SUMMARY
FGF21, a member of the fibroblast growth factor (FGF) superfamily has recently emerged as a novel regulator of metabolism and energy utilization. However, the exact mechanism(s) whereby FGF21 mediates its actions have not been elucidated. There is considerable evidence that insulin resistance may arise from aberrant accumulation of intracellular lipids in insulin responsive tissues due to lipotoxicity. In particular the sphingolipid ceramide has been implicated in this process. Here, we show that FGF21 rapidly and robustly stimulates adiponectin secretion in rodents, while diminishing accumulation of ceramides in obese animals. Importantly, adiponectin knockout mice are refractory to changes in energy expenditure and ceramide-lowering effects evoked by FGF21 administration. Moreover, FGF21 lowers blood glucose levels and enhances insulin sensitivity in diabetic Lepob/ob mice and diet-induced obese (DIO) mice, only when adiponectin is functionally present. Collectively, these data suggest that FGF21 is a potent regulator of adiponectin secretion, and that FGF21 critically depends on adiponectin to exert its glycemic and insulin sensitizing effects.
Keywords: ceramide, insulin resistance, obesity
FGF21 has garnered increasing attention for its abilities to lower blood glucose and circulating lipid concentrations, and reduce body weight via ameliorated adiposity (Coskun et al., 2008; Kharitonenkov and Larsen, 2011; Kharitonenkov et al., 2005). Endogenous FGF21 is induced physiologically by fasting (Adams and Kharitonenkov, 2012) and feeding of ketogenic diets (Badman et al., 2007), or pharmacologically through the use of PPARα, PPARγ or FXR agonists, which promote its production in liver and adipose tissue (Potthoff et al., 2012). Administration of recombinant FGF21 to cultured cells (Kharitonenkov et al., 2005), rodents (Berglund et al., 2009; Coskun et al., 2008) and primates (Kharitonenkov et al., 2007) improves glucose metabolism. However, the mechanisms governing the metabolic improvements elicited by FGF21 are not completely understood.
FGF21, like the other “endocrine” FGF sub family members FGF19 and FGF23, binds to its receptor in a heparin-sulfate-independent manner, a feature which separates these molecules from the canonical FGF’s (Itoh, 2010). In place of heparin, FGF19 and FGF21 utilize a signaling complex consisting of an activity competent FGF receptor (FGFR) and the scaffold protein β Klotho (KLB) (Kharitonenkov et al., 2008). KLB is predominantly expressed in metabolically active organs such as adipose tissue, liver, and pancreas; making these the primary sites of FGF21 action (Adams et al., 2012b). Emerging evidence suggests that adipose tissue-derived secretory factors are likely to be critical drivers of the metabolic improvements elicited by FGF21. Adipose-specific elimination of FGFR1 has been shown to completely abrogate all of the hypoglycemic, insulin lowering and triglyceride lowering effects of FGF21, but not FGF19 (Adams et al., 2012c). While complete ablation of KLB abrogates the entire spectrum of FGF21 activity in vivo (Adams et al., 2012a), adipose tissue-specific ablation of KLB prevents many, however not all of the improvements of FGF21 (Ding, 2012). Collectively, these novel findings suggest that FGF21 exerts profound effects on energy expenditure and whole-body glucose metabolism largely through activation of the FGFR1/KLB complex in adipose tissue.
Adiponectin (Adn) and leptin are both prominent adipokines which may facilitate responses to FGF21. Adiponectin potently opposes hyperglycemia, insulin resistance, inflammation, and lipotoxic damage (Turer and Scherer, 2012). Here, we address the role of adiponectin as a potential mediator of the metabolic effects of FGF21.
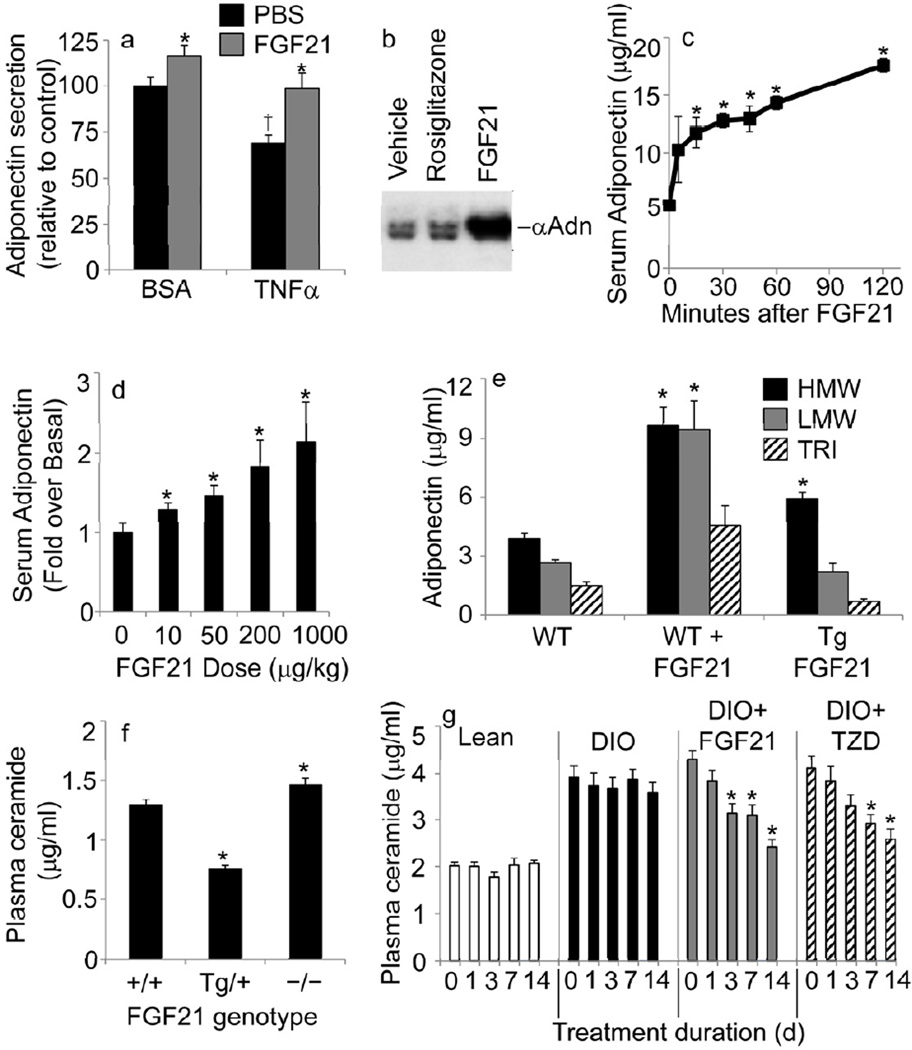
To expand upon our recent observation that circulating adiponectin levels are elevated following chronic FGF21 administration (Adams et al, 2012c), we utilized 3T3-L1 adipocytes to determine whether the effect could be observed in a cell-autonomous manner. Modest, albeit significant increases in adiponectin were detected from the media of cultured 3T3-L1 adipocytes following FGF21 treatment (p<0.05); furthermore, FGF21 potently prevented TNFα from impairing adiponectin secretion (Fig 1a). In contrast, incubation of FGF21 with primary murine adipocytes strongly upregulated adiponectin secretion into medium, and was even more potent than the PPARγ agonist rosiglitazone (Fig 1b). We further evaluated the kinetics required for a single bolus of FGF21 to increase circulating adiponectin levels in vivo. We were surprised to discover that adiponectin levels began to rise within 5 minutes of treatment and were significantly elevated within 15 minutes (Fig 1c). Increased levels persisted for at least 2 hours after acute treatment with FGF21 and remained 2.76-fold higher (p<0.05) than vehicle treated mice after 2 weeks of FGF21 administration, through continuous subcutaneous infusion via osmotic minipumps (sup Fig 1a). Dose-response studies were performed with a single bolus injection of FGF21 (Fig 1d), which produced significant increases in serum adiponectin with as little as a 46% increase in circulating FGF21 (Sup Fig 1b). Thus, recombinant FGF21 can stimulate a rapid, robust, and prolonged release of adiponectin both in vitro in cultured adipocytes and in vivo in rodents.
Figure 1.
FGF21 promotes adiponectin secretion and diminishes ceramide accumulation in serum. Results are mean ± SEM Asterisks indicate p< 0.05 for FGF21 effects. Dagger denotes p<0.05 for TNFα effect. (a) Adiponectin from media conditioned for 2 h after L1 adipocytes were cultured for 16 hours with TNFα (10 ng/mL) or BSA control, in the presence of FGF21 (100 ng/mL, grey bars) or PBS (black bars). n=8 from separate experiments (b) Primary murine adipocytes were isolated and equal aliquots were cultured for 4 hours with the TZD rosiglitazone (1 nM) or FGF21 (100 ng/mL). Conditioned media was collected and subjected to SDS-PAGE and western blots were performed with antibodies to Adiponectin (αAdn). Data are representative of 5 independent experiments from separate animals. (c) Serum adiponectin was measured from sera collected at indicated time-points after injection of FGF21 (1 mg/kg, IP) into WT mice. (d) WT mice were injected with indicated doses of human FGF21. Adiponectin was measured by ELISA on serum samples obtained before or after FGF21 treatment, and the change in adiponectin was calculated (n=5–6 per group). (e) HMW (black bars), LMW (grey bars) and trimeric (hatched bars) adiponectin from WT mice, WT mice 2-hours post-FGF21 treatment (1mg/kg, IP), or transgenic (Tg) FGF21 over-expressing mice (n=5/group). (f) Plasma ceramides from WT, FGF21 overexpressing mice, and FGF21 knockout mice (n=5/group). (g) Plasma ceramides from lean (white bars) or DIO mice after treatment with vehicle (black bars), FGF21 (1mg/kg/day, grey bars), or rosiglitazone (10 mg/kg/day, hatched bars) (n=5/group).
Adiponectin is an adipocyte derived protein known to circulate in a combination of three forms: trimers, low-molecular weight (LMW) multimers, and high-molecular weight (HMW) oligomers. Adiponectin expression is enhanced by PPARγ agonists and is essential for these agonists to reach their optimal anti-diabetic effects (Kubota et al., 2006; Nawrocki et al., 2006). The HMW form of the protein is the best biomarker for clinical efficacy of TZDs (Combs et al., 2002). Although our FGF21 transgenic mice (Kharitonenkov et al., 2005)only show a 10% increase in total circulating adiponectin (p<0.05), they exhibit more striking elevation of HMW adiponectin levels; while in WT animals, administration of recombinant FGF21 increases both HMW and LMW adiponectin (Fig. 1f). These findings corroborate recent reports that adiponectin is elevated in FGF21 transgenic mice from other groups (Ding, 2012). When compared to WT mice our FGF21−/− animals (Badman et al., 2007)on a high fat diet show significantly impaired adiponectin production (3.71±0.26mg/ml vs. 2.15±0.19mg/mL, p<0.05). Thus, FGF21 is likely critical for maintaining basal adiponectin levels, particularly during obesity.
Our previous work suggests that adiponectin exerts its numerous biological effects, in part, by enhancing the deacylation of the sphingolipid ceramide, a lipotoxic metabolite stimulated primarily by excess exposure to saturated fats or inflammatory mediators (Holland et al., 2011). With adiponectin potentially playing a critical role in FGF21-mediated glucose lowering effects, we subsequently evaluated circulating ceramide levels in FGF21 transgenic and knockout mice which have been described previously (Kharitonenkov et al., 2005) (Badman et al., 2007). FGF21 over-expressing mice display lower concentrations of circulating ceramide, while FGF21 knockout animals display mild but significant (p<0.05) increases in circulating ceramide levels (Fig. 1g).
Consistent with the results obtained with genetic mouse models, circulating ceramide levels were diminished in WT mice following treatment with recombinant FGF21 in a time dependent manner (Fig 1g). Circulating ceramide concentrations were approximately twice as high in DIO mice, and remained unchanged by vehicle treatment. Three days of FGF21 administration to DIO mice significantly decreased ceramide levels (p<0.05) and completely normalized them after 2 weeks of treatment. The PPARγ agonist rosiglitazone is a known stimulator of FGF21 production and adiponectin secretion. We and others have previously established adiponectin secretion (Kubota et al., 2006; Nawrocki et al., 2006) is essential for normal anti-diabetic responses to TZDs. It has been recently demonstrated that FGF21−/− mice fail to exhibit the anti-glycemic effects of TZD treatment, a defect linked to blunted adiponectin production (Dutchak et al., 2012); however, another FGF21 knockout line, utilized in this study (Badman et al., 2007) maintains a normal response to TZD administration (Adams and Kharitonenkov, unpublished observations). We show here that rosiglitazone effectively achieved ceramide lowering after 7 and 14 days of treatment. These data suggest that FGF21 abates ceramide accumulation, which is a key biomarker of adiponectin action. All together, these data suggest that FGF21, adiponectin, and ceramides may lie in a collinear pathway, with TZDs evoking important, yet unresolved roles in this process.
We find dramatic effects of FGF21 on adiponectin production and release, both acutely as well as in the chronic setting. Effects of such magnitude on adiponectin secretion have not been observed previously. It is difficult to induce acute enhancements of adiponectin release in adipocytes, and the effects reported in the chronic setting are on par with the fold-stimulation for prolonged TZD treatment (Nawrocki et al., 2006). We and others have demonstrated that adiponectin is essential for the full metabolic improvements induced by TZDs, as Adn−/− mice show minimal improvements to standard doses of rosiglitazone (Kubota et al., 2006; Nawrocki et al., 2006).
Prior to embarking on studies with adiponectin null mice, we sought to ensure that Adn−/− mice have all components of the FGF21-specific receptor machinery, FGFRs and KLB, and exhibit a normal signaling response to FGF21 (Kharitonenkov and Larsen, 2011). Expression of all four major FGF receptors and KLB were not significantly altered in liver or adipose by adiponectin ablation (Sup Fig 2a). Furthermore, FGF21-induced transcription of the immediate early genes EGR1 (Sup Fig 2b–c) and cFos (Sup Fig 2d –e) remained intact in adipose and liver of Adn−/− mice. Finally, FGF21 levels are normal during fed and fasted states in Adn−/− mice (Sup Fig 3). Thus, Adn−/− mice maintain essential components of FGF21 signaling in both adipose and liver and are able to respond in a normal acute fashion to FGF21 administration at the signaling level.
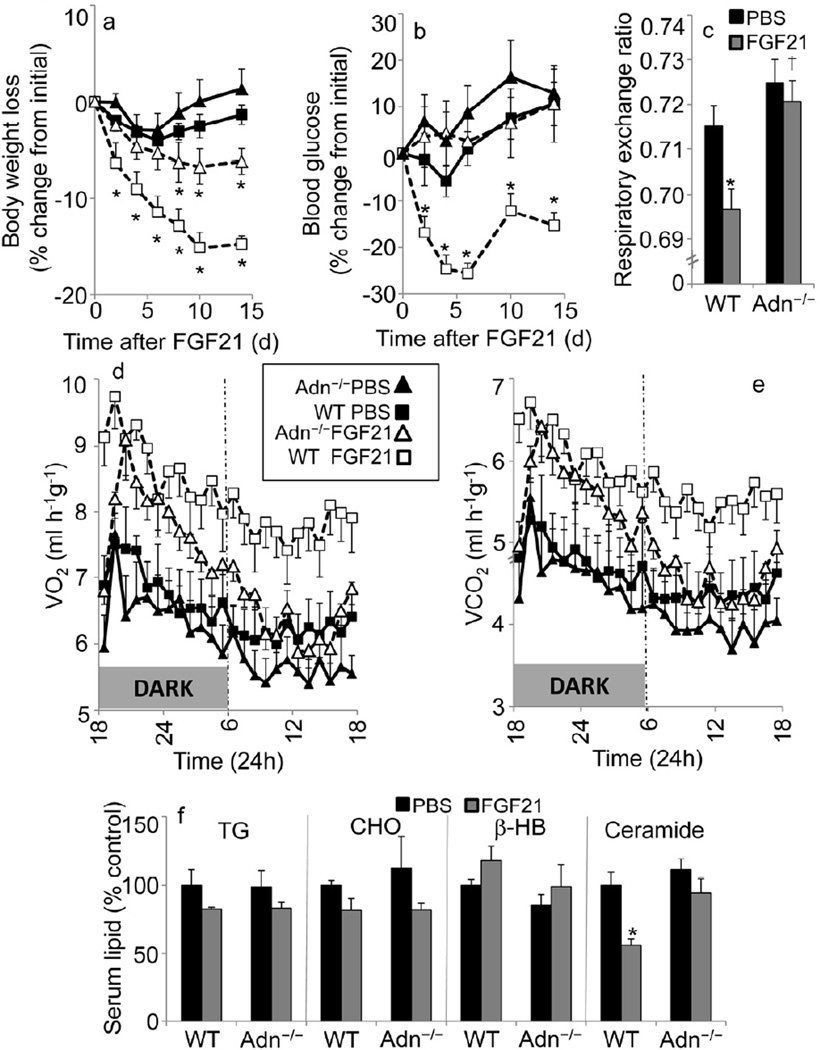
To evaluate the role of adiponectin in FGF21-mediated metabolic improvements, we treated wild-type (WT) or Adn−/− mice chronically with FGF21 via mini-osmotic pumps after they were maintained on a high-fat diet for 6 weeks to promote diet induced obesity. FGF21 treatment produced similar elevations in circulating FGF21 (25.22 ± 2.97ng/mL in wt, 24.26±4.63 ng/mL in Adn−/− mice). Consistent with the established effects of FGF21 on energy expenditure (Coskun et al., 2008), WT mice receiving FGF21 display potent decreases in body weight (Fig. 2a). Although FGF21 still promoted weight loss in Adn−/− mice, they lost significantly less body weight than WT mice (p<0.01). NMR analysis of body composition revealed a 39% reduction of fat mass in WT mice (p<0.005), whereas Adn−/− mice only lost 6.7% (p=0.52) of their fat mass during the first week of FGF21 treatment (data not shown). Analysis of glucose levels during chronic administration of FGF21 revealed a substantial lowering of serum glucose only in WT mice (Fig. 2b), with a complete failure to lower blood glucose in Adn−/− mice.
Figure 2.
Adiponectin critically regulates improvements in glucose homeostasis, but not body weight regulation in DIO mice. Results are mean ± SEM. Asterisks indicate p< 0.05 for FGF21 effects. Dagger denotes p<0.05 for Adn−/− vs. WT of same treatment. Body weight (a) and blood glucose (b) from WT (squares) or adiponectin knockout (triangles) treated with FGF21 (0.3 mg/kg/d, open shapes/dashed lines) or PBS (filled shapes/solid lines). (n=8/group) (c) Average RER during the night from WT and Adn−/− mice treated with PBS (black) or FGF21 (grey). Oxygen consumption (d), and carbon dioxide production (e) from WT (squares) or Adn−/− mice (triangles) treated with FGF21 (0.3 mg/kg/d, open shapes/dashed lines) or PBS (filled shapes/solid lines). (n=8/group) (f) Serum lipid concentrations were determined and expressed as a percentage of the PBS/WT controls [control values: 80.67±8.88 mg/dL Triglyceride (TG); 160.16± 5.49 mg/dL Cholesterol (CHO); 4.20±0.17 mg/dL β-hydroxybutyrate (β-HB); 1.64±0.15 µg/mL ceramide] following treatments with FGF21 (0.3 mg/kg/d, grey) or PBS (black) by mini-pump (n=6 per group).
Identically treated cohorts were evaluated in metabolic cages from days 2–6 after implanting mini pumps to determine energy expenditure effects. In response to FGF21, WT mice displayed the expected decreases in respiratory exchange ratio (RER) (Fig. 2c) and increases in energy expenditure, as measured by oxygen (O2) consumption (Fig. 2d) and carbon dioxide (CO2) production (Fig. 2e). The downward shift in RER, reflecting a slight increase in lipid oxidation for energy production, was not evident in Adn−/− mice. Moreover, Adn−/− animals were partially refractory to increases in energy expenditure; however their responsiveness was completely dependent on the light cycle. Although O2 consumption and CO2 production were fully enhanced by FGF21 at night (p<0.05), energy expenditure was not elevated during the light phase or on average throughout the entire day in Adn−/− mice.
We additionally analyzed profiles of several circulating lipid classes (Fig. 2f). Importantly, various anticipated effects of FGF21 on serum lipids occurred independently of adiponectin. FGF21 promoted the expected decreases in circulating triglyceride (TG) and cholesterol (CHO) in serum of either genetic group. The ketone β-hydroxybutyrate was elevated by FGF21 in both groups. Serum ceramides were diminished after 2 weeks of FGF21 treatment in WT mice (p<0.05, Sup Table 1), however ceramides were not significantly altered in adiponectin deficient mice (p<0.19). For this study, serum ceramides only showed trends towards elevatation in Adn−/− mice unlike what we saw in other studies (Fig 3 and (Holland et al., 2011). This is likely due to the surgical manipulations with the minipumps that led to a slight temporary weight loss.
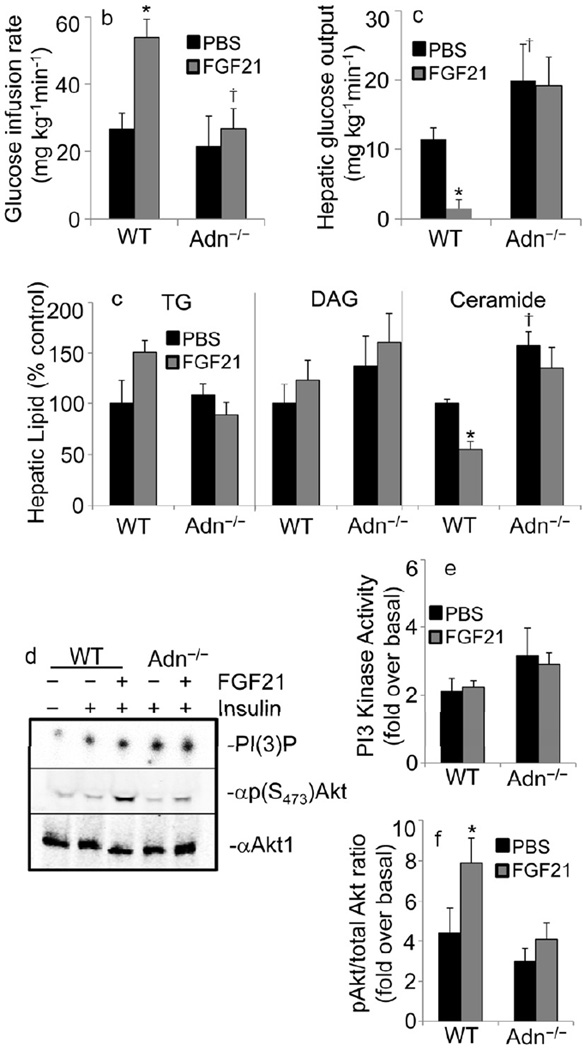
Figure 3.
Adiponectin is essential for enhanced insulin action after acute administration of FGF21. After 6-weeks of high fat diet, WT and Adn−/− mice received chronic indwelling jugular catheters and were allowed to recover to presurgical weight. FGF21 (1µg/kg/min, iv, grey bars) or PBS (black bars) was infused for 90 minutes prior to initiating hyperinsulinemia and throughout the duration of the experiment (n=4–5 per group). (a-b) Hyperinsulinemic euglycemic clamps were performed on conscious unrestrained mice a) The glucose infusion rate required to maintain euglycemia was determined and (b) the rate of glucose efflux from the liver was calculated from kinetic measurements of 3H-glucose turnover. (c) Lipids were extracted from livers obtained from mice following experiments and triglyceride (TG), diacylglycerol (DAG), and ceramide concentrations were quantified and expressed as a percentage of the PBS/WT controls (control values: 108.4±25.3 mg/g TG; 867.7±168.0 nmol/g DAG; 172.0±7.8 nmol/g Ceramide). (d-f) Hyperinsulinemic clamps were initiated for 15 minutes and tissues were snap frozen for analysis of insulin signaling intermediates. (d) Representative samples of 32P-labeled 3,4,5-trisphosphoinositol [PI(3)P] from IRS2-associated PI3 kinase assays (Top), and immunoblots against phosphorylated (serine 473) Akt and total Akt1. (e) Insulin-stimulated activation of IRS2-associated PI3 kinase activity was quantified and graphed as fold stimulation over non-insulin treated samples. (f) Insulin-stimulated phosphorylation of Akt kinase was quantified and normalized to total Akt. Data are graphed as fold stimulation over non-insulin treated samples. Results are mean ± SEM. Asterisks indicate p< 0.05 for FGF21 effects. Dagger denotes p<0.05 for Adn−/− vs. WT of same treatment.
Body mass was still significantly decreased in Adn−/− mice, which could account for the trend toward the decrease in ceramides in Adn−/− mice. Such changes in body weight also obscure interpretations of insulin sensitivity tests, as body mass profoundly effects insulin sensitivity. Given these limitations, we opted to analyze insulin action after acute administration of FGF21, which prevents the confounding effect of differences in body weight. To evaluate insulin sensitivity, hyperinsulinemic-euglycemic clamps were performed on conscious unrestrained WT or Adn−/− mice. Intravenous infusion of FGF21 into wild-type DIO mice substantially improved whole body insulin sensitivity, as measured by the amount of glucose required to maintain euglycemia (Fig. 3a). Serum adiponectin concentrations doubled (p<0.05) in response to FGF21 (18.85±3.08 µg/mL) as compared to PBS infused wild-type mice (9.75±1.60 µg/mL). Consistent with our established clamp data from adiponectin transgenic studies (Combs et al., 2001; Holland et al., 2011) and previous clamp experiments with FGF21 infusions (Berglund et al., 2009), all of the improvements could be accounted for by suppression of hepatic glucose efflux (Fig. 3b). FGF21 failed to improve insulin action in DIO Adn−/− mice on the whole body or the liver. These results could not be explained by differences in circulating hFGF21 levels (21.98±.35ng/mL in WT, 20.72±0.73 ng/mL in Adn−/−), blood glucose, or serum insulin in the clamped state. To evaluate lipid intermediates linked with insulin resistance, we assessed triglyceride (TG), diacylglycerol (DAG), and ceramide levels from livers obtained from these acute FGF21 experiments (Fig. 3c). While TG and DAG were not significantly altered by this short treatment with FGF21, hepatic ceramides were significantly lower in WT mice. Adiponectin null mice did not display the same FGF21-induced effects on hepatic ceramides.
To assess insulin signaling in this acute setting, tissues were harvested 15 minutes after initiating clamps in a separate cohort of animals. We evaluated insulin-stimulated PI3-kinase activity and Akt (PKB) kinase phosphorylation, two essential components of insulin signal transduction (Fig. 3d). In cultured cells, ceramides impair insulin signaling to Akt, while PI3- kinase activation remains intact. Consistent with these findings, FGF21 enhanced insulin-stimulated phosphorylation of Akt (Fig. 3e) in liver of WT mice, but not Adn−/− mice. Insulin-stimulated activation of IRS2-associated PI3-kinase activation was not enhanced by FGF21 in either genetic group (Fig 3f). Changes in ceramide species (Sup Table 1) were consistent with previous changes noted to correlate with insulin sensitivity in adiponectin transgenic mice and in human studies (Haus et al., 2009; Holland et al., 2011; Lopez et al., In Press). Collectively, these data from DIO mice suggest that FGF21 promotes improvements in hepatic glucose output and whole body glucose homeostasis via mechanisms that require adiponectin. However, changes in energy expenditure and body mass are partially independent of adiponectin, and vary greatly with circadian rhythms.
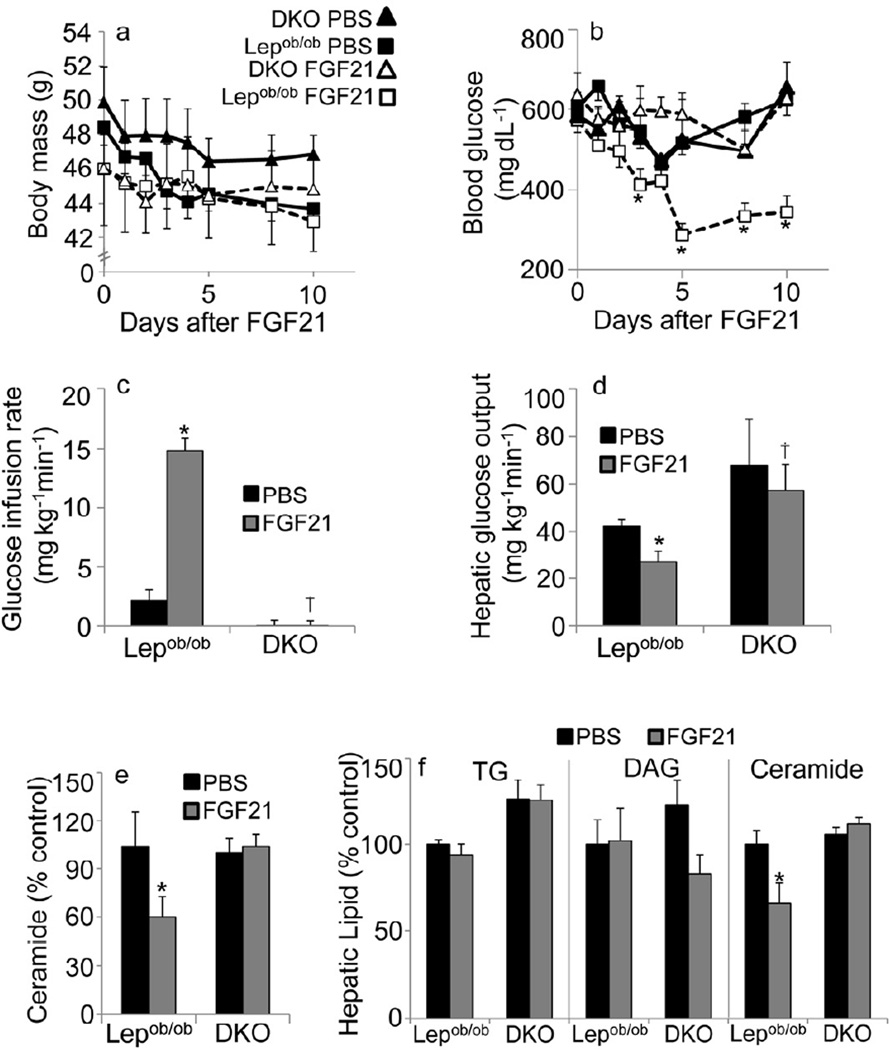
Although leptin deficiency promotes elevation in circulating FGF21 levels, Lepob/ob mice maintain exquisite sensitivity to the glucose lowering effects of recombinant FGF21. Our data suggest that at the low doses utilized for our chronic studies (0.3 mg/kg/d), FGF21 does not cause significant decreases in body weight in Lepob/ob mice, consistent with previous reports in this strain (Fig 4a) (Adams et al., 2012b). Despite the lack of change in body weight in Lepob/ob mice, they still display robust improvements in blood glucose concentrations in response to FGF21 (Fig 4b). To gauge the contribution of adiponectin to FGF21-mediated improvements in glucose homeostasis in a diabetic model, we subsequently crossed our Adn−/− mice with Lepob/ob mice on an FVB genetic background. These leptin/adiponectin double knockout mice (DKO) showed similar blood glucose concentrations and similar fasted FGF21 levels to Lepob/ob mice prior to treatments (Sup Fig. 3b). FGF21 levels were higher in DKO mice during the fed state. As with leptin-only knockouts, FGF21 did not promote significant weight loss in DKO mice. Unlike Lepob/ob mice, DKOs were completely refractory to the improvements in blood glucose caused by FGF21 (Fig 4b).
Figure 4.
Adiponectin critically regulates improvements in glucose homeostasis in Lepob/ob mice. Results are mean ± SEM. Asterisks indicate p< 0.05 for FGF21 effects. (a) Changes in body weight and (b) blood glucose during infusion of FGF21 (0.3 mg/kg/d, dashed lines, open shapes) or PBS (solid lines, filled shapes) by subcutaneous mini-pumps into Lepob/ob mice (squares) or Lepob/ob -Adn−/− mice (DKO, triangles). (n=4/group) (c-d) Hyperinsulinemic euglycemic clamps were performed during acute infusion of FGF21 (1µg/kg/min, iv, grey bars) or PBS (black bars). (c) The glucose infusion rate required to maintain euglycemia and (d) the rate of glucose efflux from the liver was determined. (n=4/group) (e) Liver ceramide content after 10-day treatment with FGF21 (grey bars) or PBS (black bars).sphingosine-2 (f) Lipids were extracted from livers obtained from mice following acute administration of FGF21 (clamp experiments) and triglyceride (TG), diacylglycerol (DAG), and ceramide concentrations were quantified and expressed as a percentage of the PBS/ Lepob/ob controls (control values: 100.8±2.8 mg/g TG; 1347.9±192.8 nmol/g DAG; 174.6±10.7 nmol/g Ceramide) (n=4/group).
Adiponectin secretion is repressed in the context of obesity. However, upon further elevation of FGF21 with intravenous infusion into Lepob/ob mice, circulating adiponectin concentrations were significantly increased (4.53±0.89 µg/mL for PBS, 12.27±1.77 µg/mL for FGF21, p<0.01). This substantially improved whole body insulin sensitivity, as measured by the amount of glucose required to maintain euglycemia (Fig 4c); all of the improvements could be accounted for by suppression of hepatic glucose efflux (Fig 4d). Strikingly, FGF21 completely failed to improve measures of whole body or hepatic insulin sensitivity in DKO mice, although similar levels of FGF21 (22.09±0.27 ng/mL for Lepob/ob ; 22.29±0.35 ng/mL for DKO) were achieved in the circulation of both genetic groups infused with FGF21. These results could not be explained by differences in blood glucose or serum insulin in the clamped state. As a measure of adiponectin action, we measured hepatic ceramides following 10-days of FGF21 treatment (Fig. 4e). Ceramides were significantly lowered in the liver by FGF21 in Lepob/ob mice, but not DKO mice. We also measured hepatic lipids following acute FGF21 treatments (Fig. 4f). These 3-hour FGF21 infusions were sufficient to lower hepatic ceramides; however, Triglyceride and diacylglycerol were not significantly affected. While it is curious that adiponectin secretion and FGF21 levels are dissociated in obesity, we think this is yet another example of hormonal resistance that occurs during metabolic dysfunction due to combinations of hypoxia, ER stress and inflammation.
Collectively, these data from Lepob/ob mice suggest that adiponectin is essential for FGF21 to enhance suppression of hepatic glucose efflux and promote whole body glucose homeostasis. The results from clamps in Lepob/ob mice and DIO mice are consistent with previous studies with adiponectin alterations. Hyperinsulinemic clamps in Adn transgenic mice (Combs et al., 2004), Adn−/− mice (Nawrocki et al., 2006), or wild-type mice treated with full-length Adn (Holland et al., 2011), uniformly suggest the liver as the primary site of enhanced insulin action evoked by the intact protein. Similarly, recombinant FGF21 has been show to aid in the opposition of glucose efflux in Lepob/ob (Berglund et al., 2009), lean (Xu et al., 2009), or DIO mice (Xu et al., 2009).
In summary, we report data that strongly suggests that enhanced secretion of adiponectin is critical in facilitating many of the metabolic improvements elicited by FGF21, including FGF21 mediated effects on ceramide metabolism that we demonstrate here for the first time.
Methods
Animal Care
Mice were maintained on a 12 h dark/light cycle and fed a normal chow diet. Unless otherwise indicated, all mice were 14–16 weeks old at the time of experiments. Mice were bred in-house in the University of Texas Southwestern Medical Center and Lilly animal facilities. The HFD consisted of 60 % (kCal) from fat (Research Diets, D12492). Lepob/ob were on an FVB background to allow for uniform development of hyperglycemia; all other mice were on C57Bl6/J backgrounds. The Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center and Lilly and Company approved all animal experimental protocols.
Animal Surgery
Anesthesia was accomplished by 2% isofluorane. For clamps, silicone catheters were aseptically placed in the jugular vein. Rimadyl (5 mg/Kg, sc) was administered for pain control and animals were allowed to recover (4–5 days) to preoperative weight prior to experiments. For chronic infusions, Alzet mini-osmotic pumps were asceptically filled, primed, and asceptically placed subcutaneously in the mid-back area.
Hyperinsulinemic Euglycemic Clamps
Clamps were performed in conscious unrestrained animals as previously described (Berglund et al., 2009; Berglund et al., 2012; Holland et al., 2011). Clamps in DIO mice were initiated by primed continuous infusion of insulin (4mU/kg/min) and glucose was maintained constant at ~150 mg/dL during the clamped state via variable infusion of dextrose (50%). Clamps in Lepob/ob mice were initiated by primed continuous infusion of insulin (10mU/kg/min) and glucose was maintained constant at ~250 mg/dL during the clamped state. FGF21 or PBS was initiated with the onset of 3H-glucose tracer infusion (5 μCi bolus + 0.05 μCi/min) to allow for a 90 minute-pretreatment. Continuous infusion of FGF21 was maintained after initiating insulin. [3-3H]glucose was increased to 0.1 μCi/min at the onset of insulin delivery to account for changes in specific activity. Glucose turnover was determined and hepatic glucose output was calculated as whole body glucose disposal less glucose infusion rate as previously described (Hill et al., 2010). To generate samples for the analysis of insulin signaling intermediates FGF21 or PBS was infused identically, and insulin stimulation was allowed to proceed for 15 minutes prior to sacrifice and rapid freezing of tissues.
Energy expenditure
Metabolic measurements were obtained continuously using TSE metabolic chambers (TSE Labmaster System, Germany) in an open circuit indirect calorimetry system. Studies were performed from days 4–6 after implanting osmotic pumps. Data were normalized to lean body mass as determined using a Bruker MQ10 NMR analyzer.
Lipid and analyte quantification
Sphingolipid were quantified as described previously by LC/ESI/MS/MS using a TSQ Quantum Ultra-triple quadrupole mass spectrometer (Thermo Fisher) equipped with an electrospray ionization (ESI) probe and interfaced with an Agilent 1100 HPLC (Agilent Technologies) (Holland et al., 2011; Li et al., 2009). Glucose was measured by Alpha Trak glucometer (Abbott Labs). Insulin and Adiponectin were quantified by ELISA (Millipore). Adiponectin distribution was measured as previously described (Schraw et al., 2008). To prevent contamination of MS equipment with 3H-containing samples, ceramides and diacyglycerols were measured on samples obtained following hyperinsulinemic euglycemic clamps using established enzymatic methods (Perry et al., 2000).
Analysis of insulin signaling intermediates
PI3 kinase assays were performed after immunoprecipitation with antibodies against IRS2 (Cell Signaling L1326) and carried out as previously described (Wang and Summers, 2003). Immunoblotting for pAkt (Ser473, 4060) and total Akt (2920) (Cell Signaling Technology, Inc.) was performed as previously described (Kusminski et al., 2012).
Statistics
The results are shown as mean±SEM. All statistical analysis was performed in SigmaStat 2.03 (SysStat Software, Point Richmont, CA). Differences between multiple groups were determined by 2-way ANOVA. Kaplan Meier plots were compared by log-rank test. For comparison between 2 independent groups the Students T-test were used. Significance was as accepted at p < 0.05.
Supplementary Material
HIGHLIGHTS.
FGF21 potently stimulates adiponectin secretion
Adiponectin is critical for the FGF21-induced increases in energy expenditure
Adiponectin is requisite for FGF21 to improve glucose homeostasis in obese mice
Like Adiponectin, TZDs and FGF21 decrease ceramide accumulation in obese mice
ACKNOWLEDGEMENTS
The authors were supported by US National Institutes of Health grants R01-DK55758, RC1- DK086629 and P01-DK088761 (P.E.S.), K99-DK094973 and an AHA Beginning Grant in Aid 12BGI-A8910006 (W.L.H.). C.M.K. was supported by a fellowship from the Juvenile Diabetes Foundation (JDRF 3-2008-130).
The abbreviations used
- FGF21
fibroblast growth factor 21
- FGF19
fibroblast growth factor 19
- FGF23
fibroblast growth factor 23
- FGFR
fibroblast growth receptor
- KLB
βKlotho
- DIO
diet induced obese
- EGR1
early growth response protein 1
- FBS
fetal bovine serum
- BSA
bovine serum albumin
- PBS
phosphate buffered saline
- TNFα
tumor necrosis factor-alpha
- TZD
thiazolidinedione
- RER
respiratory exchange ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES AND NOTES
- Adams AC, Cheng CC, Coskun T, Kharitonenkov A. FGF21 Requires betaklotho to Act In Vivo. PloS one. 2012a;7:e49977. doi: 10.1371/journal.pone.0049977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams AC, Coskun T, Irizarry Rovira AR, Schneider MA, Raches DW, Micanovic R, Bina HA, Dunbar JD, Kharitonenkov A. Fundamentals of FGF19 & FGF21 Action In Vitro and In Vivo. PloS one. 2012b;7:e38438. doi: 10.1371/journal.pone.0038438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams AC, Kharitonenkov A. FGF21: The center of a transcriptional nexus in metabolic regulation. Current diabetes reviews. 2012;8:285–293. doi: 10.2174/157339912800840505. [DOI] [PubMed] [Google Scholar]
- Adams ACYC, Coskun T, cheng C, Gimeno R, Luo Y, Kharitonenkov A. The breadth of FGF21s metabolic actions are governed by FGFR1 in adipose tissue. Molecular Metabolism. 2012c doi: 10.1016/j.molmet.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell metabolism. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Berglund ED, Li CY, Bina HA, Lynes SE, Michael MD, Shanafelt AB, Kharitonenkov A, Wasserman DH. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology. 2009;150:4084–4093. doi: 10.1210/en.2009-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund ED, Vianna CR, Donato J, Jr, Kim MH, Chuang JC, Lee CE, Lauzon DA, Lin P, Brule LJ, Scott MM, et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. The Journal of clinical investigation. 2012;122:1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. The Journal of clinical investigation. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, Nawrocki AR, Rajala MW, Parlow AF, Cheeseboro L, et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145:367–383. doi: 10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]
- Combs TP, Wagner JA, Berger J, Doebber T, Wang WJ, Zhang BB, Tanen M, Berg AH, O'Rahilly S, Savage DB, et al. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology. 2002;143:998–1007. doi: 10.1210/endo.143.3.8662. [DOI] [PubMed] [Google Scholar]
- Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- Ding XB-MJ, Owen B, Bookout A, Colbert Coate K, Mangelsdorf D, Kliewer S. βKlotho Is Required for Fibroblast Growth Factor 21 Effects on Growth and Metabolism. Cell metabolism. 2012;15:387–393. doi: 10.1016/j.cmet.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutchak PA, Katafuchi T, Bookout AL, Choi JH, Yu RT, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor-21 regulates PPARgamma activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148:556–567. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, Kirwan JP. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58:337–343. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, et al. Direct insulin and leptin action on proopiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell metabolism. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N. Hormone-like (endocrine) Fgfs: their evolutionary history and roles in development, metabolism, and disease. Cell Tissue Res. 2010;342:1–11. doi: 10.1007/s00441-010-1024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Dunbar JD, Bina HA, Bright S, Moyers JS, Zhang C, Ding L, Micanovic R, Mehrbod SF, Knierman MD, et al. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. Journal of cellular physiology. 2008;215:1–7. doi: 10.1002/jcp.21357. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Larsen P. FGF21 reloaded: challenges of a rapidly growing field. Trends in endocrinology and metabolism: TEM. 2011;22:81–86. doi: 10.1016/j.tem.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, et al. FGF-21 as a novel metabolic regulator. The Journal of clinical investigation. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Kubota T, Kumagai H, Itoh S, Satoh H, Yano W, Ogata H, Tokuyama K, Takamoto I, et al. Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and -independent pathways. J Biol Chem. 2006;281:8748–8755. doi: 10.1074/jbc.M505649200. [DOI] [PubMed] [Google Scholar]
- Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, Askew GR, Simcox JA, McClain DA, Li C, et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med. 2012;18:1539–1549. doi: 10.1038/nm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Li Y, Chakraborty M, Fan Y, Bui HH, Peake DA, Kuo MS, Xiao X, Cao G, Jiang XC. Liver-specific deficiency of serine palmitoyltransferase subunit 2 decreases plasma sphingomyelin and increases apolipoprotein E levels. J Biol Chem. 2009;284:27010–27019. doi: 10.1074/jbc.M109.042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez X, Goldfine AB, Holland WL, Gordillo R, Scherer PE. Plasma Ceramides are Elevated in Female Children and Adolescents with Type 2 Diabetes. Journal of Pediatric Endocrinology & Metabolism. doi: 10.1515/jpem-2012-0407. (In Press) [DOI] [PubMed] [Google Scholar]
- Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- Perry DK, Bielawska A, Hannun YA. Quantitative determination of ceramide using diglyceride kinase. Methods Enzymol. 2000;312:22–31. doi: 10.1016/s0076-6879(00)12897-6. [DOI] [PubMed] [Google Scholar]
- Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes & development. 2012;26:312–324. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraw T, Wang ZV, Halberg N, Hawkins M, Scherer PE. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology. 2008;149:2270–2282. doi: 10.1210/en.2007-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- Wang LP, Summers SA. Measuring insulin-stimulated phosphatidyl-inositol 3-kinase activity. Methods in molecular medicine. 2003;83:127–136. doi: 10.1385/1-59259-377-1:127. [DOI] [PubMed] [Google Scholar]
- Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.