Abstract
Background and aims:
Nonalcoholic fatty liver disease (NAFLD) is now recognized as part of the metabolic syndrome, and is specifically related to obesity and insulin resistance. Lifestyle modification is advocated for the treatment of NAFLD, but few studies have evaluated its impact on liver histology. The purpose of this study was to investigate which, if any, specific diet and exercise recommendations are associated with histopathologic changes.
Methods:
A total of 56 participants were randomly assigned to 1 of 4 lifestyle modification subgroups for 6 months: standard care, low-fat diet and moderate exercise, moderate-fat/low-processed-carbohydrate diet and moderate exercise, or moderate exercise only. All subjects had biopsy-proven NAFLD, to include nonalcoholic steatohepatitis (NASH), and received a repeat 6-month biopsy to detect histopathologic changes. Other measures included blood assay of liver enzymes (aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase), fasting glucose, serum insulin, lipid panel, body weight, dietary intake, fat mass, and fitness level.
Results:
Among the 41 participants who completed the study (88% with NASH), a significant change was found in pre- to post-NAFLD activity score in the group as a whole (p < 0.001) with no difference detected between subgroups (p = 0.31). Our results confirm that lifestyle modification is effective in improving NAFLD and NASH.
Conclusions:
Regardless of intervention group, lifestyle modification improved liver histology, as verified by repeat biopsy, after a 6-month intervention. This study reinforces the importance of lifestyle modification as the primary treatment strategy for patients with NAFLD.
Keywords: NAFLD, diet, lifestyle modification, fatty liver, treatment, low fat, diabetes
Introduction and background
The number of overweight and obese individuals in the United States (USA) is increasing. According to the National Center for Health Statistics, the prevalence of obesity has increased to 34% of US adults over the age of 20 years [Ogden et al. 2010]. Obesity is associated with multiple comorbidities, including type 2 diabetes, cardiovascular disease, hypertension, and sleep apnea [Weiss, 2007]. Nonalcoholic fatty liver disease (NAFLD) is now considered by many to be the hepatic manifestation of metabolic syndrome, and is specifically related to obesity and insulin resistance (IR) [Moore, 2010]. The spectrum of NAFLD includes both simple steatosis and nonalcoholic steatohepatitis (NASH), the latter of which may lead to fibrosis and cirrhosis of the liver [Weiss, 2007; Reid, 2001]. NASH by itself is associated with increased all-cause mortality and patients with NASH-induced cirrhosis are at increased risk for liver decompensation and the development of hepatocellular carcinoma. NAFLD is present in the majority of morbidly obese individuals [body mass index (BMI) ≥ 40 kg/m2] with or without elevated serum liver enzymes [Harrison and Day, 2007]. The role of IR combined with obesity appears to have the most prominent effect on the development of NAFLD [Bellentani and Marino, 2009; Gholam et al. 2007; Seppala-Lindroos et al. 2002; Rocha et al. 2005].
Our knowledge of the pathogenesis of NAFLD and its progression to NASH, although not yet fully elucidated, is improving. Still, many questions remain regarding the most effective treatment modality [Torres et al. 2012]. Weight loss is currently the most agreed upon intervention [Harrison and Day, 2007; Rafiq and Younossi, 2008; Torres et al. 2012; Shaffer, 2006; Yan et al. 2007; Youssef and McCullough, 2002; Petersen et al. 2005; de Luis et al. 2008]. Treatments including drug therapy, bariatric surgery, and lifestyle modification, have all demonstrated weight loss success. Research has shown that rapid weight loss from bariatric surgery may damage the liver and medication may not provide any substantial benefit that cannot be achieved with lifestyle modification alone [Harrison and Day, 2007; Huang et al. 2005; Cinar et al. 2006]. Prospective studies evaluating changes in liver histology following successful weight loss through lifestyle modification, in addition to the most beneficial type of lifestyle modification to improve liver histology in NAFLD/NASH, are lacking.
Diet and exercise guidelines are well established for conditions such as diabetes and cardiovascular disease but no such guidelines exist for NAFLD. Previous studies investigating low-fat and low-carbohydrate diets for NAFLD have reported conflicting results. Disparity also exists in defining the macronutrient distribution appropriate for a low-fat or low-carbohydrate diet in this population [Youssefand McCullough, 2002; Cinar et al. 2006; Solga et al. 2004; Schwarz et al. 2003; Sato et al. 2007; Westerbacka et al. 2005; Kechagias et al. 2008; Tendler et al. 2007]. Refined carbohydrates and saturated fat may have a greater potential to increase liver lipid content as these dietary components are known to increase hepatic production of cholesterol and triglycerides [Fletcher et al. 2005]. It is possible that low to moderate amounts of fat and moderate amounts of complex carbohydrates may be most beneficial in improving NAFLD and IR. Furthermore, moderate exercise in conjunction with dietary changes is more effective at improving liver abnormalities than diet alone, and may improve NAFLD and NASH independently of weight loss [Suzuki et al. 2005; Tamura et al. 2005; Ueno et al. 1997; Hickman et al. 2004]. Recent reviews of NAFLD treatments conclude that a multidisciplinary approach to lifestyle modification may be the most successful therapy [Harrison and Day, 2007; Bellentani et al. 2008]. The purpose of this study was to implement and evaluate the effectiveness of four different lifestyle modification approaches to NAFLD treatment through diet and exercise on liver histology with repeat biopsy.
Patients and methods
Adults (age 18–70 years) were enrolled through an open recruitment period between October 2008 and February 2010 through the Gastroenterology Clinic at Brooke Army Medical Center (BAMC). Inclusion criteria included Department of Defense (DoD) beneficiaries (active duty, dependent, and retiree) living in the area for at least 9 months and liver biopsy-confirmed NAFLD (to include NASH) within 6 months prior to enrollment. Subjects were excluded for alcohol consumption greater than 20 g/day (more than two alcoholic drinks per day), diagnosis of concurrent viral hepatitis, chronic liver disease of other etiology, inborn errors of metabolism, insulin therapy for treatment of diabetes, or current pregnancy. This study was approved by the local institutional review board and all participants gave written informed consent. Participant randomization occurred by computer-generated random-numbers list in blocks of 10 with assignments placed in sealed envelopes, numbered sequentially, and allocated to participants in the order of recruitment by the primary investigator.
Study design
This study was a 6-month randomized controlled trial of lifestyle modification with four subgroup designations: standard care (SC; control), low-fat diet (20% fat, 60% carbohydrate, 20% protein) with moderate exercise (LFDE), moderate-fat/low-processed-carbohydrate diet (30% fat, 50% carbohydrate, 20% protein) with moderate exercise (MFDE), and moderate exercise only (ME). Histopathologic liver assessment using the NAFLD activity score (NAS) before and after randomization/participant study completion served as our primary study outcome measure. Secondary outcome measures included assessing individual changes in liver histology using the Brunt grading and staging system, liver function blood tests, cholesterol panels, IR markers, body weight, dietary intake, and percentage fat mass (including android and gynoid). Data collection, performed pre and post intervention, included percutaneous liver biopsy with histopathologic evaluation, blood assay, dual energy X-ray absorptiometry (DEXA) body composition scan, diet analysis, and anthropometric analysis. Blood assay and anthropometric data were also collected at the 3-month mid-point examination. Demographic information (age, sex), past medical history, and pertinent medication use were obtained through each subject’s medical record. All of the study authors had access to accumulated data and reviewed/approved the final manuscript.
Diet
All participants completed a baseline 3-day dietary intake record. SC subgroup participants attended one healthy eating class within 2 weeks of enrollment. This class is regularly taught at BAMC for DoD beneficiaries interested in weight loss or general nutrition guidelines. LFDE and MFDE participants attended a specialized nutrition class conducted by a registered dietitian, developed for this protocol. The class provided a nutrition prescription based on individualized calorie needs and macronutrient distribution (using the MyPyramid food group serving sizes) based on group assignment. Calorie (kcal) needs were calculated using the Mifflin–St Jeor equation using initial, actual body weight, and activity factor of 1.5 (light activity) subtracting 500 kcal/day for weight loss of 1 lb per week. Participants were also instructed on estimating portion sizes using the MyPyramid serving size guidelines, received a set of measuring cups and spoons, and were provided with supplemental reference material on healthy cooking, grocery shopping, and dining out. LFDE and MFDE participants met with one of three research staff dietitians three additional times (at 2, 4, and 6 months) for continued guidance on assigned diet. ME participants did not receive any dietary guidance. All participants received brief instruction on keeping a food diary and completed a 3-day food record (including food item, preparation, and amount) upon enrollment as well as at 2-, 4-, and 6-month study intervals. All 3-day food records were analyzed using Food Processor version 9.9.1 (ESHA Research, Salem, OR, USA) diet analysis software for macronutrient distribution, and intake of dietary fiber, sugar, saturated fat, polyunsaturated fat, and monounsaturated fat.
Exercise
All participants provided self-reported physical activity habits at baseline, although an actual initial exercise assessment was only completed with the three exercise groups to tailor their activity program. SC participants were encouraged to exercise by their physician but did not receive a formal exercise prescription. Participants in the three other subgroups (LFDE, MFDE, and ME) received education on beginning an exercise program for weight loss. The initial class was taught by an exercise physiologist, who started each participant on an individualized exercise program. Exercise initiation and progression was based on the FITT (frequency, intensity, time, and type) principle as recommended by the American College of Sports Medicine [Kaminsky, 2006]. All participants in these subgroups were encouraged to exercise for 20–60 min, 4–7 days/week, and received an exercise log to track sessions, along with a Yamax Digiwalker pedometer (New Lifestyles Inc., Lees Summit, MO, USA). During the first year of enrollment, participants were asked to exercise in a supervised environment at the CardioPulmonary Rehabilitation Program at the hospital. Participants followed an 18-step program, including a warm up, exercise bicycle, walking on a treadmill, various arm and leg strength exercises, and a cool down, with gradual ramp up over the 6 months. Exercise logs and pedometer use were discussed weekly by an exercise physiologist to monitor compliance. Unfortunately, this asset became unavailable to research participants in September 2009. All subsequent participants had free access to multiple fitness centers on the military bases located in San Antonio, TX.
Histopathologic assessment
Pre- and post- study liver biopsies were performed via sterile percutaneous technique. Assessment of steatosis, inflammation, fibrosis and overall diagnosis of NASH (if present) was performed using the grading and staging system developed by Brunt [Brunt, 1999]. In addition, a NAS was assigned to each specimen (pre- and post- intervention) by a single hepatopathologist who was blinded to study randomization [Kleiner et al. 2005]. The total NAS score represents the sum of scores for steatosis, lobular inflammation, and ballooning, and ranges from 0 to 8.
Body composition
DEXA analysis was performed using a GE Lunar Prodigy densitometer (GE Healthcare, Madison, WI, USA) and analyzed with enCORE software (version 9.30.044, GE Healthcare) to measure percentage of total body fat mass and percentage of lean body mass. Fat distribution was also assessed by measuring percentage of gynoid fat and percentage of android fat. These data were recorded at the initiation and the conclusion of each subject’s study participation.
Blood assay
Blood samples were collected following a 12 h fast and analyzed for serum levels of total cholesterol, low-density lipoprotein (LDL) and high-density lipoprotein cholesterol, triglycerides, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, glycosylated hemoglobin, fasting glucose, and insulin. IR was confirmed if the quantitative insulin sensitivity check index (QUICKI) value was calculated to be less than 0.357 [Hrebicek et al. 2002].
Anthropometric data
Participant height was measured in inches using a stadiometer and recorded once upon enrollment. All weights were measured in light clothing (without shoes) to the nearest tenth (0.1) of a pound on a calibrated electronic scale. BMI was calculated from height and weight measurements using the formula, weight (kg)/height (m)2.
Statistical analysis
Statistical analysis was conducted using SPSS statistical software package version 16.0 (SPSS Inc., Chicago, IL, USA). Demographic characteristics were represented by mean ± standard deviation (SD) or by percentage of study population. All results were expressed as mean ± SD. Treatment subgroups were compared using a two-factor analysis of variance (ANOVA) (treatment, time) with repeated measures on one factor (time). Further comparison was conducted with paired two-tailed t tests using Bonferroni correction for multiple comparisons. One-way ANOVA was used to compare pre and post differences in health parameters. Pearson’s correlation coefficient was used to determine significant relationships among variables. Significance was defined as p less than 0.05. A total of 16 participants were needed to detect an effect size of 4.0 SD at 80% power and 95% confidence level, however the goal was to recruit 15 participants per subset group (60 total), which would be sufficient to detect a 1.33 SD effect size.
Results
Subjects
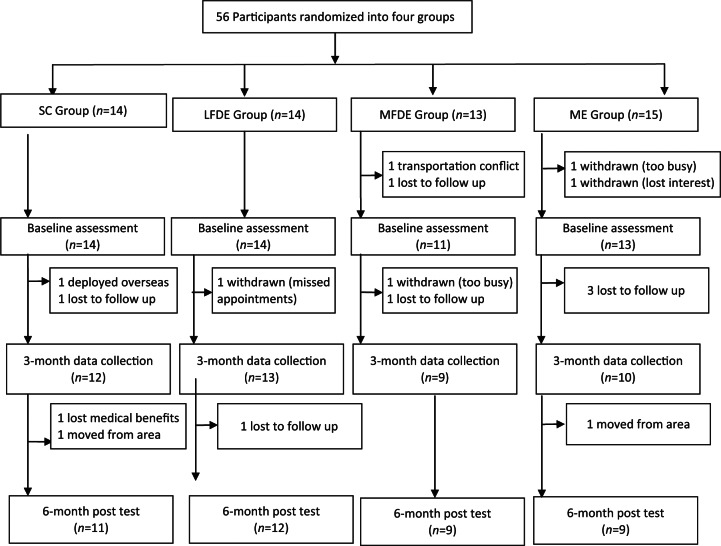
A total of 56 participants were recruited and randomized over the course of 15 months with an attrition rate of 27% (n = 15); see Figure 1. Reasons for withdrawal included scheduling conflicts, military deployment, loss of contact, loss of healthcare benefits, and transportation issues for meeting scheduled appointments. Of the 41 participants who completed the 6-month trial, 11 were assigned to the SC subgroup, 12 to the LFDE subgroup, 9 to the MFDE subgroup, and 9 to the ME subgroup.
Figure 1.
Participant flow throughout study. LFDE, low-fat diet plus moderate exercise; ME, moderate exercise only; MFDE, moderate fat diet plus moderate exercise; SC, standard care.
Baseline characteristics
Baseline characteristics of subjects are displayed in Table 1. The mean age was 50 ± 11years with 61% being men. Over half of all subjects had hyperlipidemia and hypertension while 40% had type 2 diabetes mellitus or prediabetes at baseline. More than 25% of participants were taking an antihyperglycemic agent and 33% of participants were taking cholesterol-lowering medication. The mean initial BMI was 34.7 ± 6.4 and mean initial NAS was 3.8 ± 1.2. A total of 36 of 41 (88%) met criteria for NASH, based on Brunt criteria. The majority of participants met the criteria for IR (mean QUICKI score of 0.309 ± 0.028). Mean ALT and AST levels were 66.8 ± 47.4 and 45.3 ± 25.8 respectively. Mean total cholesterol was 189.4 ± 40.3 with an LDL of 109.8 ± 32.4. Mean percent body fat (44.0% ± 8.0%) was above the recommended range (>25% for men, >30% for women). There were no significant characteristic differences between the subgroups at baseline, except for BMI (p = 0.009).
Table 1.
Baseline characteristics of all participants by subgroup.
| Characteristic (baseline) | SC (n = 11) | LFDE (n = 12) | MFDE (n = 9) | ME (n = 9) | Total (n = 41) |
|---|---|---|---|---|---|
| Age | 51 ± 11 | 44 ± 11 | 55 ± 5 | 52 ± 10 | 50 ± 11 |
| Sex (men) | 64% | 50% | 67% | 67% | 61% |
| Height (inches) | 66.7 ± 4.2 | 66.5 ± 3.8 | 64.1 ± 3.2 | 66.5 ± 3.8 | 66.0 ± 3.8 |
| Weight (lbs) | 224.9 ± 39.3 | 206.3 ± 38.4 | 234.5 ± 50.2 | 197.4 ± 34.6 | 215.5 ± 41.7 |
| BMI (kg/m2)* | 35.3 ± 3.5 | 32.7 ± 4.7 | 40.3 ± 9.3 | 31.3 ± 4.4 | 34.7 ± 6.4 |
| Existing conditions$ | |||||
| Hyperlipidemia | 82% | 50% | 67% | 56% | 63% |
| Diabetes (type 2) | 18% | 8% | 33% | 22% | 20% |
| Prediabetes | 18% | 17% | 22% | 22% | 20% |
| Hypertension | 64% | 58% | 44% | 44% | 54% |
| Medication use‡ | |||||
| Cholesterol lowering | 64% | 17% | 44% | 33% | 39% |
| Antihyperglycemic | 9% | 17% | 56% | 33% | 27% |
| NAFLD activity score | 3.6 ± 1.1 | 3.9 ± 1.7 | 4.3 ± 0.7 | 3.7 ± 1.1 | 3.8 ± 1.2 |
| Insulin (uIU/ml) | 18.1 ± 9.7 | 19.8 ± 8.5 | 20.1 ± 12.5 | 19.5 ± 5.9 | 19.3 ± 9.1 |
| Fasting glucose (mg/dl) | 101.4 ± 16.3 | 108.7 ± 25.3 | 108.7 ± 25.9 | 114.2 ± 23.2 | 107.9 ± 22.5 |
| QUICKI score | 0.319 ± 0.039 | 0.307 ± 0.026 | 0.307 ± 0.024 | 0.303 ± 0.016 | 0.309 ± 0.028 |
| HbA1C (%) | 5.8 ± 0.4 | 6.1 ± 0.5 | 6.3 ± 0.9 | 6.0 ± 0.3 | 6.0 ± 0.6 |
| ALT (IU/l) | 48.3 ± 46.6 | 71.2 ± 39.8 | 70.3 ± 50.7 | 79.9 ± 55.5 | 66.8 ± 47.4 |
| AST (IU/l) | 36.5 ± 26.7 | 47.0 ± 23.0 | 55.6 ± 43.3 | 43.3 ± 20.7 | 45.3 ± 28.8 |
| ALP (IU/l) | 89.4 ± 36.7 | 79.3 ± 21.9 | 84.3 ± 28.2 | 86.1 ± 30.6 | 84.6 ± 28.8 |
| Total cholesterol (mg/dl) | 201.9 ± 33.2 | 189.8 ± 41.4 | 190.4 ± 43.9 | 172.3 ± 43.6 | 189.4 ± 40.3 |
| LDL (mg/dl) | 112.9 ± 32.6 | 111.8 ± 36.6 | 110.3 ± 32.1 | 102.7 ± 31.0 | 109.8 ± 32.4 |
| HDL (mg/dl) | 47.6 ± 11.7 | 46.0 ± 11.0 | 48.3 ± 8.6 | 42.0 ± 9.8 | 46.1 ± 10.3 |
| TG (mg/dl) | 210.9 ± 117.5 | 160.3 ± 92.0 | 158.2 ± 79.6 | 156.8 ± 71.5 | 172.7 ± 92.8 |
| Fat mass (%) | 46.3 ± 7.5 | 42.5 ± 9.3 | 47.8 ± 6.8 | 39.8 ± 6.3 | 44.0 ± 8.0 |
| Gynoid fat mass (%) | 49.0 ± 8.9 | 45.8 ± 10.6 | 51.0 ± 8.7 | 41.9 ± 7.3 | 46.9 ± 9.3 |
| Android fat mass (%) | 55.5 ± 6.5 | 51.5 ± 7.6 | 55.5 ± 4.7 | 48.7 ± 4.7 | 52.8 ± 6.6 |
Data reported as mean ± standard deviation.
Significant difference between groups for BMI (p = 0.009).
Existing conditions: documented diagnosis in medical record; reported in cases (% of total group).
Medication use: documented prescription in medical record; reported in cases (% of total group).
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; HbA1C, glycosylated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LFDE, low-fat diet plus moderate exercise; ME, moderate exercise only; MFDE, moderate-fat diet plus moderate exercise; NAFLD, nonalcoholic fatty liver disease; QUICKI, quantitative insulin sensitivity check index; SC, standard care; TG, triglyceride.
Changes in liver histology
Pre- and post-study liver biopsy NAS values for each subgroup are displayed in Table 2. All subgroups had a decrease in NAS over the 6-month period and there was a significant decrease (–0.9 ± 1.3) in pre to post NAS in the group as a whole (p < 0.001). In addition, the LFDE (–1.3 ± 1.3) and MFDE (–1.2 ± 0.9) subgroups each experienced a significant decrease (p < 0.05) in NAS. However, no significant difference was observed in histologic improvements between the subgroups (p = 0.31). Out of 36 participants with NASH at baseline, 19 (53%) improved their Brunt grade or stage classification at 6 months, and 9 (25%) did not meet criteria for NASH at 6 months.
Table 2.
Change in liver histology (NAS) by subgroup.
| Group | Baseline | 6 months | Change | p value (95% CI) |
|---|---|---|---|---|
| SC (n = 11) | 3.6 ± 1.1 | 3.3 ± 1.6 | −0.4 ± 1.5 | 0.441 (–0.645 to 1.372) |
| LFDE (n = 12) | 3.9 ± 1.7 | 2.5 ± 1.6 | −1.3 ± 1.3* | 0.005 (0.506–2.161) |
| MFDE (n = 9) | 4.3 ± 0.7 | 3.1 ± 1.4 | −1.2 ± 0.9* | 0.005 (0.475–1.969) |
| ME (n = 9) | 3.7 ± 1.1 | 2.9 ± 1.5 | −0.7 ± 1.4 | 0.133 (–0.294 to 1.850) |
| Total (n = 41) | 3.9 ± 1.2 | 2.9 ± 1.5 | −0.9 ± 1.3* | <0.001 (0.510–1.347) |
Data reported as mean ± standard deviation.
Indicates significant change over time within group.
LFDE, low-fat diet plus moderate exercise; ME, moderate exercise only; MFDE, moderate-fat diet plus moderate exercise; NAS, nonalcoholic fatty liver disease activity score; SC, standard care.
Other outcome measures
Other secondary health outcome measures are compared in Table 3. There were no significant differences in the outcome changes between the subgroups. However, there was a significant decrease in Brunt grade (–0.3 ± 0.8), ALT (–18.3 ± 38.2), and AST (–11.6 ± 28.1) for the group as a whole. Weight loss over the 6-month period was observed in the SC, LFDE, and MFDE subgroups while slight weight gain was experienced by the ME subgroup. Decrease in percent body fat was only seen in the LFDE and MFDE subgroups. However, no subgroup achieved a significant weight loss of greater than 5% and changes in percent body fat were minimal on average.
Table 3.
Changes in health outcome measures by subgroup.
| Health measures (change from baseline to 6 months) | Group | Mean | SD | 95% CI for mean |
p value | |
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| NAFLD activity score | SC | −0.4 | 1.5 | −1.4 | 0.6 | 0.31 |
| LFDE | −1.3 | 1.3 | −2.2 | −0.5 | ||
| MFDE | −1.2 | 1.0 | −2.0 | −0.5 | ||
| ME | −0.8 | 1.4 | −1.8 | 0.3 | ||
| Total | −0.9 | 1.3 | −1.3 | −0.5 | <0.001* | |
| Brunt grade | SC | 0.1 | 0.5 | −0.3 | 0.5 | 0.059 |
| LFDE | −0.8 | 0.8 | −1.4 | −0.3 | ||
| MFDE | −0.2 | 1.1 | −1.1 | 0.6 | ||
| ME | −0.2 | 0.7 | −0.7 | 0.3 | ||
| Total | −0.3 | 0.8 | −0.6 | 0.0 | 0.022* | |
| Brunt stage | SC | 0.4 | 1.0 | −0.3 | 1.1 | 0.32 |
| LFDE | −0.7 | 1.5 | −1.6 | 0.3 | ||
| MFDE | −0.1 | 1.5 | −1.3 | 1.1 | ||
| ME | −0.4 | 1.3 | −1.5 | 0.6 | ||
| Total | −0.2 | 1.4 | −0.7 | 0.2 | 0.311 | |
| ALT (IU/l) | SC | −4.3 | 38.7 | −30.3 | 21.8 | 0.535 |
| LFDE | −27.5 | 27.9 | −45.2 | −9.8 | ||
| MFDE | −19.8 | 54.9 | −62.0 | 22.4 | ||
| ME | −21.8 | 30.6 | −45.3 | 1.8 | ||
| Total | −18.3 | 38.2 | −30.4 | −6.2 | 0.004* | |
| AST (IU/l) | SC | −2.9 | 25.8 | −20.3 | 14.4 | 0.554 |
| LFDE | −15.9 | 19.1 | −28.1 | −3.8 | ||
| MFDE | −19.6 | 47.9 | −56.4 | 17.3 | ||
| ME | −8.4 | 10.4 | −16.4 | −0.5 | ||
| Total | −11.6 | 28.1 | −20.5 | −2.7 | 0.012* | |
| QUICKI score | SC | −0.002 | 0.034 | −0.025 | 0.021 | 0.133 |
| LFDE | 0.017 | 0.021 | 0.004 | 0.030 | ||
| MFDE | −0.008 | 0.019 | −0.022 | 0.007 | ||
| ME | 0.006 | 0.026 | −0.013 | 0.026 | ||
| Total | 0.0042 | 0.0264 | −0.0041 | 0.0125 | 313 | |
| Insulin (uIU/ml) | SC | −1.08 | 6.89 | −6.01 | 3.85 | 0.245 |
| LFDE | −5.22 | 7.43 | −9.94 | −0.50 | ||
| MFDE | 2.55 | 5.95 | −2.42 | 7.52 | ||
| ME | −0.66 | 12.11 | −9.97 | 8.65 | ||
| Total | −1.51 | 8.52 | −4.27 | 1.25 | 0.275 | |
| Fasting glucose (mg/dl) | SC | 2.36 | 9.75 | −4.19 | 8.91 | 0.332 |
| LFDE | −9.58 | 23.36 | −24.43 | 5.26 | ||
| MFDE | −1.78 | 20.30 | −17.38 | 13.82 | ||
| ME | −9.80 | 14.83 | −21.20 | 1.60 | ||
| Total | −4.71 | 18.14 | −10.44 | 1.01 | 0.104 | |
| Weight (lbs) | SC | −2.5 | 5.3 | −6.0 | 1.0 | 0.43 |
| LFDE | −0.2 | 5.4 | −3.7 | 3.2 | ||
| MFDE | −3.0 | 4.7 | −6.6 | 0.6 | ||
| ME | 0.1 | 4.8 | −3.6 | 3.8 | ||
| Total | −1.4 | 5.1 | −3.0 | 0.2 | 0.089 | |
| Fat mass (%) | SC | 0.0 | 2.5 | −1.8 | 1.8 | 0.853 |
| LFDE | −0.8 | 2.6 | −2.6 | 0.9 | ||
| MFDE | −0.4 | 2.9 | −2.8 | 1.9 | ||
| ME | 0.6 | 6.2 | −4.1 | 5.3 | ||
| Total | −0.2 | 3.6 | −1.4 | 1.0 | 0.744 | |
| Android fat mass (%) | SC | −0.3 | 3.2 | −2.6 | 2.0 | 0.838 |
| LFDE | −1.0 | 3.6 | −3.5 | 1.6 | ||
| MFDE | −0.1 | 3.4 | −3.0 | 2.8 | ||
| ME | 0.4 | 2.3 | −1.5 | 2.3 | ||
| Total | −0.3 | 3.1 | −1.3 | 0.7 | 0.569 | |
| Gynoid fat mass (%) | SC | −0.7 | 2.6 | −2.5 | 1.1 | 0.573 |
| LFDE | −0.9 | 1.8 | −2.2 | 0.4 | ||
| MFDE | −1.5 | 1.7 | −2.9 | −0.1 | ||
| ME | 0.2 | 3.3 | −2.6 | 2.9 | ||
| Total | −0.7 | 2.4 | −1.5 | 0.1 | 0.068 | |
Data reported as mean ± standard deviation (SD).
Indicates significant change over time within group.
ALT, alaninine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; LFDE, low-fat diet plus moderate exercise; ME, moderate exercise only; MFDE, moderate fat diet plus moderate exercise; NAFLD, nonalcoholic fatty liver disease; QUICKI, quantitative insulin sensitivity check index; SC, standard care.
Dietary intake
In reviewing dietary intake of participants prescribed either the low-fat diet or the moderate-fat diet, it was noted that approximately 50% of participants did not comply with the prescribed macronutrient intake. Several dietary and health outcomes of the LFDE and MFDE subgroups were compared against the SC and ME subgroups. The diet plus exercise subgroups (LFDE and MFDE) had a significant decrease (p < 0.05) in total energy (kcal) intake (–15.3% ± 21.9%), total fat intake (–9.3% ± 10.1%), and total saturated fat intake (–9.9 g ± 13.2 g). While not significant, the single intervention subgroups (SC and ME) consumed more calories at the end of the 6-month study period than compared with baseline (9.2% ± 21.5%).
Discussion
This is the first randomized controlled prospective trial to compare the impact of multiple lifestyle interventions, to include nutritional modification, on nonalcoholic fatty liver histology through repeat biopsies. To the best of the authors’ knowledge, only two previous studies evaluated lifestyle modification with repeat biopsy in patients with NAFLD, although neither compared specific types of nutritional modification [Huang et al. 2005; Promrat et al. 2009]. Our results confirm that lifestyle modification, with diet and exercise intervention, is effective in improving NAFLD and NASH. An important caveat to these findings is that lifestyle modification alone may not be as effective in individuals with more advanced inflammation and fibrosis since the majority of study patients had early disease.
It does not appear that weight loss is essential to create improvement in liver histology as other researchers have formerly reported [Cinar et al. 2006; Zelber-Sagi et al. 2007; Promrat et al. 2009; Vilar et al. 2009]. The majority of our study population did not experience significant weight loss and several study participants actually gained weight with improvement in liver histology. This may be related to the transition in body composition (fat loss/lean muscle gain) with increased physical activity since muscle weighs more than fat; both LFDE and MFDE groups experienced mean loss in fat mass. None of the participants exceeded the weight loss goals recommended by the National Heart, Lung, and Blood Institute (NHLBI) and the American Dietetic Association of 1–2 lb of weight loss per week for the first 6 months of lifestyle change [Seagle et al. 2009; National Heart, Lung, and Blood Institute, 2000]. Half of the participants (51%) lost weight in the first 3 months despite weight regain by the 6-month endpoint. It may be possible that 3 months is enough time to facilitate improvements in liver histology (steatosis, lobular inflammation, hepatocyte ballooning and fibrosis) despite the lack of continued weight loss. Weight loss and its potential for improving liver histology in patients with NAFLD/NASH remains a point of contention in the current literature. Of the studies that conclude that weight loss is required for improvement in liver health, few have supported these findings with repeat biopsy [Petersen et al. 2005; de Luis et al. 2008; Huang et al. 2005; Fletcher et al. 2005; Hickman et al. 2004; Promrat et al. 2009; Vilar et al. 2009]. Promrat and colleagues conducted a similar study with comprehensive lifestyle intervention over a 48-week period, twice the length of this study, and reported a significant decrease in NAS with an average weight loss of 9.3% [Promrat et al. 2009]. Another study also noted an improvement in NAS with greater than 10% weight loss over a 6-month period with lifestyle intervention and dietary supplementation of Viusid [Vilar et al. 2009]. A recent study that evaluated liver enzymes and metabolic risk factors after a 3-month lifestyle intervention found that weight loss was not necessary to show improvement in typical screening markers for NAFLD [St George et al. 2009]. A 2011 Cochrane review of NAFLD, in which weight reduction was evaluated for its effect on NAFLD, concluded that no clear implications can be drawn with the current data, despite the related benefits and safety of lifestyle modification [Peng et al. 2011]. The natural history of NASH and the potential for spontaneous improvement or resolution of steatosis, inflammation, or even fibrosis over time with stable weight and no lifestyle intervention has also been suggested in the literature [Lindor et al. 2004]. Our study suggests that a healthier lifestyle may promote subtle changes beyond absolute weight loss that can benefit patients with NAFLD, although for global health improvement weight loss is still advantageous in overweight and obese patients with NAFLD. Future similar lifestyle modification studies with greater intervention time may be beneficial in shedding light on the question of weight loss and its role in NAFLD and NASH.
The design of this study had several strengths. The analysis of NAFLD using liver biopsy is the gold standard for evaluating this disease state and there are limited prospective randomized data in the literature utilizing liver histology to assess the effect of lifestyle modification in NAFLD to include NASH [Neuschwander-Tetri, 2005; Angulo et al. 2007; Youssef and McCullough, 2002; Nugent and Younossi, 2007]. This study involved a multidisciplinary team for health screening and lifestyle modification implementation. Current knowledge suggests that diet and exercise lifestyle modification is effective and should be the first line of treatment for NAFLD [Harrison and Day, 2007; Suzuki et al. 2005; Tamura et al. 2005; Cankurtaran et al. 2007; Chen et al. 2008; Toshimitsu et al. 2007]. Perhaps this type of approach could be of use in the future as a template for standardizing lifestyle modification in this group of patients.
We acknowledge the limitations of allowing participants to make their own food choices and our use of patient-reported data to analyze dietary intake. Our study was modeled on the idea of practicality and feasibility when implementing treatments that impact lifestyle modification. Despite this limitation, the two subgroups who received specific diet education (LFDE and MFDE) and follow-up nutrition counseling recorded decreased overall energy intake compared with the two subgroups that did not receive specific diet education or follow-up counseling (SC and ME). Many participants found it difficult to comply with exercise recommendations and meet bimonthly with a dietitian. A 2008 review of behavioral therapy for NAFLD recommends that unstructured exercise and self-monitoring programs be used to improve compliance [Bellentani et al. 2008]. Simply being diagnosed with a chronic liver disease as a motivating factor may explain why some succeeded in making lifestyle changes without specific diet or exercise instruction. It is possible that an intervention program with only basic nutrition and exercise education, similar to our SC subgroup, is all that is necessary to promote lifestyle modification. While the number of patients enrolled approached adequate power (60 in the analysis, 56 actually enrolled), there was greater patient dropout than anticipated, which could contribute to a type 2 error. Additional research on this topic is necessary to further investigate the most effective and beneficial components for a lifestyle modification program.
Conclusion
The results of this study verified that lifestyle modification can improve liver histology through repeat biopsy after a 6-month intervention in patients with NAFLD and NASH. Lifestyle modification should be used as the primary treatment strategy for patients with NAFLD. It appears that weight loss is not necessarily the key to improving liver histology. Further research is needed to determine the most effective diet and exercise interventions for development of a standardized treatment plan for these patients.
Acknowledgments
The authors thank all of the supporting departments and staff at Brooke Army Medical Center: Department of Clinical Investigation, Gastroenterology Clinic, Cardiac Rehabilitation Program, Pulmonary Clinic, Nuclear Medicine Service, and Department of Nutritional Medicine. Special thanks to MAJ Won Song, MD, Nuclear Medicine Service, Stacey Dramiga, MA, Director of Cardiopulmonary Rehabilitation, and SPC Jose Avitia, Department of Nutritional Medicine. The views expressed herein are those of the authors and do not reflect the official policy or position of San Antonio Military Medical Center, the US Army Medical Department, the US Army Office of the Surgeon General, the Department of the Army, Department of the Air Force, Department of Defense or the US Government.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Carly Eckard, Nutrition Care, US Army Medical Department-Vicenza Health Center, Vicenza, Italy.
Renee Cole, US Military Dietetic Internship, Army Medical Department Center & School, Fort Sam Houston, TX, USA.
Joshua Lockwood, Nutrition Care Division, Womack Army Medical Center, Fort Bragg, NC, USA.
Dawn M. Torres, Hepatology Clinic, Gastroenterology Service, Department of Medicine, Walter Reed Army Medical Center, Washington, DC, USA
Christopher D. Williams, Gastroenterology Service, Department of Medicine, Brooke Army Medical Center, Fort Sam Houston, TX, USA
Janet C. Shaw, Anatomical Pathology, Wilford Hall Medical Center, Lackland AFB, TX, USA
Stephen A. Harrison, Division of Gastroenterology and Hepatology, Department of Medicine, Brooke Army Medical Center, Fort Sam Houston, TX 78234, USA
References
- Angulo P., Hui J., Marchesini G., Bugianesi E., George J., Farrell G. (2007) The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45: 846–854 [DOI] [PubMed] [Google Scholar]
- Bellentani S., Dalle Grave R., Suppini A., Marchesini G. (2008) Behavior therapy for nonalcoholic fatty liver disease: the need for a multidisciplinary approach. Hepatology 47: 746–754 [DOI] [PubMed] [Google Scholar]
- Bellentani S., Marino M. (2009) Epidemiology and natural history of non-alcoholic fatty liver disease (NAFLD). Ann Hepatol 8: S4–S8 [PubMed] [Google Scholar]
- Brunt E. (1999) Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94: 2467–2474 [DOI] [PubMed] [Google Scholar]
- Cankurtaran M., Tayfur O., Yavuz B., Geyik S., Akhan O., Arslan S. (2007) Insulin resistance and metabolic syndrome in patients with NAFLD but without diabetes: effect of a 6 month regime intervention. Acta Gastroenterol Belg 70: 253–259 [PubMed] [Google Scholar]
- Chen S., Liu C., Li S., Huang H., Tsai C., Jou H., et al. (2008) Effects of therapeutic lifestyle program on ultrasound-diagnosed nonalcoholic fatty liver disease. J Clin Med Assoc 71: 551–558 [DOI] [PubMed] [Google Scholar]
- Cinar K., Coban S., Idilman R., Tuzun A., Sarioglu M., Bektas M., et al. (2006) Long-term prognosis of nonalcoholic fatty liver disease: is pharmacological therapy actually necessary? J Gastroenterol Hepatol 21: 169–173 [DOI] [PubMed] [Google Scholar]
- de Luis D., Aller R., Izaola O., Sagrado M., Conde R., Gonzalez J. (2008) Effect of a hypocaloric diet in transaminases in nonalcoholic fatty liver disease and obese patients, relation with insulin resistance. Diabetes Res Clin Pract 79: 74–78 [DOI] [PubMed] [Google Scholar]
- Fletcher B., Berra K., Ades P., Braun L., Burke L., Durstine J., et al. (2005) Managing abnormal blood lipids; a collaborative approach. Circulation 112: 3184–3209 [DOI] [PubMed] [Google Scholar]
- Gholam P., Flancbaum L., Machan J., Charney D., Kotler D. (2007) Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol 102: 399–408 [DOI] [PubMed] [Google Scholar]
- Harrison S., Day C. (2007) Benefits of lifestyle modification in NAFLD. Gut 56: 1760–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman I., Jonsson J., Prins J., Ash S., Purdie D., Clouston A., et al. (2004) Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut 53: 413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrebicek J., Janout V., Malincikova J., Horakova D., Cizek L. (2002) Detection of insulin resistance by simple quantitative insulin sensitivity check index QUICKI for epidemiological assessment and prevention. J Clin Endocrinol Metab 87: 144–147 [DOI] [PubMed] [Google Scholar]
- Huang M., Greenson J., Chao C., Anderson L., Peterman D., Jacobson J., et al. (2005) One-year intense nutritional counseling results in histological improvement in patients with nonalcoholic steatohepatitis: a pilot study. Am J Gasteroenterol 100: 1072–1081 [DOI] [PubMed] [Google Scholar]
- Kaminsky L. (2006) American College of Sports Medicine’s Guidelines for Exercise Testing and Prescription. 5th ed. Philadelphia, PA: Lippincott, Williams & Wilkins [Google Scholar]
- Kechagias S., Ernersson A., Dahlqvist O., Lundberg P., Lindstrom T., Nystrom F. (2008) Fast food based hyper-alimentation can induce rapid and profound elevation of serum alanine aminotransferase in healthy subjects. Gut 57: 649–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner D., Brunt E., Van Natta M., Behling C., Contos M., Cummings O., et al. (2005) Design and validation of histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313–1321 [DOI] [PubMed] [Google Scholar]
- Lindor K., Kowdley K., Heathcote E., Harrison M., Jorgensen R., Angulo P., et al. (2004) Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology 39: 770–778 [DOI] [PubMed] [Google Scholar]
- Moore J. (2010) Non-alcoholic fatty liver disease: the hepatic consequence of obesity and the metabolic syndrome. Proc Nutr Soc 69: 211–220 [DOI] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute (2000) The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Bethesda, MD: National Institutes of Health [Google Scholar]
- Neuschwander-Tetri B. (2005) Nonalcoholic steatohepatitis and the metabolic syndrome. Am J Med Sci 330: 326–335 [DOI] [PubMed] [Google Scholar]
- Nugent C., Younossi Z. (2007) Evaluation and management of obesity related nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol 4: 432–441 [DOI] [PubMed] [Google Scholar]
- Ogden C., Carroll M. (2010) Prevalence of Overweight, Obesity, and Extreme Obesity Among Adults: United States, Trends 1960–1962 through 2007–2008. Hyattsville, MD: Center for Disease Control and Prevention National Center for Health Statistics [Google Scholar]
- Peng L., Weng J., Li F. (2011) Weight reduction for non-alcoholic fatty liver disease. Cochrane Database Syst Rev 6: 1–32 [DOI] [PubMed] [Google Scholar]
- Petersen K., Dufour S., Befroy D., Lehrke M., Hendler R., Shulman G. (2005) Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 54: 603–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promrat K., Kleiner D., Niemeier H., Jackvony E., Kearns M., Wands J., et al. (2009) Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 51: 121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiq N., Younossi Z. (2008) Effects of weight loss on nonalcoholic fatty liver disease. Semin Liver Dis 28: 427–433 [DOI] [PubMed] [Google Scholar]
- Reid A. (2001) Nonalcoholic steatohepatitis. Gastroenterology 121: 710–723 [DOI] [PubMed] [Google Scholar]
- Rocha R., Cotrim H., Carvalho F., Siqueira A., Braga H., Freitas L. (2005) Body mass index and waist circumference in non-alcoholic fatty liver disease. J Hum Nutr Diet 18: 365–370 [DOI] [PubMed] [Google Scholar]
- Sato F., Tamura Y., Watada H., Kumashiro N., Igarashi Y., Uchino H., et al. (2007) Effects of diet-induced moderate weight reduction on intrahepatic and intramyocellular triglycerides and glucose metabolism in obese subjects. J Clin Endocrinol Metab 92: 3326–3329 [DOI] [PubMed] [Google Scholar]
- Schwarz J., Linfoot P., Dare D., Aghajanian K. (2003) Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr 77: 43–50 [DOI] [PubMed] [Google Scholar]
- Seagle H., Strain G., Makris A., Reeves R. (2009) Position of the American Dietetic Association: weight management. J Am Diet Assoc 109: 330–346 [DOI] [PubMed] [Google Scholar]
- Seppala-Lindroos A., Vehkavaara S., Hakkinen A., Goto T., Westerbacka J., Sovijarvi A., et al. (2002) Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab 87: 3023–3028 [DOI] [PubMed] [Google Scholar]
- Shaffer E. (2006) Bariatric surgery: a promising solution for nonalcoholic steatohepatitis in the very obese. J Clin Gastroenterol 40: S44–S50 [DOI] [PubMed] [Google Scholar]
- Solga S., Alkhuraishe A., Clark J., Torbenson M., Greenwald A., Diehl A., et al. (2004) Dietary composition and nonalcoholic fatty liver disease. Dig Dis Sci 49: 1578–1583 [DOI] [PubMed] [Google Scholar]
- St George A., Bauman A., Johnston A., Farrell G., Chey T., George J. (2009) Effect of a lifestyle intervention in patients with abnormal liver enzymes and metabolic risk factors. J Gastroenterol Hepatol 24: 399–407 [DOI] [PubMed] [Google Scholar]
- Suzuki A., Lindor K., St Saver J., Lymp J., Mendes F., Muto A., et al. (2005) Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol 43: 1060–1066 [DOI] [PubMed] [Google Scholar]
- Tamura Y., Tanaka Y., Sato F., Choi J., Watada H., Niwa M., et al. (2005) Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 90: 3191–3196 [DOI] [PubMed] [Google Scholar]
- Tendler D., Lin S., Yancy W., Mavropoulos J., Sylvestre P., Rockey D., et al. (2007) The effect of a low-carbohydrate, ketogenic diet on nonalcoholic fatty liver disease: a pilot study. Digest Dis Sci 52: 589–593 [DOI] [PubMed] [Google Scholar]
- Torres D., Williams C., Harrison S. (2012) Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 10: 837–858 [DOI] [PubMed] [Google Scholar]
- Toshimitsu K., Matsuura B., Ohkubo I., Niiya T., Furukawa S., Hiasa Y., et al. (2007) Dietary habits and nutrient intake in non-alcoholic steatohepatitis. Nutrition 23: 46–52 [DOI] [PubMed] [Google Scholar]
- Ueno T., Sugawara H., Sujaku K., Hashimoto O., Tsuji R., Tamaki S., et al. (1997) Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol 27: 103–107 [DOI] [PubMed] [Google Scholar]
- Vilar G., Rodriguez D., Gra O., Arus S., Llanio N., Calzadilla B., et al. (2009) Clinical trial: a nutritional supplement Viusid, in combination with diet and exercise, in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther 30: 999–1009 [DOI] [PubMed] [Google Scholar]
- Weiss R. (2007) Fat distribution and storage: how much, where, and how? Eur J Endocrinol 157: S39–S45 [DOI] [PubMed] [Google Scholar]
- Westerbacka J., Lammi K., Hakkinen A., Rissanen A., Salminen I., Aro A., et al. (2005) Dietary fat content modifies liver fat in overweight nondiabetic subjects. J Clin Endocrinol Metab 90: 2804–2809 [DOI] [PubMed] [Google Scholar]
- Yan E., Durazo F., Tong M., Hong K. (2007) Nonalcoholic fatty liver disease: pathogenesis, identification, progression, and management. Nutr Rev 65: 376–384 [DOI] [PubMed] [Google Scholar]
- Youssef W., McCullough A. (2002) Steatohepatitis in obese individuals. Best Prac Res Clin Gastroenterol 16: 733–747 [DOI] [PubMed] [Google Scholar]
- Zelber-Sagi S., Nitzan-Kaluski D., Goldsmith R., Webb M., Blendis L., Halpern Z., et al. (2007) Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study. J Hepatol 47: 711–717 [DOI] [PubMed] [Google Scholar]