Population cohort studies have found that most patients experience a chronic, relapsing course of IBD, with only 55% of patients with CD in remission at any given time and only 10.6% of patients with UC in remission in 25 years [Langholz et al. 1994; Munkholm et al. 1995]. Surgery is often indicated in patients who do not respond to medical treatment or who develop disease complications. Comparing two IBD cohorts from Copenhagen, Denmark (1962–1987 versus 1991–1993), Jess and colleagues noted that the cumulative probability of intestinal resections during the first decade after receiving a diagnosis of CD was 63% and 65% respectively [Jess et al. 2007]. In UC, the 10-year probability for undergoing proctocolectomy was 24% in each group. Despite significant advances in medical therapy over the past decade, hospitalization and surgery rates for IBD have not decreased [Bewtra et al. 2007]. On the contrary, hospitalization rates for CD have increased significantly since 1990 [Bewtra et al. 2007].
Tumor necrosis factor α (TNF) is a proinflammatory cytokine that is key to the pathogenesis of CD and UC [Bosani et al. 2009]. Abundantly expressed in the gastrointestinal tracts of patients with IBD [Breese et al. 1994; Murch et al. 1993], TNF is believed to contribute to intestinal mucosal inflammation through several mechanisms, including disruption of the epithelial barrier, induction of apoptosis of the villous epithelial cells, and secretion of chemokines from intestinal epithelial cells [Bosani et al. 2009]. Strategies aimed at reducing the activity of TNF have been used in patients with IBD. Over the past decade, anti-TNF agents have dramatically influenced the treatment of patients with refractory IBD.
Biologic agents used for the treatment of IBD have been developed as monoclonal antibodies (mAbs) or fragments thereof that are directed against TNF molecules. Antibodies, also known as immunoglobulins, are Y-shaped proteins on the surface of B cells. Antibodies are secreted into the blood or lymph in response to an antigenic stimulus and neutralize the antigen by binding specifically to it.
Infliximab (IFX, Remicade; Centocor, Horsham, PA, USA; http://www.remicade.com/remicade/assets/hcp_ppi.pdf), a chimeric immunoglobulin G (IgG) human (75%)/murine (25%) mAb administered by intravenous infusion, is indicated for induction and maintenance of remission in adult and pediatric CD and for induction and maintenance of remission of UC [Cassinotti and Travis, 2009]. IFX is also approved for other chronic inflammatory conditions such as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis. The murine component of this antibody has been implicated in the promotion of immunogenicity to the drug and formation of human antichimeric antibodies [Baert et al. 2003; Cassinotti and Travis, 2009], also known as antibodies to infliximab (ATI) [Afif et al. 2010; Sandborn, 2003]. Another anti-TNF agent, adalimumab (ADA, Humira; Abbott Laboratories, Abbott Park, IL, USA; http://www.prxabbvie.com/pdf/humira.pdf) is a self-injected, fully humanized recombinant mAb. This agent is indicated for induction and maintenance of remission in adult CD, as well as for rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis. IFX and ADA have high binding affinity for both soluble and transmembrane forms of TNF, blocking interaction of TNF with p55 and p75 cell surface TNF receptors and neutralizing its biologic activity. Certolizumab pegol (Cimzia; UCB Pharma, Brussels, Belgium; http://www.cimzia.com/pdf/prescribing_information.pdf), a third anti-TNF agent, is a pegylated, recombinant, humanized, antigen-binding fragment of a TNF mAb. Because it lacks the Fc protein of the antibody, which induces complement binding and cell lysis, certolizumab pegol has been suggested to have an improved safety profile [Barnes and Moots, 2007; Dinesen and Travis, 2007; Nesbitt et al. 2007]. This molecule has also been suggested to be less immunogenic because it is fully humanized and is bound to a polyethylene glycol moiety, which shields the protein from recognition by the immune system and potentially reduces the formation of antibodies to certolizumab (ATC) [Barnes and Moots, 2007].
Anti-TNF agents have greatly improved outcomes in immune-mediated inflammatory conditions, although they are not universally effective in all patients [Hanauer et al. 2006; Sandborn et al. 2007a]. In addition, a considerable proportion of initial responders lose response over time while others may become intolerant to these agents [Colombel et al. 2007; Hanauer et al. 2002; Sandborn et al. 2007b]. While reasons for these phenomena continue to be studied, this review details evidence accumulated thus far regarding anti-TNF treatment and response in IBD. PubMed, EMBASE, Biosis, Dialog, and Conference Papers Index were searched for reports of clinical trials in adult and pediatric patients with IBD treated with anti-TNF agents for CD or UC published from 1 January 1995 to 7 April 2012. The search strategy used for this review is depicted in Table 1. Of the resulting citations, those that discussed clinical efficacy and safety outcomes during anti-TNF treatment in conjunction with determinations of serum levels of drug and presence of anti-TNF antibodies were extracted and examined.
Table 1.
Literature search strategy.
| ‘Infliximab’ AND …; ‘Adalimumab’ AND …; ‘Certolizumab’ AND … |
|---|
| Crohn’s disease OR ulcerative colitis |
| Immunogenicity |
| Drug level |
| Antibodies OR antibody formation |
| Patient selection |
| Pharmacoeconomics |
| Pharmacoeconomics AND (Crohn’s disease OR ulcerative colitis) |
| Treatment response |
| Treatment response AND (Crohn’s disease OR ulcerative colitis) |
| Clinical utilities |
| Outcomes |
| Patient reported outcomes |
| Impact of antibody formation |
| Drug level AND patient response |
| Antibody formation AND patient response |
Pharmacokinetics of antitumor necrosis factor α biologics approved for inflammatory bowel disease
Each of the anti-TNF agents approved for use in IBD exhibit linear relationships between the dosage administered and resulting plasma concentration achieved. Both ADA and certolizumab pegol have mean elimination half lives of approximately 2 weeks, although it can range from 10 to 20 days for ADA. The median half life of IFX is shorter, ranging from 7.7 to 9.5 days.
In general, monitoring of serum drug concentrations is done primarily to ensure therapeutic concentrations are achieved and maintained and potentially toxic concentrations are avoided (Figure 1) [Fasanmade et al. 2011; Spector et al. 1988]. This monitoring is especially warranted with drugs that have narrow therapeutic windows, which are those drugs having a small interval between concentrations that are therapeutic and toxic; these drugs include aminoglycoside antibiotics, theophylline, anticonvulsants, tricyclic antidepressants, and antiarrhythmics [Spector et al. 1988].
Figure 1.

Concentrations versus time curve of infliximab in Crohn’s disease. Observed (dots) and simulated (lines) median concentration–time profiles of adult patients with Crohn’s disease (data from ACCENT I trial). Patients received treatment with infliximab 5 mg/kg at weeks 0, 2, and 6 and every 8 weeks thereafter. The serum infliximab concentrations are higher at early time points in the graph, corresponding with the induction phase of treatment (loading doses). After 14 weeks (100 days), infliximab serum concentrations tend to stabilize. ACCENT I, A Crohn’s Disease Clinical Trial Evaluating Infliximab in a New Long-term Treatment Regimen. Reprinted with permission from Fasanmade et al. [2011].
The use of serum drug monitoring is also used in the treatment of IBD to maximize the utility of anti-TNF therapeutics, as biologic drug serum levels and the presence of antidrug antibodies may help guide subsequent treatment decisions [Afif et al. 2010].
Clearance of monoclonal antibodies from the systemic circulation is incompletely understood; however, this clearance appears to occur through proteolytic catabolism after receptor-mediated endocytosis in cells comprising the reticuloendothelial system (RES) [Keizer et al. 2010]. The process whereby phagocytic cells internalize the antibody is driven primarily by Fc-γ receptors [Keizer et al. 2010] and is more efficient when the antibody is cross linked, as occurs during binding to a multivalent antigen [Raghavan and Bjorkman, 1996]. Another potential clearance mechanism is through the binding of circulating antibody to membrane-bound antigens, where the antibody is then internalized and degraded in lysosomes [Keizer et al. 2010; Tabrizi et al. 2006]. mAb and IgG homeostasis and clearance can also be influenced by neonatal Fc receptors (FcRn). Expressed both in the RES and in vascular endothelial cells, FcRn binds to soluble antigen:IgG complexes, internalizes the complex and then degrades the antigen. The antibody eventually returns to the cell surface, dissociates from the receptor, and is returned to the circulation [Mould and Green, 2010]. The FcRn receptor is essential for the maintenance of immunoglobulin and albumin homeostasis [Brambell et al. 1964].
Issues influencing treatment outcomes with antitumor necrosis factor α agents in inflammatory bowel disease
Drug concentrations and clinical response
Many attempts have been made to find correlations between serum anti-TNF drug concentrations and clinical outcomes in IBD. Current data are limited, however, by factors such as the retrospective design of some analyses, the differences in anti-TNF administration schedules (i.e. episodic versus scheduled), the presence or absence of concomitant immunosuppressant therapy, the use of predefined rather than clinically based sampling times, the use of different serum level cutoffs to predict efficacy, the inclusion of only patients with an initial response versus primary nonresponse, and the use of subjective, symptom-based end points [e.g. Crohn’s Disease Activity Index (CDAI)] rather than more objective measures such as mucosal healing to measure clinical response and remission. Subjective outcomes are more likely to be influenced by comorbidities and may not be specific to IBD [Bruining and Sandborn, 2011; Sandborn et al. 2002]. Indeed, no randomized trials have prospectively assessed the relationship between treatment and drug concentration, and no trials have compared the effects of continued conventional dosing versus dose escalation, for example in a patient with a trough IFX level that is low (e.g. 1 µg/ml), to determine whether increasing the drug level leads to a better chance of remission [Bruining and Sandborn, 2011; Sandborn et al. 2002].
Infliximab
Crohn’s disease
Studies have shown a correlation between serum concentrations of anti-TNF agents and clinical response in IBD. Baert and colleagues prospectively investigated IFX in 125 patients with refractory luminal (n = 87) or fistulizing (n = 38) CD over a median follow up of 36 months [Baert et al. 2003]. Patients with active luminal disease received a single infusion of IFX 5 mg/kg, and those with fistulizing disease were treated with three infusions (0, 2, and 6 weeks). Initial responders who relapsed received repeat IFX infusion. Overall, 89 patients (71%) responded to IFX. IFX concentrations were directly correlated with duration of response; concentrations of at least 12 μg/ml were associated with median duration of response of 81.5 days, and concentrations less than 12 μg/ml were associated with median duration of response of 68.5 days (p < 0.01). Patients receiving immunosuppressants were more likely to have IFX concentrations greater than 12.0 μg/ml [relative risk (RR) 1.93; 95% confidence interval (CI) 1.40–2.60]. Logistic regression analysis showed that immunosuppressive agents were the only variable of significance (p < 0.001) predictive of IFX concentrations [Baert et al. 2003].
Similar correlations were noted by Maser and colleagues in a prospective study of IFX in 105 consecutive patients with refractory inflammatory and/or perianal fistulizing CD who had IFX concentrations determined at trough, a median of 88 weeks after the baseline infusion [Maser et al. 2006]. Following IFX induction, 82 patients received scheduled maintenance IFX and 23 received infusions at relapse. Direct relationships were found between detectable trough serum IFX concentrations and the interval of clinical remission (R 2 = 0.61; p < 0.001) and the change in endoscopic score from baseline (R 2 = 0.46; p < 0.001). An inverse relationship was found between serum IFX concentrations and C-reactive protein (CRP) levels (R 2 = 0.26; p < 0.001) [Maser et al. 2006].
Van Assche and colleagues conducted an open-label nonplacebo-controlled study to assess immunosuppressant discontinuation in 80 patients aged at least 16 years who had CD controlled during concomitant treatment with IFX and immunosuppressants (azathioprine/6-mercaptopurine or methotrexate) for a minimum of 6 months [Van Assche et al. 2008]. The results from the total study population showed that IFX trough levels correlated with CRP levels and CDAI values (r = 0.387 for CRP and r= 0.205 for CDAI; p < 0.01). When IFX trough levels were divided into quartiles and matched with corresponding CRP and CDAI values, quartile 1 trough levels (0–0.90 μg/ml) were associated with higher median CDAI scores (p < 0.05), while quartile 1 and 2 trough levels (0–2.23 μg/ml) were associated with higher median concentrations of CRP (p < 0.0001). Patients who continued to receive combination IFX/immunosuppressive therapy had higher median trough levels of IFX and lower CRP concentrations than those who discontinued immunosuppressant therapy; however, no clear clinical benefit of combination therapy was observed beyond 6 months [Van Assche et al. 2008].
In the Study of Biologic and Immunomodulator Naïve Patients in Crohn’s Disease (SONIC) [Colombel et al. 2010], 508 adults with moderate-to-severe CD who had not undergone previous immunosuppressive or biologic therapy were andomized to receive IFX or azathioprine monotherapy or these agents in combination. Corticosteroid-free clinical remission, defined as an absolute CDAI score of less than 150, was achieved in 75 of 169 (44.4%) patients receiving IFX monotherapy, 51 of 170 (30.0%) receiving azathioprine monotherapy, and 96 of 169 (56.8%) receiving combination therapy. In patients receiving IFX as monotherapy or in combination with azathioprine (n = 202), corticosteroid-free remission was noted at week 26 in 110 of 151 (72.8%) patients who had median IFX trough levels of at least 1 µg/ml at week 30. Patients receiving IFX who had lower median IFX levels of 0–1 µg/ml had a similar rate of corticosteroid-free remission (32 of 55; 58.2%) in this study. Among patients with mucosal ulceration at baseline (n = 325), 47 of 107 (43.9%) receiving combination therapy, 28 of 93 (30.1%) receiving IFX alone, and 18 of 109 (16.5%) receiving azathioprine alone demonstrated mucosal healing at week 26. No prospective, randomized trial has assessed treatment to drug concentration versus treatment to mucosal healing [Colombel et al. 2010].
Ulcerative colitis
The predictive value of trough serum IFX concentrations was studied by Seow and colleagues in 115 patients with moderately severe to severe UC [Seow et al. 2010]. Patients were treated with a three-dose induction regimen followed by scheduled maintenance IFX over a median of 13.9 months. Clinical response to IFX induction was observed in 59% of patients, and clinical remission was achieved in 32% of patients at week 10. Patients with detectable trough serum IFX concentrations had significantly higher clinical remission rates (69%) than those with undetectable concentrations (15%; p < 0.001) and higher endoscopic remission rates (27% versus 8%; p = 0.021). Using multivariate logistic regression analysis, detectable serum IFX concentrations were shown to be significant positive predictors for clinical remission [odds ratio (OR) 12.5; 95% CI 4.6–33.9; p < 0.001] and endoscopic improvement (OR 7.3; 95% CI 2.9–18.4; p < 0.001). Undetectable IFX serum concentrations predicted an increased risk for colectomy (55% versus 7%; OR 9.3; 95% CI 2.9–29.9; p < 0.001) [Seow et al. 2010].
A recent post hoc analysis [Reinisch et al. 2012] evaluated the association of serum IFX levels with clinical outcomes from the Acute Ulcerative Colitis Treatment 1 and 2 (ACT 1 and ACT 2) trials in patients treated with 5 mg/kg IFX dosing [Rutgeerts et al. 2005]. ACT 1 and ACT 2 evaluated induction and maintenance therapy with IFX in patients with moderate to severe refractory UC. These patients were randomized to receive placebo, IFX 5 mg/kg, or IFX 10 mg/kg at weeks 0, 2, and 6 and then every 8 weeks through week 22 (ACT 2) or week 46 (ACT 1). To assess outcome, investigators determined the Mayo score in ACT 1 and ACT 2 at weeks 8 and 30 and again in ACT 1 at week 54. Overall median IFX concentrations in patients receiving the IFX 5 mg/kg induction and maintenance regimen were 33.0 µg/ml at week 8, 2.4 µg/ml at week 30, and 3.6 µg/ml at week 54. In patients achieving clinical response (decrease from baseline Mayo score by ≥30% and 3 points, with either a decrease from baseline in rectal bleeding subscore ≥1 or a rectal bleeding score of 0 or 1), clinical remission (up to 2 points, with no individual subscore >1), or mucosal healing (endoscopic subscore 0 or 1) at weeks 8, 30, and 54, median IFX concentrations were higher than in those who did not achieve clinical response. The proportion of patients achieving clinical remission at each of these time points increased with increasing quartiles of IFX concentrations, reaching statistical significance at weeks 30 (p < 0.0001) and 54 (p = 0.0066) and a trend-level association at week 8 (p = 0.0504). Similar results were noted for clinical response and mucosal healing outcomes [Reinisch et al. 2012].
Crohn’s disease and ulcerative colitis
A retrospective review of medical records from the Mayo Clinic looked at the clinical utility of measuring IFX concentrations and its impact on patient therapy [Afif et al. 2010]. Of 155 patients identified who had ATI and IFX concentration testing for a diagnosis of UC or CD, 127 (82%) had received induction therapy followed by scheduled dosing. Approximately 44.5% of patients were receiving immunomodulator therapy (azathioprine/6-mercaptopurine, methotrexate) at the time of the initial test. The median time to initial testing after IFX initiation was 50 weeks, and the median number of infusions per patient before testing was eight. Overall, 100 (65%) patients achieved an initial complete response, 45 (29%) patients had a partial response, and 10 (6%) had no response to IFX. A total of 63 patients had subtherapeutic concentrations of IFX, defined as less than 12 μg/ml 4 weeks after infusion or less than 1.4 μg/ml at trough. In patients who were negative for ATI, an IFX dosage increase was associated with a greater incidence of complete/partial response (86%) than a switch to another anti-TNF agent (33%; p < 0.016 ), underscoring the utility of measuring IFX trough concentrations and testing for ATI and the potential to positively affect treatment decisions [Afif et al. 2010].
Adalimumab
In the Clinical Assessment of Adalimumab Safety and Efficacy Studied as Induction Therapy in Crohn’s Disease I trial (CLASSIC I) [Hanauer et al. 2006], 299 patients with moderate to severe CD who were naïve to anti-TNF therapy were randomized to receive one of three ADA regimens or placebo: 160/80 mg, 80/40 mg, or 40/20 mg given subcutaneously at weeks 0 and 2 respectively. Significantly higher rates of remission (defined as a CDAI score < 150) were achieved by patients in the two highest dose ADA groups (36% and 24% respectively) versus placebo (12%; p = 0.004). As expected, mean ADA serum concentrations were higher among patients receiving the highest doses of ADA (12.61 ± 5.25, 5.65 ± 3.06, and 2.79 ± 1.48 µg/ml respectively). The results from CLASSIC I demonstrate that serum ADA concentrations achieved with the 160/80 mg dose were clearly effective for induction of remission [Hanauer et al. 2006].
A recent analysis [Li et al. 2012] evaluated correlations between serum ADA concentrations and clinical remission from CLASSIC I and its long-term follow-on study (CLASSIC II-2) [Hanauer et al. 2006; Sandborn et al. 2007b]. In the CLASSIC II trial, 276 participants from the CLASSIC I trial received open-label ADA 40 mg at weeks 0 (week 4 of CLASSIC I) and 2. Patients achieving clinical remission at weeks 0 and 4 were then rerandomized to receive ADA 40 mg either weekly or every other week or placebo (1:1:1) for a total of 56 weeks. With a flare or nonresponse, rerandomized patients could switch to open-label treatment with 40 mg ADA every other week or weekly, if indicated. Patients who were not in remission at both weeks 0 and 4 enrolled in an open-label regimen of ADA 40 mg every other week, which could be increased to 40 mg/week as indicated. Serum trough ADA levels were available for 258 patients from CLASSIC I and II. There was considerable overlap in ADA trough serum levels between those in remission and those not achieving remission. On logistic regression analysis at week 4 (CLASSIC I), a small, but statistically significant correlation between ADA trough levels and remission (p = 0.01) was observed. In CLASSIC II, however, no correlations were found between serum ADA levels and remission at weeks 4, 24, or 56. Threshold analysis did not reveal a serum ADA concentration that was predictive of remission [Li et al. 2012].
Similar to the CLASSIC trials, the Gauging Adalimumab Efficacy in Infliximab Nonresponders (GAIN) trial [Sandborn et al. 2007c] enrolled 325 adults with moderate to severe CD (CDAI 220–450) who had either lost response to or were intolerant of IFX. Patients were randomized to receive induction doses of ADA at weeks 0 (160 mg) and 2 (80 mg) or placebo. At week 4, remission was achieved in 34 of 159 (21%) patients receiving ADA compared with 12 of 166 (7%) of those receiving placebo (p < 0.001). The mean ADA concentration at week 4 in patients treated with ADA was 12.6 ± 6.0 µg/ml, which is identical to that achieved with 160/80 mg ADA in the CLASSIC I trial [Hanauer et al. 2006].
Bodini and colleagues reported on an open-label study that prospectively investigated serum ADA concentrations and antibodies to adalimumab (ATA) and their relationship to clinical response as assessed by Harvey-Bradshaw Index (HBI) score and CRP blood levels [Bodini et al. 2012]. These investigators followed 22 IFX-naïve patients with CD for 2 years. The 10 (45%) patients who had sustained clinical remission (HBI < 5) at 2 years had significantly higher ADA trough levels than patients who developed some level of disease activity (p < 0.01). Likewise, four patients who discontinued ADA because of loss of response had significantly lower ADA trough serum concentrations than those who continued ADA therapy [2.1 ng/ml (range 0–3.9 ng/ml) versus 6.7 ng/ml (range 3.2–9.2 ng/ml); p < 0.01]. ATA were detected in 9% of patients and did not seem to affect ADA serum concentrations in this small study [Bodini et al. 2012].
Drastich and colleagues analyzed the association between IFX and ADA trough levels and deep remission (defined as clinical remission and endoscopically proven mucosal healing) during open-label treatment of 100 patients with CD [Drastich et al. 2011]. A total of 24 of the 48 patients in the ADA group (all receiving 40 mg every 2 weeks) achieved deep remission, and 21 of these patients (84%) had trough levels greater than 2 µg/ml. However, 17 of the 24 patients (70.8%; p = nonsignificant) who did not achieve deep remission were also noted to have trough ADA levels of at least 2 µg/ml [Drastich et al. 2011].
Ferrante and colleagues evaluated the long-term efficacy of open-label ADA in 50 patients with moderate to severe UC who had received prior IFX treatment but had discontinued because of loss of response, infusion reactions, or inability to tolerate treatment [Ferrante et al. 2011]. At 4 weeks, ADA induction (160/80 mg) brought about a clinical response in 68%, a complete response (absence of diarrhea and blood) in 22%, and a partial response (marked clinical improvement with persistent rectal blood loss) in 46%. A total of 47 patients continued ADA therapy beyond week 4. After ADA initiation, 20 patients required dose escalation to 40 mg weekly and, after a median of 16 weeks, another 18 patients lost response and required dose escalation. Successful dose escalation was associated with significant increases in median ADA serum concentrations, from 4.75 to 7.95 µg/ml (p = 0.023). Colectomy-free survival was associated with both short-term clinical response (p = 0.030) and response to dose escalation (p < 0.001). The high percentage of patients responding to ADA dose escalation prompted these researchers to suggest that higher ADA doses are needed to treat UC [Ferrante et al. 2011].
Certolizumab pegol
Initially, clinical trials showed no correlation between certolizumab pegol concentrations and clinical efficacy [Sandborn et al. 2010a, 2010b]. In the Pegylated Antibody Fragment Evaluation in Crohn’s Disease Safety and Efficacy (PRECiSE 4) trial, 124 patients with moderate to severe CD who had responded to certolizumab induction therapy at week 6 but relapsed before week 26 during the PRECiSE 2 trial received open-label certolizumab pegol for 52 weeks [Sandborn et al. 2010b]. Patients who had relapsing disease on continuous maintenance therapy in PRECiSE 2 received a single extra 400 mg dose of certolizumab pegol, while those who had relapsing disease after drug interruption underwent certolizumab reinduction followed by maintenance certolizumab therapy every 4 weeks. Approximately two-thirds of patients regained clinical response or remission after certolizumab pegol 400 mg reinduction or recapture with an additional certolizumab 400 mg dose. By week 52, the geometric mean plasma concentrations of certolizumab pegol were similar for the two groups (continuous maintenance, 6.769 µg/ml; drug interruption, 7.0111 µg/ml). Although, mean plasma concentrations of certolizumab pegol did not correlate significantly with clinical response, as determined by HBI score, at virtually all points from weeks 0 to 52, the authors noted that the study was not designed to detect such a correlation and that the patient population was small and heterogeneous with respect to concomitant immunosuppressive therapy [Sandborn et al. 2010b].
The WELCOME trial [Sandborn et al. 2010a] enrolled 539 patients with active moderate to severe CD who had lost response to IFX or had developed hypersensitivity as evidenced by acute or delayed infusion reactions. These patients received open-label certolizumab induction 400 mg at weeks 0, 2, and 4; responders (CDAI reduction of ≥100 points) at week 6 were then randomized to double-blind maintenance certolizumab treatment at 400 mg every 2 or 4 weeks. After induction, 334 of 539 (62%) patients responded at week 6 and 329 were randomized to receive double-blind maintenance. No significant difference in response rate was observed at week 26 between those treated every 2 weeks (36.6%; 59/161) versus every 4 weeks (39.9%; 67/168; p = 0.55). Subgroup analyses indicated that serum certolizumab concentrations did not affect clinical response [Sandborn et al. 2010a].
Patients in the WELCOME trial [Sandborn et al. 2010a] who completed the 6-week induction period but who still had active disease (CDAI score of 220–450) were eligible to enter an open-label study [Sandborn et al. 2012] of certolizumab and received 400 mg at weeks 0, 2, and 4 and then every 4 weeks thereafter. A post hoc analysis of data from these patients sought to correlate certolizumab levels with clinical remission (CDAI score ≤ 150) analyzed from baseline of the open-label period. In 203 patients receiving open-label induction therapy, remission rates at weeks 0, 2, 4, and 6 in the open-label period were higher among patients whose certolizumab levels fell within the two highest quartiles (27.5 to <33.8 µg/ml and ≥33.8 µg/ml respectively) [Sandborn et al. 2012].
Immunogenicity
Immunogenicity is defined as the potential for an antigen to induce an immune response after being recognized by a preexisting T-cell or B-cell receptor [Cassinotti and Travis, 2009]. All exogenous proteins have the capacity to induce immunogenicity, and the concept that ‘humanness’ of a biologic agent, as in fully humanized antibodies, renders it nonimmunogenic is unfounded [Cassinotti and Travis, 2009]. In fact, numerous studies have documented antibody development with ADA [Hanauer et al. 2006; Karmiris et al. 2009; Sandborn et al. 2007b; West et al. 2008] and certolizumab pegol [Sandborn et al. 2007a, 2010a; Schreiber et al. 2005, 2007], despite their respective degrees of ‘humanness’. The development of immunogenicity depends on intrinsic patient factors, characteristics of the drug being used, and route of administration [Cassinotti and Travis, 2009].
Despite confirmation that immunogenicity occurs during anti-TNF therapy use in clinical studies [Hanauer et al. 2006; Karmiris et al. 2009; Sandborn et al. 2007a, 2007b, 2010a; Schreiber et al. 2005, 2007; West et al. 2008], the standard assays used to measure antibodies, which are based on enzyme-linked immunosorbent assays (ELISAs), have methodologic limitations (Figure 2). For example, anti-TNF drug concentrations interfere with assessment of antidrug antibodies [Cassinotti and Travis, 2009; Hanauer et al. 2002; Schreiber et al. 2007], often leading to inconclusive findings [Hanauer et al. 2002; Maser et al. 2006; Seow et al. 2010], particularly when sera are collected soon after anti-TNF administration. Measurement of these antibodies is optimized when the drug is no longer detectable in serum [Cassinotti and Travis, 2009]. Most antibody detection assays are based on a validated ELISA; these solid-phase ELISAs may also produce false-positive results due to nonspecific binding of other immunoglobulins [Ainsworth et al. 2008; Cassinotti and Travis, 2009]. Fluid-phase radioimmunoassay (Figure 3) may provide an alternative to solid-phase ELISA, as this test is not affected by artifacts induced by solid-phase adsorption of proteins but is complicated by the use of radioisotopes [Cassinotti and Travis, 2009]. In addition, a wide variety of clinical, analytical, and technical factors can complicate detection of immunogenicity and comparisons between agents [Cassinotti and Travis, 2009]. These complications underlie at least part of the variability seen in clinical trial reports detailing anti-TNF antibody levels.
Figure 2.

Enzyme-linked immunosorbent assay (ELISA). Although most commonly used for measuring antitumor necrosis factor α (TNF) levels and antidrug antibodies, ELISA has methodologic limitations that include risk of false-positive results because of nonspecific binding to immunoglobulins, and serum levels of the respective anti-TNF may interfere with the assay and produce inconclusive results potentially leading to underestimation of antidrug antibody levels.
Figure 3.

Radioimmunoassay (RIA). This assay provides greater sensitivity than enzyme-linked immunosorbent assay (ELISA). RIA measures functional serum drug levels via radiolabeled tumor necrosis factor α binding or by measuring antibody levels. RIA has less interaction with other immunoglobulins compared with ELISA.
Impact of antitumor necrosis factor α antibody formation on clinical response
Infliximab
Numerous studies suggest that development of immunogenicity from anti-TNF therapies affects their efficacy in IBD. The development of ATI and its correlation to IFX response has been extensively investigated (Table 2). In the prospective cohort study by Baert and colleagues, 76 of 125 (61%) patients with CD developed detectable ATI after the fifth IFX infusion [Baert et al. 2003]. However, the incidence of ATI development was significantly lower among patients who were receiving immunosuppressants (43%) than among those who were not (75%; p < 0.01). Overall, ATI concentrations were found to correlate with duration of response, with ATI concentrations of at least 8 μg/ml predictive of a shorter duration of response (35 days) and those less than 8 μg/ml predictive of a longer duration of response (71 days; p < 0.001) [Baert et al. 2003].
Table 2.
Published studies in adults on infliximab and antibodies to infliximab in Crohn’s disease and ulcerative colitis.
| Reference | Study design | Population | Regimen | Follow up | ATI incidence | Impact of ATI |
|
|---|---|---|---|---|---|---|---|
| Clinical response | Safety | ||||||
| Baert et al. [2003] | Prospective cohort | Refractory luminal and fistulizing CD (n = 125) | Episodic (luminal: 1 infusion; fistulizing: 3 infusions + on demand) | Median 36 months |
|
|
|
| Farrell et al. [2003] | Prospective, observational | Refractory luminal and fistulizing CD (n = 53) | Episodic (luminal: 1-2 infusions; fistulizing: 3 infusions + on demand) | Median 20 weeks |
|
|
|
| Farrell et al. [2003] | Randomized, double blind, placebo controlled | Refractory luminal and fistulizing CD (n = 80) | Episodic | 16 weeks |
|
|
|
|
Hanauer et al. [2002]; ACCENT I |
Randomized, double blind | Moderate to severe CD (n = 573) | Induction regimen followed by maintenance therapy | 54 weeks |
|
|
|
|
Hanauer et al. [2004]; ACCENT I subanalysis |
Randomized, double blind | Moderate to severe CD (n = 573) | Induction regimen followed by maintenance therapy | 72 weeks |
|
|
|
| Rutgeerts et al. [2005]; ACT 1 | Randomized, double blind, placebo controlled | Moderate to severe UC (n = 229) |
Induction regimen followed by maintenance therapy | 54 weeks |
|
|
|
| Rutgeerts et al. [2005]; ACT 2 | Randomized, double blind, placebo controlled | Moderate to severe UC (n = 188) |
Induction regimen followed by maintenance | 30 weeks |
|
|
|
| Maser et al. [2006] | Prospective cohort | Refractory inflammatory and/or perianal fistulizing CD (n = 105) | Induction regimen followed by maintenance (n = 82) or episodic (n = 23) therapy | Median 88 weeks |
|
|
|
| Vermeire et al. [2007] | Prospective, proof of concept | Refractory luminal and fistulizing CD (n = 174) | Episodic (luminal: 1 infusion; fistulizing: 3 infusions; + on demand) | Median 42 weeks |
|
|
|
| Van Assche et al. [2008] | Randomized, open label | Moderate to severe CD (n = 80) | Maintenance therapy | 2 years |
|
|
NR |
| Colombel et al. [2010]; SONIC | Randomized, double blind | Moderate to severe CD; naive to immunomodulator and anti-TNF therapy(n = 508) | IFX alone or AZA alone or IFX + AZA combination therapy | 50 weeks |
|
|
NR |
| Seow et al. [2010] | Prospective, cohort | Moderate to severe UC (n = 115) |
Induction regimen followed by maintenance | 54 weeks |
|
|
|
| Afif et al. [2010] | Retrospective review | Patients with CD, UC, or indeterminate colitis who underwent ATI and IFX concentration measurement (n = 155) |
– | – |
|
|
– |
ACCENT I, A Crohn’s Disease Clinical Trial Evaluating Infliximab in a New Long-term Treatment Regimen I; ACT 1 and 2, Acute Ulcerative Colitis Treatment 1 and 2; ATI, antibodies to infliximab; AZA, azathioprine; CD, Crohn’s disease; CI, confidence interval; IFX, infliximab; IS, immunosuppressant; MTX, methotrexate; NR, not reported; OR, odds ratio; SONIC, Study of Biologic and Immunomodulator Naive Patients in Crohn’s Disease; TNF, tumor necrosis factor; UC, ulcerative colitis.
Farrell and colleagues conducted a prospective observational study to assess the clinical efficacy of IFX and the potential effect of ATI formation in 53 patients with refractory CD [Farrell et al. 2003]. Nearly 40% of patients were receiving concomitant immunomodulator therapy. After a median follow up of 20 weeks, 19 of 53 (36%) patients developed ATI. The incidence of ATI formation was significantly lower among patients receiving concomitant immunosuppressants (24%) than among those who were not (63%; p = 0.007). ATI-positive patients had a significantly higher rate of nonresponse to subsequent infusions than ATI-negative patients (52% versus 14% respectively; p = 0.0005) and a significantly shorter duration of response (28 versus 61 days; p = 0.007) [Farrell et al. 2003].
In a second study, these investigators randomized 80 consecutive patients with refractory CD to receive intravenous hydrocortisone 200 mg premedication or placebo immediately before receiving their first IFX 5 mg/kg infusion [Farrell et al. 2003]. Approximately 34% of those randomized to placebo and 38% of those randomized to hydrocortisone were receiving concurrent azathioprine/6-mercaptopurine or methotrexate. Patients who received hydrocortisone had significantly lower ATI levels than those who received placebo at weeks 8 (2.9 versus 11.1 µg/ml respectively; p = 0.002) and 16 (1.6 versus 3.4 µg/ml; p = 0.02). Overall, ATI developed in fewer patients in the hydrocortisone group than in the placebo group at weeks 8 (21% versus 29%; p = 0.14) and 16 (26% versus 42%; p = 0.06). Although clinical response and remission rates were higher at weeks 8 and 16 in patients receiving hydrocortisone, the differences were not statistically significant [Farrell et al. 2003].
Hanauer and colleagues performed a subanalysis on data from A Crohn’s Disease Clinical Study Evaluating Infliximab in a New Long-Term Treatment Regimen I trial (ACCENT I) [Hanauer et al. 2002, 2004], a randomized, double-blind investigation assessing maintenance IFX therapy in 573 patients with moderate to severe CD who improved after an initial infusion. Approximately 29% of the overall population was receiving immunomodulator therapy at baseline. Patients received one infusion of IFX 5 mg/kg before being randomized to receive 46 weeks of treatment with IFX 5 mg/kg at weeks 2, 6, and every 8 weeks thereafter; 5 mg/kg at weeks 2, 6, and then 10 mg/kg every 8 weeks thereafter; or placebo. Overall, 64 of 442 (14%) patients developed ATI; however, ATI developed in a smaller proportion of patients receiving corticosteroids (with or without immunomodulators) [Hanauer et al. 2002]. In the subanalyses of these data, the incidence of ATI was significantly lower among patients given regular maintenance treatment (8%) than among those given episodic treatment (30%; p < 0.001) [Hanauer et al. 2004]. In the episodic treatment group, reductions in serum IFX concentrations were more pronounced among ATI-positive patients than among ATI-negative patients, although rates of clinical response and remission were similar between groups (33% versus 36% respectively) and independent of antibody status [Hanauer et al. 2004].
The SONIC trial was a randomized, double-blind, 30-week study (with a 20-week extension) in 508 adults with moderate to severe CD without prior immunosuppressive or biologic therapy exposure [Colombel et al. 2010]. Patients were randomized to receive IFX infusions, azathioprine 2.5 mg/kg, or combination therapy. At week 30, ATI were detected in 15 of 103 (14.6%) patients receiving IFX and in 1 of 116 (0.9%) receiving combination therapy [Colombel et al. 2010].
Development of immunogenicity to IFX was also explored in patients with UC. The ACT 1 and ACT 2 trials each randomized 364 patients with moderate to severe UC to receive IFX 5 or 10 mg/kg or placebo at weeks 0, 2, and 6 and every 8 weeks thereafter through week 46 (ACT 1) or week 22 (ACT 2) [Rutgeerts et al. 2005]. Patients were followed for 54 weeks (ACT 1) or 30 weeks (ACT 2). The proportion of patients receiving immunomodulator therapy ranged from 43.8% to 54.5% in ACT 1 and from 41.7% to 43.9% in ACT 2. At week 54, ATI developed at some point after the first IFX infusion in 6.1% of ACT 1 patients and 6.4% of ACT 2 patients. In ACT 1, 21.4% of ATI-positive patients achieved a clinical response compared with 8.3% (3/36) of ATI-negative patients and 57.5% (103/179) of patients with inconclusive ATI findings. In ACT 2, 57.9% of ATI-positive patients achieved a clinical response compared with 57.0% (45/79) of ATI-negative patients and 77.2% of patients with inconclusive ATI findings. Unlike patients with CD, patients with UC who had positive or inconclusive findings for ATI were more likely to have had a clinical response at weeks 54 (ACT 1) and 30 (ACT 2) than those who had negative ATI findings. The authors suggest that the lower rate of clinical response associated with ATI-negative tests was due to undetectable serum IFX concentrations [Rutgeerts et al. 2005]. These findings, along with the very high rates of inconclusive ATI results, illustrate the need for more sensitive testing modalities.
Similar to the ACT 2 findings on clinical response, Seow and colleagues found that ATI-positive and ATI-negative patients with UC had similar rates of clinical remission (14% versus 18% respectively; p = 0.95) and endoscopic improvement (25% versus 35%; p = 0.61) after IFX induction and maintenance therapy [Seow et al. 2010]. In contrast, patients whose ATI status was inconclusive had significantly higher rates of remission (69%; p < 0.001), endoscopic improvement (76%; p = 0.004) and lower rates of colectomy (7%; p < 0.001) than those found in ATI-positive and ATI-negative patients [Seow et al. 2010].
In their retrospective chart review of 155 patients with IBD, Afif and colleagues found that 35 of 155 (23%) patients were positive for ATI [Afif et al. 2010]. Switching to an alternate anti-TNF agent achieved complete/partial response in 11 of 12 (92%) ATI-positive patients but only 1 in 6 (17%; p < 0.004) ATI-positive patients who received IFX dose escalation. None of these patients achieved therapeutic IFX concentrations with dose escalation, suggesting that further IFX treatment after development of ATI may lower clinical response rates [Afif et al. 2010].
Vande Casteele and colleagues retrospectively analyzed 788 serum samples from 57 IFX-treated patients with IBD in whom ATI had been detected at least once during follow up [Vande Casteele et al. 2012a]. Each sample was analyzed for ATI and IFX trough levels using a new, proprietary fluid-phase mobility shift assay (Prometheus Laboratories, San Diego, CA, USA). Decisions regarding IFX treatment were made during follow up based on clinical findings and CRP values, not serum IFX levels or presence of ATI. These investigators observed that ATI disappeared over time in 19 (33%) patients while persisting in 38 (67%). Patients with transient ATI had significantly lower median ATI levels (6 U/ml; range 3.1–12.2 U/ml) than those with persistent ATI (18 U/ml; range 8.7–34.8 U/ml). In patients with transient ATI, these antibodies disappeared in 58% after IFX dose optimization; ATI disappeared spontaneously in 42%. More patients with persistent (74%) than transient ATI (26%) discontinued treatment. These findings indicate that transient ATI can disappear spontaneously or can be overcome by dose optimization, particularly when concentrations are low, whereas sustained high ATI levels lead to permanent loss of response and necessitate treatment discontinuation. These researchers also concluded that measurement of ATI is necessary when IFX trough levels are low or undetectable [Vande Casteele et al. 2012a].
At least two studies investigated the IFX immunogenicity in pediatric patients. Miele and colleagues conducted a retrospective review of records from 132 children and adolescents who received a total of 621 IFX infusions [Miele et al. 2004]. Of 56 patients with ATI assay results, 22 had indeterminate findings owing to detectable circulating IFX, and 12 of the remaining 34 (35%) were positive for ATI. The trend was toward lower rates of ATI formation among patients under 14 years of age (p = 0.13). In contrast to adult patients, these pediatric patients who had shorter intervals between IFX infusions showed a tendency toward higher levels of ATI (≥8.0 µg/ml; p = 0.08) [Miele et al. 2004]. In the second pediatric study, Candon and colleagues investigated the clinical and biologic consequences of IFX immunogenicity in a retrospective cohort of 28 children (aged 6 months–15 years) who received IFX for refractory luminal or fistulizing CD [Candon et al. 2006]. A total of 18 children were receiving concomitant methotrexate at baseline, while 16 were receiving azathioprine. Overall, 10 children (35.7%) developed ATI during the course of therapy. The risk of developing ATI was influenced by the type of induction therapy; ATI were detected in 77.7% of patients treated with a single infusion and 15.7% of patients treated with a three-infusion induction (p = 0.0028; OR 18.6). Further, a direct relation was observed between loss of response to IFX and presence of ATI. Among 14 children who maintained response at the end of the study, 78.5% did not develop ATI, whereas 6 of the 8 (75%) children who were nonresponders did. The authors concluded that regular detection and quantification of ATI may be useful in identifying patients at risk for loss of clinical response [Candon et al. 2006].
Adalimumab
Karmiris and colleagues investigated the relationship of the formation of ATA to clinical outcome in 168 consecutive patients with CD who had lost response or become intolerant to IFX [Karmiris et al. 2009]. ADA treatment consisted of 160/80 mg (n = 126) or 80/40 mg (n = 28) induction therapy at weeks 0 and 2 respectively, or 40 mg every 2 weeks with no loading dose (n = 14). Approximately 37% of patients were receiving concomitant azathioprine/6-mercaptopurine or methotrexate at baseline. Overall, 70.5% of patients responded to ADA induction therapy by week 4, and 61.5% demonstrated sustained clinical benefit (defined as the presence of symptom control and ongoing ADA maintenance therapy at the end of follow up) over a median follow up of 20.3 months. The development of ATA was detected in 12 of 130 (9.2%) patients for whom ATA were measured. Median ADA trough serum concentrations were lower at week 4 in ATA-positive patients (2.1 μg/ml) than ATA-negative patients (6.1 μg/ml; p < 0.02). No relationship was found between ADA trough serum concentration or presence of ATA and short-term clinical response. Sustained clinical benefit was significantly decreased when patients had a trough serum ADA concentration of less than 0.33 μg/ml at least once, although the small size of this group (n = 16) warrants interpretive caution. Over the long term, lower trough serum ADA concentration was associated with discontinuation [Karmiris et al. 2009].
Certolizumab pegol
Although data are limited, clinical trials exploring certolizumab immunogenicity have not found a relationship between the development of ATC and clinical response. In a randomized, double-blind, placebo-controlled, dose–response study [Schreiber et al. 2005], 292 patients with moderate to severe CD received placebo or subcutaneous injections of certolizumab 100, 200, or 400 mg at weeks 0, 4, and 8. Among the 291 patients in the intention-to-treat (ITT) population, 35.1–40.3% received concomitant immunomodulator therapy and 21.6% had prior anti-TNF therapy. Nine of 73 patients (12.3%) in the certolizumab 400 mg group developed ATC during the 12-week study. Although plasma certolizumab concentrations were lower in ATC-positive than ATC-negative patients, clinical response at week 12 was not affected by antibody status (44% clinical response in both groups) [Schreiber et al. 2005].
The PRECiSE 2 trial [Schreiber et al. 2007] was a 26-week randomized, placebo-controlled maintenance and withdrawal trial in patients with moderate to severe CD who had a clinical response to open-label induction therapy (400 mg at weeks 0, 2, and 4). Of 668 receiving induction, 425 who responded by week 6 were randomized to receive placebo or certolizumab maintenance injections at week 8 and every 4 weeks thereafter through week 24 and were followed through week 26. At baseline, 103 of the 425 patients (24%) in the ITT population had received prior IFX therapy and 41% were receiving concomitant immunosuppressive agents with or without glucocorticoids. ATC were detected in 58 of 668 (9%) patients who entered the induction phase but did not appear to negatively affect efficacy; 12 of the 17 (71%) ATC-positive patients assigned to certolizumab maintenance therapy had a response through week 26 compared with 121 of 196 (62%) ATC-negative patients [Schreiber et al. 2007].
In the WELCOME trial [Sandborn et al. 2010a], which assessed the use of certolizumab pegol in patients with secondary failure to IFX, ATC were detected in approximately 6% of patients receiving monthly certolizumab maintenance injections. Patients treated with certolizumab experienced a consistent clinical benefit regardless of antibody status [Sandborn et al. 2010a].
Impact of antitumor necrosis factor α antibody formation on safety
Infusion reactions are potentially serious adverse events associated with IFX that occur during or within 1–2 h after infusion and typically manifest as fever, chills, nausea, dyspnea, and headache [Han and Cohen, 2004]. These reactions have been associated with high therapy withdrawal rates, as well as reduced remission rates in the subsequent 2 years [Moss et al. 2008]. ADA and certolizumab are not administered by infusion and are therefore not associated with these reactions.
Infusion reactions have been directly correlated with the presence of ATI in adults with CD [Baert et al. 2003; Farrell et al. 2003; Hanauer et al. 2004] and UC [Rutgeerts et al. 2005]. Two studies demonstrated a correlation between ATI levels of at least 8 µg/ml and significantly higher risk of infusion reactions [Baert et al. 2003; Farrell et al. 2003]. In a subgroup analysis of Farrell’s observational study, ATI-positive patients had a significantly higher incidence of subsequent serious infusion reactions than ATI-negative patients (28% versus 0%; p = 0.0001) [Farrell et al. 2003]. A subanalysis of the ACCENT I study found that positive ATI levels were associated with an absolute increase of 12% in the incidence of infusion reactions [Hanauer et al. 2004]. In contrast, in an open-label study of 112 pediatric patients with moderate-to-severe CD, the number of infusion reactions did not differ between ATI-positive and ATI-negative patients, although 77.1% of patients had inconclusive ATI status [Hyams et al. 2007].
Impact of immunosuppression on antitumor necrosis factor α antibody formation
Despite a general belief that use of concomitant immunosuppression therapy decreases formation of ATI in patients receiving IFX therapy [Farrell et al. 2003; Hanauer et al. 2004; Sandborn, 2003], the data are inconsistent (Table 3). Vermeire and colleagues conducted a prospective, proof-of-concept study in 174 patients with refractory luminal or fistulizing CD to determine which immunosuppressant—methotrexate or azathioprine—was superior in reducing risk of ATI formation [Vermeire et al. 2007]. After IFX induction therapy with either a three-dose regimen for fistulizing disease or a single dose for luminal disease, patients received on-demand IFX with concomitant azathioprine or 6-mercaptopurine, methotrexate, or no immunosuppressants. The incidence of ATI formation was found to be lower with concomitant immunosuppressant (46%) than without (73%; p < 0.001), although no specific agent proved superior in reducing this risk [Vermeire et al. 2007]. The critique of this study is that it is grossly underpowered as a superiority trial.
Table 3.
Impact of immunomodulation on the development of anti-drug antibodies and anti-TNF drug levels.
| Study design | Population | Anti-TNF drug antibody incidence | Impact on anti-TNF drug levels |
Impact on efficacy |
Impact on safety | |
|---|---|---|---|---|---|---|
| Miele et al. [2004] | Retrospective IBD database review |
|
|
NR | NR |
|
| Vermeire et al. [2007] | Prospective cohort study on ATI formation | Patients receiving on demand (i.e. episodic) IFX with or without IMs (n = 174)
|
IMs were associated with lower ATI incidence (46% versus 73% no IMs; p < 0.001)
|
|
|
|
| Van Assche et al. [2008] | 104-week, open-label, randomized controlled trial in patients randomized to continue or discontinue IMs during continued IFX therapy | Patients with CD controlled on ≥6 months episodic or scheduled IFX (≥8-week intervals) and IMs (n = 80) |
|
|
|
|
| Afif et al. [2010] | Retrospective medical record review of 155 patients with IBD, 127 of whom had induction IFX followed by scheduled maintenance IFX | Patients with IBD (n = 69; 47%)
|
|
|
NR | NR |
| Hanauer et al. [2006]; CLASSIC I | 4-week, randomized, double-blind, placebo-controlled trial of ADA in 299 patients | Patients with CD naïve to anti-TNF therapy (n = 88; 29.4%)
|
Only 2 patients developed ATA (NR by IM status):
|
|
NR | NR |
| Sandborn et al. [2007b]; CLASSIC II | 56-week, open-label study of ADA in 276 patients from CLASSIC I; 55 in remission were randomized to ADA 40 mg/week or eow or placebo; 204 not in remission received open-label ADA 40 mg eow | Randomized patients (n = 55; 19.9%)
|
|
NR |
|
NR |
| Sandborn et al. [2007a]; PRECiSE 1 | 26-week, randomized, double-blind, placebo-controlled study in 662 adults |
|
|
NR |
|
NR |
| Schreiber et al. [2007]; PRECiSE 2 | 26-week, randomized double-blind, placebo-controlled trial of certolizumab in 428 patients; certolizumab responders were randomized to placebo or certolizumab maintenance every 4 weeks with follow up through week 26 |
|
|
NR |
|
|
| Karmiris et al. [2009] | Observational cohort study |
|
|
|
|
|
| Lichtenstein et al. [2009] | Subgroup analysis of IFX efficacy, safety, concentration and immunogenicity by baseline IM therapy across 4 large, prospective phase III trials
|
All randomized: patients on IMs
|
|
|
|
|
6-MP, 6-mercaptopurine; ACCENT I and II, A Crohn’s Disease Clinical Trial Evaluating Infliximab in a New Long-term Treatment Regimen I and II; ACT 1 and 2, Acute Ulcerative Colitis Treatment 1 and 2; ADA, adalimumab; ATA, antibodies to adalimumab; ATC, antibodies to certolizumab; ATI, antibodies to infliximab; AZA, azathioprine; CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; CI, confidence interval; CLASSIC I and II, Clinical Assessment of Adalimumab Safety and Efficacy Studied as Induction Therapy in Crohn’s Disease I and II; CRP, C-reactive peptide; eow, every other week; GC, glucocorticoid; IBD, inflammatory bowel disease; IFX, infliximab; IM, immunomodulators; MTX, methotrexate; NR, not reported; NS, nonsignificant; OL, open label; PPV, positive predictive value; PRECiSE 1 and 2, Pegylated Antibody Fragment Evaluation in Crohn’s Disease Safety and Efficacy 1 and 2; RR, relative risk; SAE, serious adverse event; TNF, tumor necrosis factor α; UC, ulcerative colitis.
The median duration of response was lower in patients with ATI who were not receiving immunosuppressants (11.71 weeks) than in those who were (13.8 weeks), although this difference was not significant. In a retrospective review, Afif and colleagues found that the incidence of ATI was significantly lower among patients who were receiving concurrent immunosuppressive agents (14%) than among those who were not (29%; p < 0.032) [Afif et al. 2010]. Further, concomitant immunosuppressants were associated with significantly higher therapeutic IFX concentrations (48% versus 21% respectively; p < 0.001) [Afif et al. 2010]. Similarly, Miele and colleagues observed a lower risk of developing ATI among pediatric patients with IBD receiving concomitant immunosuppressants (RR 0.34; 95% CI 0.17–0.72; p = 0.02) [Miele et al. 2004].
In contrast, Van Assche and colleagues observed only a modest effect of immunosuppression on ATI development in their open-label comparison of continued immunosuppression and IFX monotherapy [Van Assche et al. 2008]. Two of 40 patients (5%) who continued immunosuppressant therapy developed ATI compared with 5 of 40 (12.5%) who discontinued (p = 0.43). Although significantly higher median IFX trough levels were observed among patients treated with concomitant immunosuppressants, clinical outcomes were similar between groups [Van Assche et al. 2008].
Limited data are available regarding the use of immunosuppressants and their impact on ATA formation in patients with IBD. In the CLASSIC I trial [Hanauer et al. 2006], concomitant immunosuppression did not affect serum ADA concentrations; the effect on ATA formation was not assessed. In CLASSIC II [Sandborn et al. 2007b], of the 84 patients who received concomitant immunosuppressants, none were positive for ATA. However, the study lacked adequate statistical power to accurately determine whether immunosuppressants influenced antibody formation. Karmiris and colleagues reported that concomitant immunosuppression did not influence serum ADA concentrations or decrease the development of ATA [Karmiris et al. 2009].
Similarly, few trials have explored whether concomitant immunosuppression is protective against ATC development. Data from the PRECiSE 1 and 2 trials [Sandborn et al. 2007a; Schreiber et al. 2007] indicated that patients who were treated with concomitant immunosuppressants had modestly lower rates of ATC than patients not receiving them at baseline (4% versus 10% respectively in PRECiSE 1; 2% versus 12% in PRECiSE 2).
Using therapeutic drug monitoring in clinical practice
Traditionally, therapeutic drug monitoring has been done with patients receiving drugs that have narrow therapeutic windows. In clinical practice, such monitoring occurs with the use of a variety of agents, including tricyclic antidepressants, antiepilepsy drugs, certain antibiotics, antiarrhythmics, lithium, digoxin, heparin, and theophylline [Spector et al. 1988]. The impetus for therapeutic drug monitoring during anti-TNF treatment of IBD is based, at least in part, on the concentration–effect relationship that has been demonstrated in various chronic immunoinflammatory diseases, including CD [Baert et al. 2003; Maser et al. 2006; Seow et al. 2010; Van Assche et al. 2008; West et al. 2008]. Additionally, the substantial variability in pharmacokinetic and bioavailability parameters found between individuals or within a given individual is also operant. Perhaps the most important reason for therapeutic monitoring during anti-TNF therapy, however, is the potential to individualize treatment, especially in patients with secondary nonresponse, to improve patient outcomes.
Interpreting serum antitumor necrosis factor α concentration levels and the presence of antidrug antibodies
The measurement of serum anti-TNF drug concentrations and antidrug antibodies has been limited by assay characteristics. Despite these limitations, however, useful information pertaining to the efficacy and safety of this class of agents has been derived. In general, high serum concentrations of anti-TNF agents, measured just before the administration of the next dose, have been associated with clinical improvement, whereas low levels are associated with poor responses. Higher trough serum anti-TNF levels have been associated with longer median duration of response [Baert et al. 2003], a longer interval of clinical remission [Hanauer et al. 2006; Maser et al. 2006; Seow et al. 2010] and change in endoscopic score [Maser et al. 2006; Seow et al. 2010], lower CRP levels [Van Assche et al. 2008], and sustained clinical remission [Bodini et al. 2012]. These findings are not always consistent as some studies have not shown such a correlation between trough anti-TNF levels and clinical improvement in IBD [Drastich et al. 2011; Sandborn et al. 2010b]. Defining optimal trough anti-TNF serum levels to achieve and maintain clinical remission remains an area of active research.
The development of antidrug antibodies during anti-TNF treatment has been associated with reduced efficacy and safety issues. Evidence shows that patients receiving concomitant immunosuppressants [Farrell et al. 2003; Hanauer et al. 2002] or hydrocortisone [Farrell et al. 2003] have a lower incidence of ATI formation than patients not receiving these agents. In CD, ATI levels of at least 8 µg/ml predicted a shorter duration of response [Baert et al. 2003], whereas in UC, clinical response was similar between patients who had ATI versus those who did not [Rutgeerts et al. 2005]. Anti-TNF concentrations are reduced in the presence of antidrug antibodies [Karmiris et al. 2009]. From a safety perspective, the presence of ATI correlates with the occurrence of infusion reactions [Baert et al. 2003; Farrell et al. 2003; Hanauer et al. 2004; Rutgeerts et al. 2005]. Clinical data have given rise to suggested algorithms for revising biologic therapy in patients with ongoing inflammation or uncontrolled disease (Figure 4).
Figure 4.
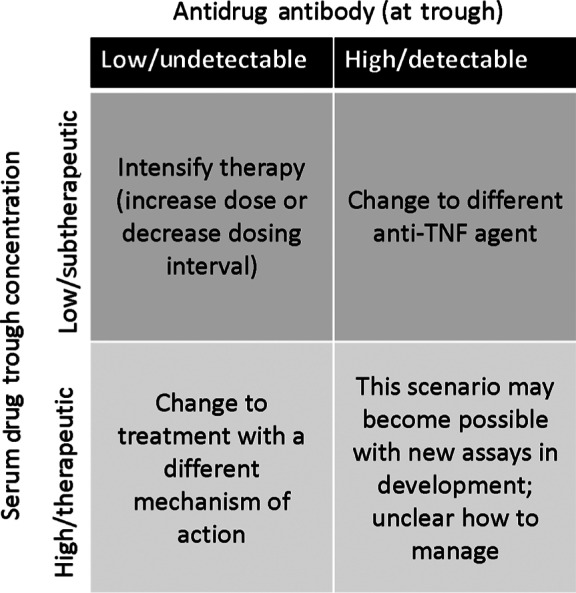
Suggested clinical algorithm for infliximab-treated patients with clinical symptoms. TNF, tumor necrosis factor. Reprinted with permission from Colombel et al. [2011].
Future research directions
In patients treated with biologic agents for IBD, therapeutic drug monitoring has the potential to positively affect treatment, especially in patients who lose response over time. However, the optimal intervention in these cases remains to be determined. While a concentration–effect relationship in which higher anti-TNF serum trough concentrations are generally associated with improved clinical status has been described [Baert et al. 2003; Bodini et al. 2012; Maser et al. 2006; Seow et al. 2010; Van Assche et al. 2008], the optimal ‘threshold’ level of anti-TNF drug in serum needed to achieve response or remission is unclear. Likewise, the development of antidrug antibodies is associated with shorter duration of response [Baert et al. 2003], higher rates of nonresponse [Farrell et al. 2003], and treatment discontinuation [Vande Casteele et al. 2012b] in some studies, whereas in other studies, a relationship did not appear to exist between antidrug antibody formation and clinical response [Rutgeerts et al. 2005; Schreiber et al. 2005]. Other pharmacokinetic parameters, such as the area under the plasma–concentration time curve, may prove more predictive of clinical course and this possibility should be examined. Additionally, the optimal timing of therapeutic drug monitoring is yet to be determined as does the utility of serial therapeutic monitoring during a treatment course. Finally, correlations between findings of serum drug concentrations, immunogenicity development and more objective patient outcomes such as complete mucosal healing remain to be defined.
Discussion
Although not completely understood, the development of antibodies to anti-TNF agents may have important clinical implications in patients with IBD [Anderson, 2005]. Antibody formation lessens the bioavailability of the anti-TNF agent and may compromise the ability of anti-TNF agents to reduce inflammation, potentially compromising clinical response. Indeed, antibody formation against anti-TNF agents has been associated with shortened clinical response [Baert et al. 2003; Farrell et al. 2003], potentially prompting the need for a higher doses, more frequent administration, use of concomitant immunosuppressants, and changes in therapy. Moreover, formation of antibodies to anti-TNF agents can compromise their safety; several studies showed a link between an increased risk of infusion reactions and anti-TNF antibody formation [Baert et al. 2003; Farrell et al. 2003; Hanauer et al. 2004].
Despite the inconsistencies in the literature regarding treatment response and its relation to TNF drug level measurements and immunogenicity testing, at least one study demonstrated that measuring IFX concentrations and testing for the presence of ATI can influence management decisions and is useful in clinical practice [Afif et al. 2010]. Based on this study and other available evidence, a treatment algorithm for the use of IFX based on these parameters has been proposed (Figure 4) [Colombel et al. 2011]. Because the presence of ATI clearly signals development of immunogenicity and suggests that further treatment may lead to decreasing returns in terms of clinical response or possibly lead to an acute infusion or delayed hypersensitivity reaction, changing to another anti-TNF may be a more successful strategy than increasing the IFX dose. In contrast, IFX dose escalation may be an appropriate strategy for patients with subtherapeutic IFX concentrations in the absence of ATI formation, given that clinical response was significantly increased with dose escalation versus changing to another anti-TNF agent (86% versus 33%; p < 0.016). However, preliminary data suggest that low levels of antibodies can be overcome with dose escalation while sustained high levels of antibodies are associated with permanent loss of response and the need for treatment discontinuation [Vande Casteele et al. 2012a]. For patients with therapeutic IFX serum concentrations who have lost response, radiologic/endoscopic testing should be performed to confirm the presence of active inflammation. Alternate therapies with a different mechanism of action should be considered for patients with persistent disease on endoscopy/radiology, while alternative etiologies should be investigated in those with inactive disease.
Despite these evolving data, limitations of the current body of evidence make it difficult to assess the true incidence of antibody formation to anti-TNF agents and its impact on treatment outcomes in IBD. Many studies assessing the incidence of immunogenicity to these agents have been conducted with small sample sizes, making statistical differences between treatment groups difficult to discern. The frequency of antibody development may be underestimated by short-term, 4-week studies [Hanauer et al. 2006; Sandborn et al. 2007c], as the development and identification of antibodies to anti-TNF agents may require longer follow up. Similarly, the follow-up times of some studies may have been too short to detect an effect of antibody formation on treatment outcome [West et al. 2008]. Further, the heterogeneity of the populations studied may influence the rate of antibody formation observed in clinical trials [West et al. 2008]. For example, studies have been conducted in patients who were naïve to anti-TNF therapy [Hanauer et al. 2002, 2006], as well as those who had previously received anti-TNF therapies [Sandborn et al. 2007c, 2010a]. Previous IFX therapy may be an important immunizing factor in patients who are subsequently treated with other anti-TNF agents [West et al. 2008]. Other potential confounding factors that may influence immunogenicity include concomitant medications, particularly immunosuppressive agents, and underlying disease [Anderson, 2005].
Differences in study design relative to antibody assays used and technical limitations of the analytic methods for antibody detection present challenges in assessing and comparing the relative immunogenicity of the anti-TNF agents in IBD [Anderson, 2005; Cassinotti and Travis, 2009]. In the future, new, more reliable tests that do not require the use of radioisotopes and avoid cross reactivity with immunoglobulins may help determine the true frequency of antibody formation. Anti-TNF antibody testing that is without temporal restrictions and is not affected by levels of the anti-TNF agent itself may lead to more definitive results and actionable data versus the large percentage of tests that are now termed inconclusive [Hanauer et al. 2002; Maser et al. 2006; Seow et al. 2010].
Conclusion
Immunogenicity can occur with any of the biologic agents used in IBD, although the underlying mechanisms by which it develops are not fully elucidated. Humanization of the agent does not eliminate this risk. Immunogenicity can change the pharmacokinetics of biologic therapeutics, resulting in suboptimal therapeutic levels of drug and response failure. The presence of antidrug antibodies has also been associated with infusion reactions.
Rates of immunogenicity to anti-TNF therapies used in IBD vary widely. Antibody formation is not unique to IFX and its chimeric structure; on the contrary, the relative degrees of ‘humanness’ of ADA and certolizumab do not eliminate the risk of immunogenicity. The incidence of antidrug antibodies noted in the IBD literature include ranges of 14–73% for ATI [Afif et al. 2010; Ainsworth et al. 2008; Baert et al. 2003; Colombel et al. 2010; Farrell et al. 2003; Hanauer et al. 2002, 2004; Maser et al. 2006; Rutgeerts et al. 2005; Seow et al. 2010; Van Assche et al. 2008; Vermeire et al. 2007], 0–17% for ATA [Hanauer et al. 2006; Karmiris et al. 2009; Sandborn et al. 2007b, 2007c; West et al. 2008], and 2–12.3% for ATC [Lichtenstein et al. 2010; Sandborn et al. 2007a, 2010a; Schreiber et al. 2005, 2007]. Reasons for the variations observed include differing assay methodologies used to detect antidrug antibodies, as well as the influence of patient-related factors including severity of disease, concomitant medications (e.g. immunomodulators), and the route of drug administration. The development of antibodies to anti-TNF agents may have important clinical implications in patients with IBD, including shortened clinical response and higher risk of infusion reactions. Given the limitations of commercially available ELISAs used to assess immunogenicity, including their being subject to matrix effects and blood component and drug interference and their failure to measure IgG4, new assays are needed to minimize inconclusive results, allow relevant comparisons across studies and agents, and characterize the impact of antibody formation on treatment outcomes in IBD.
A new fluid phase mobility shift assay was recently developed to provide improved sensitivity and specificity over the commonly used ELISA, to obviate the difficulties and complexities associated with RIA, and to allow simultaneous detection of IFX serum levels and ATI. The clinical utility of this new assay was recently reported by Feagan and colleagues [Feagan et al. 2012]. These investigators analyzed 1487 serum samples from 483 patients with CD from four prospective randomized controlled trials or cohort studies of maintenance therapy with IFX to evaluate the relationship between serum IFX levels and the formation of ATI with disease activity, as assessed by CRP. Both IFX level and the presence of ATI were noted to be related to median CRP levels in this analysis. An IFX cutoff of 3 μg/ml was found to accurately predict inflammatory status as measured by CRP. In a regression analysis, positive ATI status and IFX of at least 3 µg/ml correlated significantly with CRP level, which was 59% higher in patients who were ATI positive than in those who were ATI negative (p = 0.0038) and 52% lower in patients with IFX levels of at least 3 µg/ml than in those with levels less than 3 µg/ml (p = 0.0001) [Feagan et al. 2012]. These findings suggest that the benefits of IFX are diminished in the presence of ATI, even if drug concentrations are optimal. It is expected that the mobility shift assay may provide more accurate data on both anti-TNF drug levels and measurement of antidrug antibodies compared with prior assays and that the results obtained may provide guidance for individual treatment decisions during the management of anti-TNF therapy. Informed treatment decisions may translate into improved patient outcomes.
Acknowledgments
The authors acknowledge John Simmons, MD, and Peloton Advantage, LLC, for their editorial assistance in preparing this manuscript. The author is fully responsible for all content, editorial decisions, and opinions expressed in this paper.
Footnotes
Funding: Funding for editorial assistance in preparation of this manuscript was provided by Prometheus Laboratories.
Conflict of interest statement: Gary R. Lichtenstein, MD, is a consultant for Abbott Corporation, Alaven, Centocor Ortho Biotech, Elan, Ferring, Hospira, Meda Pharmaceuticals, Millennium Pharmaceuticals, Pfizer, Proctor and Gamble, Prometheus Laboratories, Salix Pharmaceuticals, Santarus, Schering-Plough, Shire Pharmaceuticals, Takeda, UCB, Warner Chilcotte, and Wyeth, and has served as a researcher for Alaven, Bristol-Myers Squibb, Centocor Ortho Biotech, Ferring, Proctor and Gamble, Prometheus Laboratories, Salix Pharmaceuticals, Shire Pharmaceuticals, UCB, and Warner Chilcotte.
References
- Afif W., Loftus E., Jr, Faubion W., Kane S., Bruining D., Hanson K., et al. (2010) Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol 105: 1133–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth M., Bendtzen K., Brynskov J. (2008) Tumor necrosis factor-alpha binding capacity and anti-infliximab antibodies measured by fluid-phase radioimmunoassays as predictors of clinical efficacy of infliximab in Crohn’s disease. Am J Gastroenterol 103: 944–948 [DOI] [PubMed] [Google Scholar]
- Anderson P. (2005) Tumor necrosis factor inhibitors: clinical implications of their different immunogenicity profiles. Semin Arthritis Rheum 34(Suppl. 1): 19–22 [DOI] [PubMed] [Google Scholar]
- Baert F., Noman M., Vermeire S., Van Assche G., D’Haens G., Carbonez A., et al. (2003) Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med 348: 601–608 [DOI] [PubMed] [Google Scholar]
- Barnes T., Moots R. (2007) Targeting nanomedicines in the treatment of rheumatoid arthritis: focus on certolizumab pegol. Int J Nanomedicine 2: 3–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewtra M., Su C., Lewis J. (2007) Trends in hospitalization rates for inflammatory bowel disease in the United States. Clin Gastroenterol Hepatol 5: 597–601 [DOI] [PubMed] [Google Scholar]
- Bodini G., Savarino V., Fazio V., Assandri L., Dulbecco P., Gemignani L., et al. (2012) Relationship between drug serum concentration and clinical activity in patients with Crohn disease who achieved remission with adalimumab – a prospective study [abstract Sa2045]. Gastroenterology 142: S388 [Google Scholar]
- Bosani M., Ardizzone S., Porro G. B. (2009) Biologic targeting in the treatment of inflammatory bowel diseases. Biologics 3: 77–97 [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Brambell F., Hemmings W., Morris I. (1964) A theoretical model of gamma-globulin catabolism. Nature 203: 1352–1354 [DOI] [PubMed] [Google Scholar]
- Breese E., Michie C., Nicholls SW., Murch S., Williams C., Domizio P., et al. (1994) Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology 106: 1455–1466 [DOI] [PubMed] [Google Scholar]
- Bruining D., Sandborn W. (2011) Do not assume symptoms indicate failure of anti-tumor necrosis factor therapy in Crohn’s disease. Clin Gastroenterol Hepatol 9: 395–399 [DOI] [PubMed] [Google Scholar]
- Candon S., Mosca A., Ruemmele F., Goulet O., Chatenoud L., Cezard J. (2006) Clinical and biological consequences of immunization to infliximab in pediatric Crohn’s disease. Clin Immunol 118: 11–19 [DOI] [PubMed] [Google Scholar]
- Cassinotti A., Travis S. (2009) Incidence and clinical significance of immunogenicity to infliximab in Crohn’s disease: a critical systematic review. Inflamm Bowel Dis 15: 1264–1275 [DOI] [PubMed] [Google Scholar]
- Colombel J., Feagan B., Sandborn W., Van Assche G., Robinson A. (2011) Therapeutic drug monitoring of biologics for inflammatory bowel disease. Inflamm Bowel Dis 18: 349–359 [DOI] [PubMed] [Google Scholar]
- Colombel J., Sandborn W., Reinisch W., Mantzaris G., Kornbluth A., Rachmilewitz D., et al. (2010) Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 362: 1383–1395 [DOI] [PubMed] [Google Scholar]
- Colombel J., Sandborn W., Rutgeerts P., Enns R., Hanauer S., Panaccione R., et al. (2007) Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 132: 52–65 [DOI] [PubMed] [Google Scholar]
- Crohn’s & Colitis Foundation of America (2007) Living with Crohn’s disease. Available at: http://www.ccfa.org/about/press/ibdfacts (accessed 9 September 2011).
- Dinesen L., Travis S. (2007) Targeting nanomedicines in the treatment of Crohn’s disease: focus on certolizumab pegol (CDP870). Int J Nanomedicine 2: 39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drastich P., Kozeluhova J., Jaresova M., Spicak J. (2011) Infliximab serum trough levels and deep remission in patients with IBD [abstract Sa1371]. Gastroenterology 140: S292 [Google Scholar]
- Farrell R., Alsahli M., Jeen Y., Falchuk K., Peppercorn M., Michetti P. (2003) Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn’s disease: a randomized controlled trial. Gastroenterology 124: 917–924 [DOI] [PubMed] [Google Scholar]
- Fasanmade A., Adedokun O., Blank M., Zhou H., Davis H. (2011) Pharmacokinetic properties of infliximab in children and adults with Crohn’s disease: a retrospective analysis of data from 2 phase III clinical trials. Clin Ther 33: 946–964 [DOI] [PubMed] [Google Scholar]
- Feagan B, Singh S., Lockton S., Hauenstein S., Ohrmund L., Greenberg G., et al. (2012) Novel infliximab (IFX) & antibody-to-infliximab (ATI) assays are predictive of disease activity in patients with Crohn’s disease (CD) [abstract 565]. Presented at 2012 Digestive Disease Week, 19–22 May 2012, San Diego, CA, USA [Google Scholar]
- Ferrante M, Karmiris K., Compernolle G., Ballet V., Vermeire S., Noman M., et al. (2011) Efficacy of adalimumab in patients with ulcerative colitis: restoration of serum levels after dose escalation results in a better long-term outcome [abstract OP312]. Presented at the Annual United European Gastroenterology Week, 22–26 October 2011, Stockholm, Sweden [Google Scholar]
- Han P., Cohen R. (2004) Managing immunogenic responses to infliximab: treatment implications for patients with Crohn’s disease. Drugs 64: 1767–1777 [DOI] [PubMed] [Google Scholar]
- Hanauer S., Feagan B., Lichtenstein G., Mayer L., Schreiber S., Colombel J., et al. (2002) Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 359: 1541–1549 [DOI] [PubMed] [Google Scholar]
- Hanauer S., Sandborn W., Rutgeerts P., Fedorak R., Lukas M., MacIntosh D., et al. (2006) Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology 130: 323–333 [DOI] [PubMed] [Google Scholar]
- Hanauer S., Wagner C., Bala M., Mayer L., Travers S., Diamond R., et al. (2004) Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn’s disease. Clin Gastroenterol Hepatol 2: 542–553 [DOI] [PubMed] [Google Scholar]
- Hyams J., Crandall W., Kugathasan S., Griffiths A., Olson A., Johanns J., et al. (2007) Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology 132: 863–873 [DOI] [PubMed] [Google Scholar]
- Jess T., Riis L., Vind I., Winther K., Borg S., Binder V., et al. (2007) Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: a population-based study from Copenhagen, Denmark. Inflamm Bowel Dis 13: 481–489 [DOI] [PubMed] [Google Scholar]
- Karmiris K., Paintaud G., Noman M., Magdelaine-Beuzelin C., Ferrante M., Degenne D., et al. (2009) Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn’s disease. Gastroenterology 137: 1628–1640 [DOI] [PubMed] [Google Scholar]
- Keizer R., Huitema A., Schellens J., Beijnen J. (2010) Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 49: 493–507 [DOI] [PubMed] [Google Scholar]
- Langholz E., Munkholm P., Davidsen M., Binder V. (1994) Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology 107: 3–11 [DOI] [PubMed] [Google Scholar]
- Li J., Paulson S., Chiu Y., Robinson A., Lomax K., Pollack P. (2012) Evaluation of potential correlations between serum adalimumab concentration and remission in patients with Crohn’s disease in classic I and II [abstract 741]. Gastroenterology 142: S101 [Google Scholar]
- Lichtenstein G., Diamond R., Wagner C., Fasanmade A., Olson A., Marano C., et al. (2009) Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther 30: 210–226 [DOI] [PubMed] [Google Scholar]
- Lichtenstein G., Thomsen O., Schreiber S., Lawrance I., Hanauer S., Bloomfield R., et al. (2010) Continuous therapy with certolizumab pegol maintains remission of patients with Crohn’s disease for up to 18 months. Clin Gastroenterol Hepatol 8: 600–609 [DOI] [PubMed] [Google Scholar]
- Maser E., Villela R., Silverberg M., Greenberg G. (2006) Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol 4: 1248–1254 [DOI] [PubMed] [Google Scholar]
- Miele E., Markowitz J., Mamula P., Baldassano R. (2004) Human antichimeric antibody in children and young adults with inflammatory bowel disease receiving infliximab. J Pediatr Gastroenterol Nutr 38: 502–508 [DOI] [PubMed] [Google Scholar]
- Moss A., Fernandez-Becker N., Jo K., Cury D., Cheifetz A. (2008) The impact of infliximab infusion reactions on long-term outcomes in patients with Crohn’s disease. Aliment Pharmacol Ther 28: 221–227 [DOI] [PubMed] [Google Scholar]
- Mould D., Green B. (2010) Pharmacokinetics and pharmacodynamics of monoclonal antibodies: concepts and lessons for drug development. BioDrugs 24: 23–39 [DOI] [PubMed] [Google Scholar]
- Munkholm P., Langholz E., Davidsen M., Binder V. (1995) Disease activity courses in a regional cohort of Crohn’s disease patients. Scand J Gastroenterol 30: 699–706 [DOI] [PubMed] [Google Scholar]
- Murch S., Braegger C., Walker-Smith J., MacDonald T. (1993) Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut 34: 1705–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt A., Fossati G., Bergin M., Stephens P., Stephens S., Foulkes R., et al. (2007) Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor alpha agents. Inflamm Bowel Dis 13: 1323–1332 [DOI] [PubMed] [Google Scholar]
- Raghavan M., Bjorkman P. (1996) Fc receptors and their interactions with immunoglobulins. Annu Rev Cell Dev Biol 12: 181–220 [DOI] [PubMed] [Google Scholar]
- Reinisch W., Feagan B., Rutgeerts P., Adedokun O., Cornillie F., Diamond R., et al. (2012) Infliximab concentration and clinical outcome in patients with ulcerative colitis [abstract 566]. Gastroenterology 142: S–114 [Google Scholar]
- Rutgeerts P., Sandborn W., Feagan B., Reinisch W., Olson A., Johanns J., et al. (2005) Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 353: 2462–2476 [DOI] [PubMed] [Google Scholar]
- Sandborn W. (2003) Preventing antibodies to infliximab in patients with Crohn’s disease: optimize not immunize. Gastroenterology 124: 1140–1145 [DOI] [PubMed] [Google Scholar]
- Sandborn W., Abreu M., D’Haens G., Colombel J., Vermeire S., Mitchev K., et al. (2010a) Certolizumab pegol in patients with moderate to severe Crohn’s disease and secondary failure to infliximab. Clin Gastroenterol Hepatol 8: 688–695 [DOI] [PubMed] [Google Scholar]
- Sandborn W., Feagan B., Hanauer S., Lochs H., Lofberg R., Modigliani R., et al. (2002) A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology 122: 512–530 [DOI] [PubMed] [Google Scholar]
- Sandborn W., Feagan B., Stoinov S., Honiball P., Rutgeerts P., Mason D., et al. (2007a) Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med 357: 228–238 [DOI] [PubMed] [Google Scholar]
- Sandborn W., Hanauer S., Pierre-Louis B., Lichtenstein G. (2012) Certolizumab pegol plasma concentration and clinical remission in Crohn’s Disease [abstract Su2079]. Gastroenterology 142: S563 [Google Scholar]
- Sandborn W., Hanauer S., Rutgeerts P., Fedorak R., Lukas M., MacIntosh D., et al. (2007b) Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut 56: 1232–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborn W., Rutgeerts P., Enns R., Hanauer S., Colombel J., Panaccione R., et al. (2007c) Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med 146: 829–838 [DOI] [PubMed] [Google Scholar]
- Sandborn W., Schreiber S., Hanauer S., Colombel J., Bloomfield R., Lichtenstein G. (2010b) Reinduction with certolizumab pegol in patients with relapsed Crohn’s disease: results from the PRECiSE 4 Study. Clin Gastroenterol Hepatol 8: 696–702 [DOI] [PubMed] [Google Scholar]
- Schreiber S., Khaliq-Kareemi M., Lawrance I., Thomsen O., Hanauer S., McColm J., et al. (2007) Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med 357: 239–250 [DOI] [PubMed] [Google Scholar]
- Schreiber S., Rutgeerts P., Fedorak R., Khaliq-Kareemi M., Kamm M., Boivin M., et al. (2005) A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn’s disease. Gastroenterology 129: 807–818 [DOI] [PubMed] [Google Scholar]
- Seow C., Newman A., Irwin S., Steinhart A., Silverberg M., Greenberg G. (2010) Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut 59: 49–54 [DOI] [PubMed] [Google Scholar]
- Spector R., Park G., Johnson G., Vesell E. (1988) Therapeutic drug monitoring. Clin Pharmacol Ther 43: 345–353 [DOI] [PubMed] [Google Scholar]
- Tabrizi M., Tseng C., Roskos L. (2006) Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today 11: 81–88 [DOI] [PubMed] [Google Scholar]
- Van Assche G., Magdelaine-Beuzelin C., D’Haens G., Baert F., Noman M., Vermeire S., et al. (2008) Withdrawal of immunosuppression in Crohn’s disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology 134: 1861–1868 [DOI] [PubMed] [Google Scholar]
- Vande Casteele N, Cuypers L., Singh S., Hauenstein S., Ohrmund L., Chuang E., et al. (2012a) Transient versus sustained antibodies to infliximab: possibility to overcome low titer antibody responses by dose optimization [abstract P253]. Presented at the Annual Congress of the European Crohn’s and Colitis Organisation, 16–18 February 2012, Barcelona, Spain [Google Scholar]
- Vande Casteele N., Cuypers L., Singh S., Hauenstein S., Ohrmund L., Van Assche G., et al. (2012b) Antibodies to infliximab can either be persistent or transient: a retrospective case-control study in IBD patients treated with infliximab maintenance therapy [abstract]. Presented at 2012 Digestive Disease Week, 19–22 May 2012, San Diego, CA, USA [Google Scholar]
- Vermeire S., Noman M., Van Assche G., Baert F., D’Haens G., Rutgeerts P. (2007) Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut 56: 1226–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R., Zelinkova Z., Wolbink G., Kuipers E., Stokkers P., van der Woude C. (2008) Immunogenicity negatively influences the outcome of adalimumab treatment in Crohn’s disease. Aliment Pharmacol Ther 28: 1122–1126 [DOI] [PubMed] [Google Scholar]


