Abstract
Gastroesophageal reflux disease (GERD) results from incompetency of the lower esophageal sphincter that allows the contents of the stomach to reflux into the esophagus, the airways, and the mouth. The disease affects about 10% of the western population and has a profound negative impact on quality of life. The majority of patients are successfully treated with proton-pump inhibitors, but up to 40% have incomplete relief of symptoms even after dose adjustment. The laparoscopic Nissen fundoplication represents the surgical gold standard, but is largely underused because of the level of technical difficulty and the prevalence of side effects. These factors have contributed to the propensity of patients to continue with medical therapy despite inadequate symptom control and complications of the disease. As a consequence, a significant ‘therapy gap’ in the treatment of GERD remains evident in current clinical practice. The LINX® Reflux Management System (Torax Medical, St. Paul, MN, USA) is designed to provide a permanent solution to GERD by augmenting the sphincter barrier with a standardized, reproducible laparoscopic procedure that does not alter gastric anatomy and is easily reversible. Two single-group trials confirmed that a magnetic device designed to augment the lower esophageal sphincter can be safely and effectively implanted using a standard laparoscopic approach. The device decreased esophageal acid exposure, improved reflux symptoms and quality of life, and allowed cessation of proton-pump inhibitors in the majority of patients.
Keywords: adenocarcinoma, Barrett’s, esophagitis, fundoplication, gastroesophageal reflux disease, heartburn, lower esophageal sphincter augmentation, Nissen, proton-pump inhibitors, regurgitation
Gastroesophageal reflux disease (GERD) is a chronic disorder of the alimentary tract in which the barrier function of the lower esophageal sphincter (LES) fails and gastric juice is allowed to reflux into the esophagus causing symptoms and anatomical lesions. GERD is typically characterized by symptoms of heartburn and regurgitation, and can lead to significant complications such as erosive esophagitis, Barrett’s esophagus, and esophageal adenocarcinoma. The Montreal definition and classification of GERD addresses the entire clinical spectrum of the disease, ranging from typical esophageal symptoms and lesions to a series of atypical cardiac, laryngeal, and pulmonary manifestations [Vakil et al. 2006]. Chronic GERD is prevalent in at least 10% of the western population [Dent et al. 2005], has a profound impact on patients’ quality of life [Becher and El-Serag, 2011], and is the number one reason for a patient to visit an outpatient clinic. As a consequence, GERD places a significant resource and cost burden on the healthcare system and the general economy, with over US$9 billion in annual direct costs in the USA alone, the highest cost of any digestive disorder [Shaheen et al. 2006].
Comparison of current therapeutic options for gastroesophageal reflux disease
The two primary treatment options for patients with GERD are long-term medical acid suppression therapy or a surgical antireflux procedure most commonly referred to as fundoplication.
Acid suppression therapy with proton-pump inhibitors (PPIs) is an effective first-line therapy in most cases. However, nearly 40% of patients experience breakthrough symptoms with once daily PPI use [Fass and Sifrim, 2009; Zerbib et al. 2013] and pharmacological therapy is often inadequate to maintain a symptom-free state in patients with a mechanically defective LES due to persistent nonacid reflux and nocturnal acid breakthrough [Lord et al. 2009]. Despite medical therapy, in some patients disease may progress and lead to serious complications, such as volume regurgitation with aspiration and Barrett’s metaplasia, a precursor to adenocarcinoma. The recently updated results of the ProGERD study, a large European open cohort multicenter study, showed that 9.7% of patients with GERD under routine medical care progress to Barrett’s esophagus at 5 years of follow up [Malfertheiner et al. 2012]. Potential risks associated with PPIs include B12 vitamin deficiency, Clostridium difficile infection, community-acquired pneumonia, hip fractures, and osteoporosis [Katz et al. 2012]. Other consequences of prolonged PPI therapy include hypergastrinemia, enterochromaffin-like cell hyperplasia, and parietal cell hypertrophy, leading to rebound acid hypersecretion [Heidelbaugh et al. 2012; McColl and Gillen, 2009].
The transabdominal Nissen fundoplication, introduced more than half a century ago, still represents the surgical standard of care and a treatment option usually reserved for patients whose condition has failed to respond to medical therapy or who desire to be free from dependence on medical therapy. The current laparoscopic version of the Nissen operation is a safe, effective, and durable therapy if performed in specialized and high-volume centers [Galmiche et al. 2011]. Despite remarkably low 30-day morbidity and mortality rates, the operation is underused due to the fear of long-term side effects and failure, which impact referral patterns [Niebisch et al. 2012]. Also, wide variability in outcomes has limited the adoption of this procedure [Richter and Dempsey, 2008; Vakil et al. 2003]. A Cochrane analysis found that laparoscopic fundoplication surgery is more effective than medical management for the treatment of GERD in short- to medium-term follow up [Wileman et al. 2010], although studies on the cost effectiveness of Nissen fundoplication versus pharmacological therapy with PPIs have been largely inconclusive [Thijssen et al. 2011].
Patients undergoing a Nissen fundoplication are at risk of potential side effects of the procedure, such as the gas bloat syndrome, the inability to belch and vomit, and the occurrence of persistent dysphagia that may require revisional surgery [Hunter et al. 1999]. Toupet fundoplication has been shown to have fewer of these side effects but does not eliminate their occurrence [Jobe et al. 1997]. These are the main reasons why gastroenterologists tend to limit their referrals for fundoplication only to patients with severe disease and large hiatal hernias.
The limitations of pharmacologic therapy and fundoplication leave many patients and clinicians in the equivocal position to either tolerate a lifetime of drug dependence with incomplete symptom relief or to undertake the risk of a surgical procedure that alters gastric anatomy and may have considerable side effects. Currently, fewer than 30,000 Nissen fundoplication procedures are performed annually in the USA, corresponding to less than 1% of the GERD population [Finks et al. 2006]. Therefore, a large proportion of patients with incomplete symptom relief could benefit from a simple sphincter augmentation procedure rather than choosing lifelong medical therapy or an anatomic altering fundoplication.
Rationale and methods of the new surgical procedure
The LINX Reflux Management System was developed to address the existing ‘therapy gap’ through a simple procedure, performed laparoscopically, that does not alter gastric anatomy, augments the physiologic barrier to reflux, and can easily be reversed if necessary, thereby preserving the option of fundoplication or other therapies in the future. Importantly, the LINX procedure is designed to limit technical variability, which will hopefully result in more standardization of antireflux surgery and more consistent clinical outcomes.
The LINX device applies magnetic force to augment the barrier function of the LES. For reflux to occur, the intragastric pressure must overcome both the patient’s native LES pressure and the magnetic bonds of the device, creating a resistance to opening. The LINX device, while augmenting the LES, allows for expansion to accommodate a swallowed bolus or the escape of elevated gastric pressure associated with belching or vomiting. This provides control of reflux without compromising the physiologic function of the LES [Ganz et al. 2013].
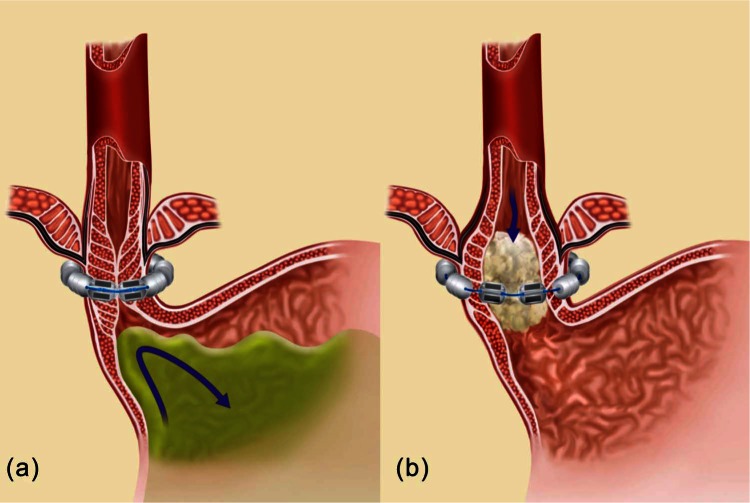
The LINX device consists of a series of titanium beads with magnetic cores hermetically sealed inside. The beads are interlinked with independent titanium wires to form a flexible and expandable ring with a ‘Roman arch’ configuration. Interestingly, each bead can move independently of the adjacent beads, creating a dynamic implant that mimics the physiological movement of the esophagus without limiting its range of motion (Figure 1). The strength of the magnetic core contained in each bead is calibrated by mass to provide a resisting force that precisely augments the sphincter’s function. This attractive force between closed beads is approximately 40 g; this decreases exponentially with distance such that attractive force at full separation is approximately 7 g. The device is manufactured in different sizes, from 10 to 18 beads, and is capable of nearly doubling its diameter when all beads are separated. The magnetic attraction force to be counteracted to allow bead separation is independent of the number of beads contained in the device.
Figure 1.
The LINX Reflux Management System encircling the distal esophagus in the closed position (a) and in the open position (b) [Bonavina, 2013]. Reproduced with kind permission from Springer Science and Business Media.
The LINX device is implanted laparoscopically under general anesthesia. Surgical dissection begins by dividing the peritoneum on the anterior surface of the gastroesophageal junction below the insertion of the inferior leaf of the phrenoesophageal ligament and above the junction of the hepatic branch to the anterior vagus nerve. The lateral surface of the left crus is freed from the posterior fundic wall without dividing any short gastric vessel. The gastrohepatic ligament is opened above and below the hepatic branch to facilitate the preparation of the retro-esophageal window. Gentle dissection from the right side is made towards the left crus just above the crural decussation to identify the posterior vagus nerve. The phrenoesophageal ligament should be left intact during these maneuvers (Figure 2). A tunnel is created between the vagus and the posterior esophageal wall and a penrose drain is passed in a left to right direction. The circumference of the esophagus is then measured to choose the proper size of the LINX device to be implanted. The sizing tool is a laparoscopic instrument with a soft, circular curved tip actuated by coaxial tubes through a handset. The handset contains a numerical indicator that corresponds to the size range of the LINX device. The sizing tool is placed around the esophagus in the dissected space between the posterior esophageal wall and the posterior vagus nerve bundle. Once the appropriate device has been selected, it is introduced through the posterior tunnel. The opposing ends are then brought to the anterior surface of the esophagus and connected together; this completes the implant procedure (Figure 3). The decision of whether to proceed with a posterior crural repair depends on the size of the hiatus hernia that is found intraoperatively: a sliding hernia up to 3 cm in size can be effectively repaired by approximating the crura with interrupted stitches and then the device can safely be implanted.
Figure 2.
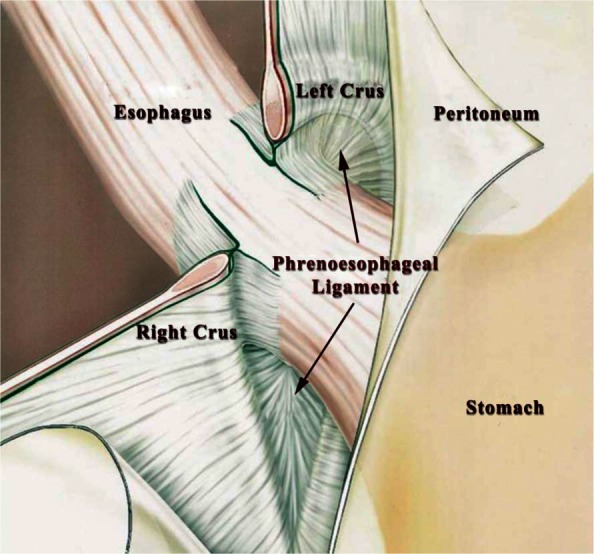
Minimal dissection of the gastroesophageal junction with preservation of the phrenoesophageal ligament is highly recommended during implantation of the LINX device.
Figure 3.
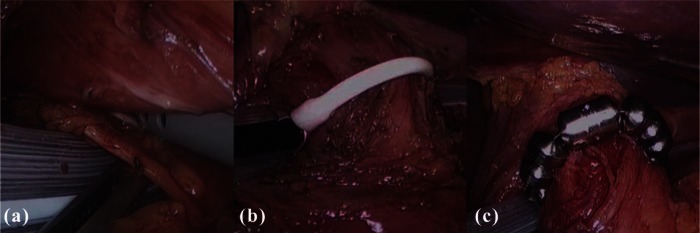
Intraoperative images show tunneling between the posterior vagus nerve and the esophageal wall (a); the sizing device in place to measure the esophageal circumference (b); and the LINX device positioned at the gastroesophageal junction (c) [Bonavina, 2013]. Reproduced with kind permission from Springer Science and Business Media.
Surgery time is about half an hour. Patients are discharged on the same day or on the first postoperative day and are told to return to a normal diet as tolerated and discontinue use of acid suppression medication.
Clinical experience with the LINX device
Two prospective, multicenter, clinical studies have been conducted under a US Food and Drug Administration (FDA) investigational device exemption to evaluate the LINX System. The first study (feasibility trial) [ClinicalTrials.gov identifier: NCT01057992] evaluated 44 patients implanted with the LINX System in four centers between February 2007 and October 2008; the short-term, mid-term, and 4-year results of this study have been previously published [Bonavina et al. 2008, 2010; Lipham et al. 2012]. The second study (pivotal trial) [ClinicalTrials.gov identifier: NCT00776997] evaluated 100 patients implanted with the device in 13 centers between January and September 2009 [Ganz et al. 2013]. A European registry of antireflux surgery is currently enrolling patients treated with either the LINX System or fundoplication.
The LINX Reflux Management System has recently been reviewed by the Gastroenterology and Urology Advisory Panel of the FDA. This Panel voted unanimously that there was reasonable assurance of safety and effectiveness, and that the benefits of treatment outweighed the risks. This information is in the public domain and available on the FDA website.
Summary of the feasibility trial
The primary criteria for inclusion in the trial were the following: aged over 18 and under 85 years, typical reflux symptoms at least partially responsive to PPI therapy, abnormal esophageal acid exposure, and normal contractile amplitude and waveform in the esophageal body. The primary criteria for exclusion from the trial were the following: history of dysphagia, previous upper abdominal surgery, previous endoluminal antireflux procedures, sliding hiatal hernia greater than 3 cm, esophagitis greater than grade A, and the presence of histologically documented Barrett’s esophagus. Patients with abnormal manometric findings (distal esophageal motility <35 mmHg peristaltic amplitude on wet swallows or <70% propulsive peristaltic sequences) were also excluded from the study. Patients served as their own control to assess the effect of treatment on esophageal acid exposure, symptoms, and use of PPIs.
Preoperative evaluation consisted of a symptom questionnaire, upper gastrointestinal endoscopy, barium swallow, esophageal manometry, and 24 h esophageal pH monitoring. The Gastro-Esophageal Reflux Disease Health Related Quality of Life (GERD HRQL) validated questionnaire [Velanovich, 1998] was administered prior to any diagnostic test and off PPI therapy. The questionnaire consists of six heartburn questions, two swallowing questions, one gas bloat question, and one question about medication use. The responses to these questions are scored on a scale of 0 (no symptoms) to 5 (incapacitating symptom).
The presence of esophagitis was assessed by upper gastrointestinal endoscopy using the Los Angeles or Savary–Miller classification. The length of hiatal hernia, if present, was measured as the distance between the gastroesophageal junction, defined by the proximal limit of the gastric folds, and the crural impression. The LES resting pressure and length were measured by esophageal manometry using a station pull-through technique. The percentage of LES relaxation and the LES residual pressure were assessed with five wet swallows. The amplitude of esophageal contractions was measured by averaging 10 wet swallows of 5 ml each, taken 30 s apart. Abnormal motility was defined as mean amplitude of less than 35 mmHg and a greater than 30% prevalence of simultaneous, dropped, or interrupted waves. Prolonged (24–48 h) esophageal pH monitoring was used to measure esophageal acid exposure off PPI therapy. The probe or Bravo capsule was placed 5 cm above the upper border of the LES as determined by manometry or 6 cm above the z line determined by endoscopy.
Postoperative assessment included a standard chest film and a modified barium swallow study to verify the position and function of the device. The GERD HRQL questionnaire, upper gastrointestinal endoscopy, modified barium esophagram, esophageal pH monitoring, and esophageal manometry were obtained at 3 and 12 months after surgery. The GERD HRQL questionnaire and esophageal pH monitoring were repeated up to 4 years after surgery.
All devices were successfully implanted through a laparoscopic approach. The median operative time was 40 min (range 19–104). No intraoperative complications occurred. Patients were instructed to resume a regular diet after a chest film and radiological assessment of the esophageal transit were performed (Figure 4). All patients except one were discharged within 48 h.
Figure 4.
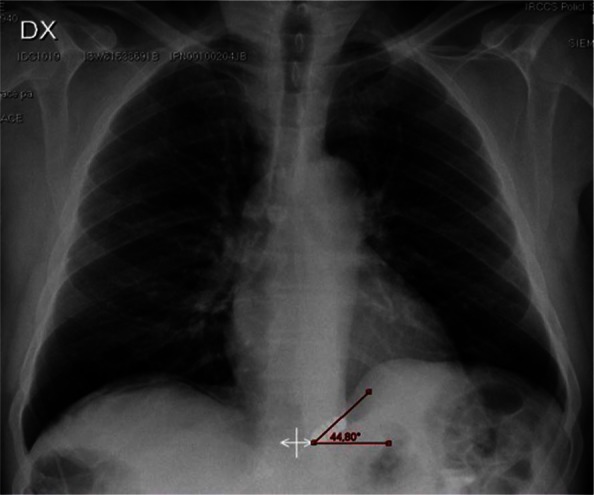
Postoperative chest film confirming the subdiaphragmatic position and the typical inclination angle of the LINX device
The impression of the device was observed at the level of the z line in all patients undergoing upper gastrointestinal endoscopy during the postoperative period. The passage of a standard 9 mm endoscope through the gastroesophageal junction was smooth and no increased resistance was felt at the junction. No mucosal erosions of the device have been reported.
The LES resting pressure increased from 6.5 to 14.6 mmHg (p < 0.005) in the nine patients with a hypotensive LES pressure. No significant changes in pressure occurred in the 23 patients with normal LES pressure at baseline. There were no statistically significant changes in the length of the LES or in the amplitude of esophageal contractions.
Esophageal pH testing was completed in 20 patients at 3 years after surgery. The mean total percentage time pH was less than 4 decreased from a preoperative baseline of 11.9% to 3.8% (p < 0.001). All the other components of the 24 h pH test and the DeMeester composite score were significantly reduced compared with baseline The esophageal acid exposure was normalized in 80% of patients (Figure 5).
Figure 5.
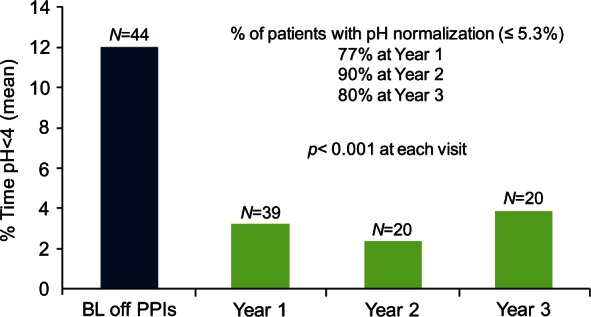
Esophageal acid exposure. Results of sequential esophageal pH monitoring studies up to 3 years of follow up showing significant and durable control of gastroesophageal reflux over time. BL, baseline; PPI, proton-pump inhibitor.
At 4 years, the mean total GERD HRQL score at 4 years or more was 3.3 compared with the baseline score of 25.7 (p < 0.0001); all patients had at least a 50% reduction in the total GERD HRQL score (Figure 6). Interestingly, 87.5% of patients were satisfied with their present condition, and 80% of patients were free from daily dependence on PPIs (Figure 7).
Figure 6.
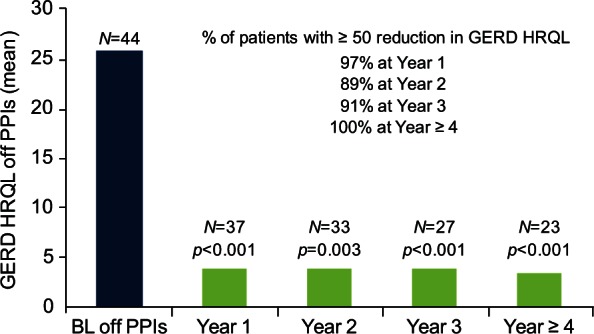
GERD HRQL score off PPIs. The Gastro-Esophageal Reflux Disease Health Related Quality of Life (GERD HRQL) total score significantly decreased and remained stable after the LINX implant. BL, baseline; PPI, proton-pump inhibitor.
Figure 7.
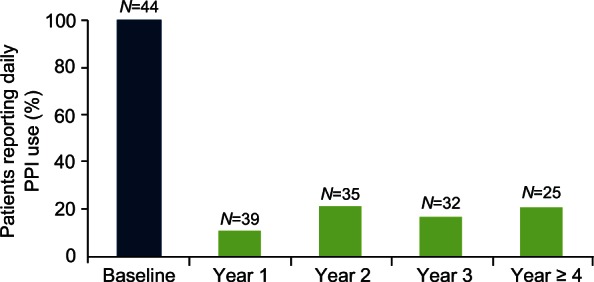
Daily PPI use. The proportion of patients requiring daily use of proton-pump inhibitors (PPIs) significantly decreased and remained stable after the LINX implant.
Forty-three percent of patients complained of mild dysphagia during the postoperative period; in all individuals symptoms resolved by 90 days without treatment. Three patients were explanted: one because of persistent dysphagia, one because of the need to undergo a magnetic resonance imaging study, and one elected to have a Nissen fundoplication for persisting GERD symptoms. Inability to belch or vomit was reported by less than 5% of patients.
Summary of the pivotal trial
The primary inclusion/exclusion criteria and the pre- and postoperative patient evaluation were similar to the feasibility trial. The magnetic device was implanted through a standard laparoscopic approach by academic or community surgeons who had experience with fundoplication. The primary endpoints were the number of patients in whom acid exposure was normalized or was decreased by 50% or more. The secondary endpoints were the number of patients with a reduction of 50% or more in the total score for quality of life, and a reduction of 50% or more in the PPI dose. Endpoint analyses were performed according to the intention-to-treat principle.
The median time required for device implantation was 36 min. No reversions to fundoplication or intraoperative complications occurred. All patients were discharged home within 1 day. The primary endpoint (i.e. normalization or a 50% reduction in esophageal acid exposure) was obtained in 64% of patients (95% confidence interval 54–73). The secondary endpoint (i.e. a 50% reduction in the quality of life score) was obtained in 92% of patients (95% confidence interval 56–96). Finally, 93% of patients reported a 50% or more reduction in the average daily PPI dose. The median quality of life score was significantly decreased at 1 year post implantation and remained stable at 2 and 3 years (p < 0.005). All individual components of pH monitoring significantly improved after surgery (p < 0.001).
Dysphagia was the most frequent adverse event and was reported by 68% of patients. Endoscopic dilatation was performed in 19 patients and was successful in 16. Removal of the device was necessary in six patients because of persistent dysphagia (n = 3), persistent reflux symptoms (n = 1), persistent chest pain (n = 1), and intermittent vomiting (n = 1). The removal procedure was uneventful; in three of the six patients a Nissen fundoplication was performed. There was no evidence of device migration or erosion up to 2 years after implantation.
Future perspectives in the treatment of gastroesophageal reflux disease
The limitations of current therapeutic strategies have left a large proportion of patients with GERD dissatisfied and in an equivocal position, that is, to continue with a lifetime dependence on a medication that does not provide complete symptom relief or to undergo a surgical procedure that requires significant alteration of gastric anatomy, may deteriorate over time, and may have significant side effects. Based on the clinical experience to date, the LINX System represents a new therapeutic option that addresses some of the limitations of existing therapies and may provide a permanent, easily reversible, and more physiologic solution to GERD.
The results of the feasibility and pivotal trials have shown that augmentation of the gastroesophageal junction barrier using the LINX System is highly effective in decreasing esophageal acid exposure, reducing typical GERD symptoms, reducing daily PPI dependence, and improving patients’ quality of life. The LINX System also appears to have a standardized surgical procedure for insertion. Since the first implants, consistent performance and reproducibility have been observed in multiple centers worldwide. The LINX System is also easily reversible. Once healing is complete after the implant, the device is encapsulated in fibrous tissue but is not incorporated into the esophageal wall; this makes removal of the device possible without damage to the esophagus and without compromising future treatment options. Overall, the device has demonstrated a high level of efficacy and has met patients’ expectations, with few side effects or serious adverse events. Notably, safety issues such as device erosions or migrations have not emerged after up to 6 years of follow up at our institution (Bonavina et al. unpublished data).
The potential limitations of the new technique are the lack of long-term results and comparative trials. The procedure also has untested efficacy in the presence of sliding or paraesophageal herniation, short esophagus, and Barrett’s esophagus. Other limitations of this new technology are contraindication to magnetic resonance imaging, and the potential long-term consequences of a permanent foreign body implant.
The LINX System is intended to be used in patients with unsatisfactory response to medical therapy who would not usually be considered candidates for fundoplication because they have early, uncomplicated disease. Considering the significant ‘therapy gap’ that exists between patients currently treated with medical and surgical therapy, and the fact that less than 1% of the GERD population are treated by fundoplication, a less invasive surgical option seems desirable. Importantly, given the standardized laparoscopic procedure required for the implant, the LINX System has the potential to be broadly adopted by the surgical community.
In conclusion, the LINX System remains a promising new method that has the potential to overcome the current limitations of the fundoplication procedures. However, to change the treatment paradigm in GERD, long-term clinical studies are needed to confirm the safety and effectiveness of this innovative technology [Bonavina, 2012; Wilson, 2006].
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: All authors received consulting fees from Torax® Medical, Inc.
Contributor Information
Luigi Bonavina, Division of General Surgery, Department of Biomedical Sciences for Health, University of Milano School of Medicine, via Morandi 30, 20097 San Donato Milanese, Milan, Italy.
Greta Saino, Division of General Surgery, Department of Biomedical Sciences for Health, University of Milano School of Medicine, Milan, Italy.
John C. Lipham, Department of Surgery, University of Southern California, Los Angeles, CA, USA
Tom R. DeMeester, Department of Surgery, University of Southern California, Los Angeles, CA, USA
References
- Becher A., El-Serag H. (2011) Systematic review: the association between symptomatic response to proton pump inhibitors and health-related quality of life in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther 34: 618–627 [DOI] [PubMed] [Google Scholar]
- Bonavina L. (2012) Assessment of surgical innovation. Innovation in Esophageal Surgery. Berlin: Springer [Google Scholar]
- Bonavina L. (2013) Selected commentary to “Esophageal sphincter device for gastroesophageal reflux disease”. Eur Surg. DOI: 10.1007/s10353-013-0206-z [DOI] [Google Scholar]
- Bonavina L., DeMeester T., Fockens P., Dunn D., Saino G., Bona D., et al. (2010) Laparoscopic sphincter augmentation device eliminates reflux symptoms and normalizes esophageal acid exposure: one- and 2-year results of a feasibility trial. Ann Surg 252: 857–862 [DOI] [PubMed] [Google Scholar]
- Bonavina L., Saino G., Bona D., Lipham J., Ganz R., Dunn D., et al. (2008) Magnetic augmentation of the lower esophageal sphincter: results of a feasibility clinical trial. J Gastrointest Surg 12: 2133–2140 [DOI] [PubMed] [Google Scholar]
- Dent J., El-Serag H., Wallander M., Johansson S. (2005) Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 54: 710–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R., Sifrim D. (2009) Management of heartburn not responding to proton pump inhibitors. Gut 58: 295–309 [DOI] [PubMed] [Google Scholar]
- Finks J., Wei Y., Birkmeyer J. (2006) The rise and fall of antireflux surgery in the United States. Surg Endosc 20: 1698–1701 [DOI] [PubMed] [Google Scholar]
- Galmiche J., Hatlebakk J., Attwood S., Ell C., Fiocca R., Eklund S., et al. (2011) Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA 305: 1969–1977 [DOI] [PubMed] [Google Scholar]
- Ganz R., Peters J., Horgan S., Bemelman W., Dunst C., Edmundowicz S., et al. (2013) Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med 368: 719–727 [DOI] [PubMed] [Google Scholar]
- Heidelbaugh J., Kim A., Chang R., Walker P. (2012) Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol 5: 219–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter J., Smith C., Branum G., Waring J., Trus T., Cornwell M., et al. (1999) Laparoscopic fundoplication failures: patterns of failure and response to fundoplication revision. Ann Surg 230: 595–604; discussion 604–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe B., Wallace J., Hansen P., Swanstrom L. (1997) Evaluation of laparoscopic Toupet fundoplication as a primary repair for all patients with medically resistant gastroesophageal reflux. Surg Endosc 11: 1080–1083 [DOI] [PubMed] [Google Scholar]
- Katz P., Gerson L., Vela M. (2012) Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 108: 308–328 [DOI] [PubMed] [Google Scholar]
- Lipham J., DeMeester T., Ganz R., Bonavina L., Saino G., Dunn D., et al. (2012) The LINX(R) reflux management system: confirmed safety and efficacy now at 4 years. Surg Endosc 26: 2944–2949 [DOI] [PubMed] [Google Scholar]
- Lord R., DeMeester S., Peters J., Hagen J., Elyssnia D., Sheth C., et al. (2009) Hiatal hernia, lower esophageal sphincter incompetence, and effectiveness of Nissen fundoplication in the spectrum of gastroesophageal reflux disease. J Gastrointest Surg 13: 602–610 [DOI] [PubMed] [Google Scholar]
- Malfertheiner P., Nocon M., Vieth M., Stolte M., Jaspersen D., Koelz H., et al. (2012) Evolution of gastro-oesophageal reflux disease over 5 years under routine medical care – the ProGERD study. Aliment Pharmacol Ther 35: 154–164 [DOI] [PubMed] [Google Scholar]
- McColl K., Gillen D. (2009) Evidence that proton-pump inhibitor therapy induces the symptoms it is used to treat. Gastroenterology 137: 20–22 [DOI] [PubMed] [Google Scholar]
- Niebisch S., Fleming F., Galey K., Wilshire C., Jones C., Litle V., et al. (2012) Perioperative risk of laparoscopic fundoplication: safer than previously reported – analysis of the American College of Surgeons National Surgical Quality Improvement Program 2005 to 2009. J Am Coll Surg 215: 61–68 [DOI] [PubMed] [Google Scholar]
- Richter J., Dempsey D. (2008) Laparoscopic antireflux surgery: key to success in the community setting. Am J Gastroenterol 103: 289–291 [DOI] [PubMed] [Google Scholar]
- Shaheen N., Hansen R., Morgan D., Gangarosa L., Ringel Y., Thiny M., et al. (2006) The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol 101: 2128–2138 [DOI] [PubMed] [Google Scholar]
- Thijssen A., Broeders I., de Wit G., Draaisma W. (2011) Cost-effectiveness of proton pump inhibitors versus laparoscopic Nissen fundoplication for patients with gastroesophageal reflux disease: a systematic review of the literature. Surg Endosc 25: 3127–3134 [DOI] [PubMed] [Google Scholar]
- Vakil N., Shaw M., Kirby R. (2003) Clinical effectiveness of laparoscopic fundoplication in a US community. Am J Med 114: 1–5 [DOI] [PubMed] [Google Scholar]
- Vakil N., van Zanten S., Kahrilas P., Dent J., Jones R. (2006) The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 101: 1900–1920; quiz 1943. [DOI] [PubMed] [Google Scholar]
- Velanovich V. (1998) Comparison of generic (SF-36) vs. disease-specific (GERD-HRQL) quality-of-life scales for gastroesophageal reflux disease. J Gastrointest Surg 2: 141–145 [DOI] [PubMed] [Google Scholar]
- Wileman S., McCann S., Grant A., Krukowski Z., Bruce J. (2010) Medical versus surgical management for gastro-oesophageal reflux disease (GORD) in adults. Cochrane Database Syst Rev (3): CD003243. [DOI] [PubMed] [Google Scholar]
- Wilson C. (2006) Adoption of new surgical technology. BMJ 332: 112–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbib F., Sifrim D., Tutuian R., Attwood S., Lundell L. (2013) Modern medical and surgical management of difficult-to-treat GORD. United Eur Gastroenterol J 1: 21–31 [DOI] [PMC free article] [PubMed] [Google Scholar]