Abstract
Thanks to integrative physiology, new relationships between organs and homeostatic functions have emerged. This approach to physiology based on a whole organism approach has allowed the bone field to make fundamental progress.
In the last decade, clinical observations and scientific evidences in vivo have uncovered that fat with leptin controls bone mass through brain including a hypothalamic relay and sympathetic nervous system.
The finding that energy metabolism affects bone remodelling suggested that in an endocrine perspective, a feedback loop should exist. Beside its classical functions, bone can now be considered as a true endocrine organ secreting osteocalcin, a hormone pharmacologically active on glucose and fat metabolism. Indeed osteocalcin stimulates insulin secretion and β-cell proliferation. Simultaneously, osteocalcin acts on adipocytes to induce Adiponectin which secondarily reduce insulin resistance. This cross regulation between bone and energy metabolism offers novel therapeutic targets in type 2 diabetes and osteoporosis.
Keywords: Bone, osteocalcin, leptin, SNS, energy metabolism
1. Energy Metabolism Affects Bone Metabolism
1.1 Bone remodelling: a survival function requiring energy
The skeleton is essential for locomotion and should be able to absorb load even of high intensity during physical activity. This is critical for vertebrates to maintain high bone quality and excellent biomechanical properties. Bone has the ability to constantly renew itself through a mechanism called bone remodelling which repairs micro- and macro-damages. Thus bone remodelling served a survival function early on during evolution. Bone remodelling is a biphasic process including first destruction of pre-existing bone, the resorption and then formation (Harada and Rodan, 2003, Rodan and Martin, 2000, Teitelbaum, 2000). Both processes are exerted by specialized bone specific cells: osteoclasts for bone resorption and osteoblasts for bone formation. In normal adults, bone resorption and bone formation are well balanced. Experimentally, the gold standard to directly study bone remodelling is histomorphometry, a specific histology method that determines bone mass, osteoclast and osteoblast number per bone surface and also dynamic parameters such as the bone formation rate. This technique informs on both aspects of bone remodelling and the combined global result. It can be completed by indirect assays such as bone mineral density, CT-Scan and bone biological markers in blood and urine. Bone remodelling occurs constantly and simultaneously in numerous points of the body, thus this is a physiological function requiring a large amount of energy. This is a first physiological clue to link bone and energy metabolism. Clinical observation provides some other interesting arguments. Obesity or high body mass index reduces fracture risk whereas anorexia enhances it. Gonadal failure, either estrogens or androgens, leads invariably to bone loss and frequently to osteoporosis. Together these observations suggest that bone remodelling, appetite and reproduction might be regulated by the same hormones.
1.2 Leptin is a key hormone for appetite, reproduction and bone mass
Leptin is a 16 kDa protein hormone (Spiegelman and Flier, 1996, Zhang et al., 1994) produced by white adipocytes and acting in the brain through a single receptor. Leptin enters central nervous system in proportion to its plasma concentration and is considered to give the brain input regarding energy storage. Leptin inhibits appetite and favours energy expenditure and fertility. Consequently, mice lacking either leptin (ob/ob) or its receptor (db/db) are obese and hypogonadal. Since leptin is a key molecule in appetite regulation, metabolism and fertility (Auwerx and Staels, 1998), it is a good candidate to determine whether there is a common regulatory link between bone remodelling and energy metabolism. There was another reason to study leptin in this context which is that it appears during evolution with bone remodelling.
Histomorphometric analysis on vertebrae of leptin deficient mice ob/ob shows a high bone mass due to a massive increase in bone formation parameters that trumped the increase in osteoclast number and bone resorption parameters also observed in these mice (Ducy et al., 2000). This increase in bone resorption was expected since ob/ob mice are hypogonadal. A very similar phenotype is observed in leptin receptor deficient mice db/db. What makes this observation so important biologically is that first it relies on the study of a model already well characterized and second that leptin signalling-deficient mice are the only model exhibiting hypogonadism and high bone mass. Could obesity explain the high bone mass of ob/ob and db/db mice? The answer is provided by another mouse model, this one lacking adipocytes, the “fat-free” mice. Because of their virtual absence of adipocytes the “fat-free” mice are not only lean but also leptin-deficient. Yet they display the same phenotype of high bone mass that the one observed in ob/ob mice thus demonstrating that the high bone mass of the ob/ob mice is consequence of their lack of leptin not of their obesity (Ducy et al., 2000). That their bone phenotype can be rescued by a leptin transgene established that leptin is the only adipocyte-derived secreted molecule regulating bone mass (Elefteriou et al., 2004).
1.3 Leptin controls bone mass through a central relay
To elucidate the mode of action of leptin on bone, leptin treatment was provided through intracerebroventricular infusion (ICV) in ob/ob mice. This ICV infusion was performed at low rate to avoid any detectable leak in bloodstream, thus given the absence of leptin in the ob/ob mice this experiment allowed to determine whether the control of bone mass by leptin is, like its other functions, occurring through a central relay (Ahima, 2004, Ahima et al., 2000). In the experiment, leptin ICV infusion fully corrected the high bone mass phenotype of the ob/ob mice (Ducy et al., 2000) demonstrating that leptin uses a central relay to regulate bone mass. That the ICV infusion could correct fully the bone phenotype caused by the leptin deficiency was a strong argument to believe that, as it is the case for its other functions, leptin acts only through a neural relay to regulate bone mass. Over the years, many other experimental data demonstrated that it was indeed the case. For instance, in wild type osteoblast primary cultures treated by leptin, a Stat 3 phosphorylation can only be detected at very high dose but not at physiological dose. Moreover, the fact that cultured osteoblasts from db/db mice do not produce more extracellular matrix than wild type shows that there is no osteoblast defect in these mice. Moreover, transgenic mice expressing specifically leptin in osteoblasts do not have skeletal abnormalities (Takeda et al., 2002). The most compelling evidence came again from a mouse genetic approach: while the deletion of the leptin receptor in osteoblasts does not affect bone mass its deletion from neurons recapitulates fully the high bone mass observed in ob/ob mice (Shi et al., 2008). Taken together, this data establish that leptin acts solely through a central relay to regulate bone mass.
In order to identify the anatomical basis of the leptin-dependant central control of bone mass, analysis of the pattern of expression of leptin receptor (ObRb) is essential. ObRb is expressed in different locations of the brain such as brainstem and hypothalamus (Tartaglia et al., 1995). In the hypothalamus, ObRb expression is maximal in three specific nuclei: the arcuate (Arc), the ventromedial hypothalamic (VMH) and the paraventricular nuclei (PVN). Using various chemicals it is possible to destroy specifically hypothalamic nuclei. Monosodium glutamate (MSG) specifically destroys the arcuate nuclei and gold thioglucose (GTG) the VMH. This kind of chemical lesioning experiment allows individualizing the putative function of each nucleus downstream of leptin. For instance MSG treatment in wild type mice increases appetite but does not affect bone mass. Moreover, leptin ICV in these mice could still decrease their bone formation and bone mass (Takeda et al., 2002) indicating that neurons of the arcuate nuclei do not play a major role in the leptin regulation of bone mass. The second experiment used GTG to destroy neurons and to affect neuronal connections within the VMH nuclei. Wild type mice treated by GTG harboured a high bone mass phenotype, similar to the one observed in ob/ob mice. Importantly, in GTG-treated wild type or ob/ob mice, leptin ICV infusion could not decrease bone mass. These experiments highlight two points. First, leptin inhibits appetite and bone mass through two different hypothalamic pathways. Second, neuronal connections going though VMH nuclei are implicated in the central control of bone mass by leptin.
1.4 Neuronal mediation of central control of bone mass
The next question was to determine the nature of the relay between the hypothalamus and the bone cells. Once more, clinical observation suggested a mechanism. Reflex sympathetic dystrophy is a painful localized syndrome involving all structures of the area: cutaneous, subcutaneous, periarticular, articular, and bone tissues. This syndrome is encountered in various rare clinical circumstances like brain tumours, phenobarbital therapy but more commonly follows a direct trauma, a fracture or a surgery. Reflex sympathetic dystrophy is characterized by a rapid onset osteoporosis and a dysregulation of sympathetic tone with swelling, hyperesthesia, pain and vasomotor signs. When treated by beta-blockers to correct high sympathetic activity, a significant amount of patients improve rapidly sympathetic signs and correct local osteoporosis (Friez et al., 1982, Simson, 1974, Visitsunthorn and Prete, 1981). These points are of particular interest since it has been observed for a long time that ob/ob mice which present a high bone mass, do have a low sympathetic tone (Coleman and Hummel, 1969). These observations suggested, before one could delete the leptin receptor in neurons only, a neuronal mediation to the leptin-dependent central control of bone mass. This was verified experimentally.
The first key experiment was a parabiosis. In parabiosis two mice undergo surgical procedure to realize an anastomosis between their vascularisation. In this particular occurrence two ob/ob mice were used hence, there was no leptin in any of the two mice at the onset of the experiment. One of the mice was then infused by leptin ICV. A month later, bone analysis shows that bone mass was corrected in the mouse injected ICV but not in the other one. This experiment demonstrated that the central control of bone mass uses neuronal mediation (Takeda et al., 2002).
The second step was to indeed demonstrate the role of sympathetic nervous system in this pathway. To that end, different genetic models were used. Dopamine beta-hydroxylase (DBH) is an enzyme involved in synthesis of epinephrine and norepinephrine, the two mediators of sympathetic nervous system. Dbh-deficient mice have a high bone mass due to an increase in bone formation parameters and intracerebroventricular infusion of leptin in Dbh-deficient mice did not affect bone mass. Similarly, after the demonstration that osteoblasts express only the beta 2 adrenergic receptor (β2Ar) (Imai et al., 1988, Moore et al., 1993, Rodan and Rodan, 1986, Takeda et al., 2002), analysis of the β2Ar-deficient mice shows a high bone mass phenotype due, in part, to an increase in bone formation parameters. Like Dbh-deficient mice, the β2Ar–deficient mice could not loose bone following leptin ICV infusion (Elefteriou et al., 2005). One needs to emphasize that mice deficient simultaneously in β1Ar and β2Ar do not display the high bone mass seen in β2Ar–deficient mice (Elefteriou et al., 2004, Pierroz et al., 2004). The reason for this observation is unclear yet it indicates that signalling through β1Ar inhibits the action of sympathetic tone on osteoblasts occurring through β2Ar. This may explain why some clinical studies using beta-blockers do not find any fracture risk reduction (Levasseur et al., 2005). To complete the genetic demonstration, pharmacological treatments were applied. First administration of a sympathomimetic drug, isoproterenol, to ob/ob mice do not affect appetite and body weight but corrects the bone phenotype. In contrast, treatment of wild type mice with beta-blocker, propanolol, increases bone mass without affecting appetite and prevents bone loss in ovariectomized wild type mice (Takeda et al., 2002). Taken together all these data clearly indicate that leptin regulates bone formation through a central relay and sympathetic nervous system.
1.5 Clinical relevance: beta-blockers reduce fracture risk
There is a steadily growing body of evidence indicating that the sympathetic nervous system-bone pathway operates also in humans. The first cohort was conducted in United Kingdom using the UK-based General Practice Research Database (UGPRD) enrolling more than 3 million people. Data of patients aged between 30 and 79 were extracted to identify all patients with a fracture recorded between January 1993 and December 1999. Patients with medical disorders affecting bone metabolism and fracture risk or previously treated by antiresorptive drugs were excluded. For one case, four match controls were randomly selected. Potential confounding factors were recorded. 30 601 cases with a fracture were identified and 120 819 were included as controls. In this study use of beta blockers is associated with a statistically significant decreased fracture risk in both men and women. The adjusted odd-ratio was 0.77 [95% CI, 0.72-0.83] (Schlienger et al., 2004).
The Australian Geelong Osteoporosis Study is a second population-based, case control study. The study was conducted in women of fifty years or more during two years between February 1994 and February 1996. 569 cases and 775 controls matched were included. Subject morphology characteristics were similar in both groups. Use of beta blockers was significantly associated with a 30% reduction in fracture risk. The adjusted odd-ratio was of 0.68 [95% CI, 0.49-0.96]. In this study an increased bone mineral density was noticed in beta-blocker group that might explain reduction of fracture risk (Pasco et al., 2004). These data were reinforced by a well-characterized prospective women cohort study: the Study of Osteoporotic Fractures (SOF) Study. 8127 patients were enrolled between 1992 and 1994. Fractures were recorded over a mean follow-up period of more than seven years. Among total population, 1099 women were beta-blocker users. In this study, risk for hip fracture is decreased in selective beta-blocker users with a hazard ratio of 0.66 [CI 95%, 0.49-0.90] after adjustment (Reid et al., 2005a).
A more recent prospective case control study showed that in elderly population not receiving antiresorptive treatment, bone mineral density of fifty beta-blocker users was significantly greater for total hip and spine compared with a hundred non-users (Turker et al., 2006). Nevertheless some well conducted studies such as that like by Reid and colleagues (Reid et al., 2005b) do not show the effect in large cohorts. The role of beta-blockers in humans is still in debate in part because not all studies relied on the use of molecules inhibiting selectively signalling through the β2 adrenergic receptor.
1.6 Beta 2 adrenergic signalling pathway in the osteoblast
To go further in the molecular understanding of β2 adrenergic signalling in osteoblasts, it is essential to appreciate that bone remodelling, through its succession of resorption and formation is a true homeostatic function and that all homeostatic functions are regulated in a circadian manner. This hypothesis is supported by the observation that osteoblast specific proteins like type I collagen or osteocalcin displays a significant periodicity during a 24 hour cycle (Gundberg et al., 1985, Joseph et al., 2007). The main molecular clock is central and located in supra-chiasmatic nucleus of the hypothalamus. This central clock controls a multitude of peripheral molecular clocks operating in virtually all cells of the body. At molecular level, the clock comprises many genes. The most preeminent in functional terms are two transcription factors: Bmal1 and Clock. These transcription factors heterodimerize to regulate other downstream circadian genes such as Per1 and Per2, Cry1 and Cry2. Among their various actions Per and Cry associate to exert a negative feed back regulation loop on Bmal1 and Clock expression. Analysis of different genetic deletions in clock genes has revealed that in osteoblasts, expression of clock genes was regulated by the sympathetic nervous system. For example Per1 -/-, Per2 -/-, Cry1 -/- or Cry2 -/- mice display a high bone mass without any change in fertility, in appetite when fed a normal diet, and in other endocrine parameters. This high bone mass was due to an increase in bone formation itself caused by an increase in osteoblast proliferation. ICV infusion of leptin in these mice failed to correct their bone phenotype establishing a functional link between the leptin regulation of bone mass and the molecular clock in the osteoblast. Further molecular studies showed that, under the control of leptin, the sympathetic tone acts through the β2 adrenergic receptor in osteoblasts, and that clock genes mediate the antiproliferative function of sympathetic signalling by inhibiting G1 cyclin expression (Fu et al., 2005).
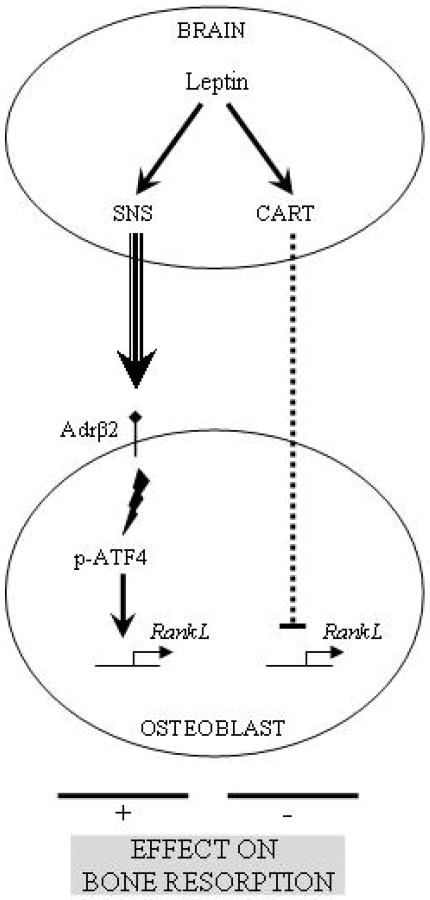
1.7 Leptin regulation of bone resorption by SNS and CART
As we have already mentioned bone remodelling is a biphasic process involving bone formation and bone resorption. That the bone formation aspect of bone remodelling is regulated through neural means raised the prospect that it might be the same for bone resorption. The in vivo verification that it was the case came from the analysis of the Adrβ2 -/- mice. Indeed their study showed that in addition to an increase in bone formation parameters, these mutant mice displayed a decrease in bone resorption parameters. That their bone phenotype could not be rescued by infusion of leptin ICV indicated that integrity of the sympathetic nervous system was required. In co-culture between osteoblasts and osteoclast progenitors a sympathomimetic such as isoproterenol could increase osteoclast differentiation whether the osteoclast progenitor cells were wild type or Adrβ2-deficient. In contrast isoproterenol could not enhance osteoclast differentiation when Adrβ2 -/- osteoblasts were used in this experiment. Gene expression analysis revealed that isoproterenol induced RankL. Several lines of evidence identified that ATF4 was the involved transcription factor regulating Rankl expression under the control of the sympathetic tone (Elefteriou et al., 2004).
Taken together this series of experiments along with a bone marrow transplantation assay showed that the sympathetic tone, under the control of leptin, targets cells of the osteoblast lineage to inhibit bone mass accrual through two complementary mechanisms: it decreases bone formation and it favours bone resorption (Elefteriou et al., 2005).
To determine the biological importance of the sympathetic regulation of bone resorption, Elefteriou et al. asked what would be the consequence of an ovariectomy in mice lacking sympathetic signalling in osteoblasts. Surprisingly in Adrβ2 -/- mice, there was no increase in bone resorption in comparison to wild type following this procedure indicating that post-menopausal osteoporosis is in part a neurological disease requiring the integrity of the sympathetic nervous system to appear. Notwithstanding this important point regarding the pathogenesis of osteoporosis, this experiment uncovered a discrepancy between the bone phenotypes of the Adrβ2 -/- and the ob/ob mice. In the former case, ovariectomy does not increase bone resorption whereas in the latter animal model it does. This indicates that the increase in bone resorption seen in the ob/ob mice could not be ascribed to their lack of function in gonads, otherwise this increase in bone resorption would have also be seen in the Adrβ2 -/- mice. This discrepancy instead suggested that leptin was regulating bone resorption by affecting expression of other genes controlling bone resorption. An analysis of mutant mice lacking various leptin regulated genes showed that the neuropeptide CART (Cocaine- and Amphetamine-Regulated Transcript), which does not regulate appetite when mice are fed a normal diet or fertility, inhibits osteoclast differentiation under the control of leptin through a mechanism that is not fully elucidated yet (Elefteriou et al., 2005). Thus leptin signalling regulates bone resorption through two antagonistic mechanisms (figure1). The first one stimulating bone resorption goes through sympathetic nervous pathway to increase RankL expression in osteoblasts. The second one reducing bone resorption uses CART to decrease RankL expression in osteoblasts through a yet incompletely deciphered pathway (Elefteriou et al., 2005). That bone resorption appears to be decreased in patients with an inactivating mutation in Mc4r who have a high serum level of CART, add a much needed human verification to this model (Ahn et al., 2006).
Figure 1.
Leptin central control of bone resorption.
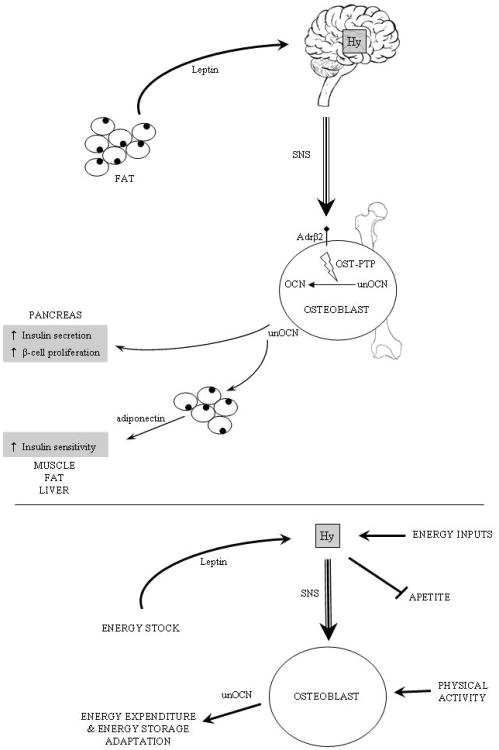
Together this body of work performed in humans and in genetically modified mouse models verified the hypothesis that a hormone affecting energy metabolism is also a major determinant of bone remodelling (Karsenty, 2006). The fact that leptin information relays through the hypothalamus offers a possibility of modulation by other systems involved in energy metabolism such as steroids, stress and sleep. The osteoblast itself offers another level of modulation in integrating load and physical activity. In the end, the osteoblast receives simple information which summarizes available energy in the body (stock and intake) and spent energy. This work has opened a new field exploring other aspects of the central control of bone mass and raises many questions that are now being studied. The most challenging one intellectually is to understand, given the fact that the pathways used by leptin to regulate bone remodelling and energy metabolism are different, if there is centrally, a common molecular link between these two aspects of the biology of this hormone. Related to this question is the currently discussed nature of the leptin-sensitive antiosteogenic neurons.
2. Skeleton Modulates Energy Metabolism
In the first part of this review we have reviewed the experimental evidences demonstrating that leptin controls bone mass through a central relay. Beyond the biology of leptin itself, this body of work suggests that the osteoblast may, through a classical feedback loop, modulate energy metabolism. To determine whether it was the case, a systematic search for osteoblast-specific molecules regulating, in vivo, energy metabolism was launched. Luckily there are few osteoblast specific genes. One of them Esp or Ptprv encodes a receptor-like protein tyrosine phosphatase: OST-PTP. The function of OST-PTP was still unknown at that time.
Esp expression is restricted to osteoblasts, sertoli cells and embryonic stem cells (Dacquin et al., 2004, Mauro et al., 1994). Interestingly for our purpose, Esp was not expressed in pancreas and fat (Lee et al., 2007). Two mutant strains have been generated. The first one is a classical knock in construct to study global Esp-deficient mice. The second construct harbours LoxP sites on Esp. This floxed construct was used to cross with α1-(1) collagen Cre mice (Dacquin et al., 2002) to specifically delete Esp in osteoblasts using the LoxP/Cre recombinase method (Lewandoski, 2001). Recombination efficiency in osteoblast was high (over 90%) and not leaky in testes (Dacquin et al., 2004). It soon appeared that Esp -/- and Esp obs -/- mice display the same metabolic phenotype, indicating that the phenotype reflected a function of Esp in osteoblasts.
2.1 Esp -/- mice display a favourable metabolic phenotype
The initial clue came from the observation that crossing heterozygous Esp-deficient mice resulted in 25% of homozygous mutant mice at birth but very few of these remaining at weaning. Since Esp is an osteoblast specific gene, the initial hypothesis was a defect in skeletogenesis but skeletal preparations failed to detect any abnormality. Facing sudden postnatal increased mortality, the second hypothesis was a metabolic origin. Independently of gender or genetic background, Esp -/- pups had a lower blood glucose at birth and at 2 and 4 weeks of age in comparison to wild type littermates. (Lee et al., 2007). Assessment of insulin revealed that Esp -/- pups had hyperinsulinemia which was confirmed in adults. Peptide C was also high. Interestingly glucagon was normal, instead of being elevated as expected, probably because of the antagonistic action of hyperinsulinemia (Maruyama et al., 1984, Raju and Cryer, 2005).
To confirm these findings, 1-month old adult mice were challenged by glucose tolerance test (GTT). Result of this test integrates both insulin secretion and insulin sensitivity. In Esp -/-, glycaemia rose less than in wild type and return more quickly to basal value. These data indicated that Esp -/- mice present an improved glucose handling in comparison to wild type littermates. Several tests were then performed to study insulin secretion and insulin sensitivity. Intraperitoneal glucose stimulated insulin secretion tests (GSIS) revealed that Esp -/- mice have higher insulin and a sustained insulin secretion compared to wild type. Linked to insulin secretion, pancreas β-cell analysis showed that Esp -/- mice have more and bigger islets and that β-cell mass was increased, a finding confirmed by Ki67 proliferation test. Hence based on the result of cell-specific gene deletion experiments, it appears that Esp, through its osteoblast expression, regulates insulin secretion and β-cell proliferation. The second aspect investigated was insulin sensitivity. In vivo, following an insulin injection, blood glucose levels dropped more quickly and profoundly in Esp -/- than in wild type mice demonstrating an increase in peripheral insulin sensitivity in Esp -/- mice despite their increase in insulin secretion. This was confirmed molecularly by the existence of an increase in the expression of the main insulin target genes in liver, fat and muscle. Finally hyperinsulinemic euglycemic clamps verified in vivo the increased insulin sensitivity. Gene deletion approach showed that osteoblasts express at least one gene regulating energy metabolism.
A second important key to deciphering this physiology came from the striking observation that the Esp deficient mice remained lean with ageing. Thus measurements of fat pad mass showed a decrease at 1-and 3-months in Esp -/- mice compared to wild type. Serum triglycerides were also lower. This was not expected since Esp -/- are hyperinsulinemic and insulin is a lipogenic hormone. Assuming that insulin signalling pathway was functional in these Esp -/- mice, the only explanation of the lower fat mass was an increase in energy expenditure. That was effectively the case in vivo since Esp -/- maintain a normal appetite but have an increased energy expenditure and an increased Ucp1 expression in white and brown fat. Ucp1 has been reported as a key gene reflecting energy expenditure (Enerback et al., 1997, Kopecky et al., 1995, Lowell et al., 1993, Rosen and Spiegelman, 2006).
This set of results was sufficiently surprising that it required verification. If indeed Esp through its expression in osteoblasts regulate that many aspects of energy metabolism then overexpressing it in osteoblasts should result in a mouse model with glucose intolerance and increased fat mass. That is exactly what was observed in α1(I)-Collagen-Esp transgenic mice. These animals have an impaired glucose tolerance test (GTT), a reduced insulin secretion in response to intraperitoneal glucose injection (GSIS) and decreased insulin sensitivity (ITT). In summary, Esp deletion in mice improves glucose handling and decreases fat mass. These data verified genetically the hypothesis that the osteoblast is an endocrine cell type controlling energy metabolism (Lee and Karsenty, 2008). The next issue was to determine how, at the molecular level OST-PTP did act.
2.2 Co-cultures uncover that osteoblast is an endocrine cell
OST-PTP, the gene product of Esp, is a transmembrane tyrosine phosphatase which cannot directly affect distant tissues like fat and β-cells. This raises the hypothesis that OST-PTP should affect synthesis, processing and/or secretion of an osteoblast-derived secreted molecule affecting glucose metabolism and energy expenditure. If such a molecule exists then a simple co-culture experiment should suffice to identify it. In this assay the most important control is obviously the negative one which has to be a cell type closely related to osteoblasts but not present in bone. This cell type is the fibroblast.
In a first approach each cell type was co-cultured with wild type primary islets. Gene expression analysis in these islets showed a 40% increase of insulin expression when they were co-cultured with osteoblasts but not when they were co-cultured with fibroblasts. In both cases, glucagon expression was not affected. Moreover, and as expected given the phenotype of these mutant mice when the co-culture was performed with osteoblasts originating from Esp -/- mice, the Insulin enhancement was even higher.
To demonstrate that the transmitted signal from osteoblast to β-cells was not due to cell-cell interactions, co-cultures were carried out with a filter separating both cell types. Results were similar to the initial experiment. Finally, β-cells were cultured with conditioned media from the osteoblasts and definitively demonstrated that osteoblasts secrete mediator affecting Insulin expression in β-cells. The same strategy was used with adipocytes instead of β-cells. Results show that osteoblasts affect Adiponectin expression in adipocytes but not Leptin. These data indicate that the osteoblast is an endocrine cell secreting molecule acting on pancreas and β-cells, i.e. a hormone. Bioactivity of this hormone is regulated by OST-PTP.
2.3 Osteocalcin regulates glucose handling
As we already discussed, in comparison with fibroblasts there are very few osteoblast specific genes (Ducy et al., 2000). One of them, Osteocalcin, is a good candidate since it is the most osteoblast–specific gene. Osteocalcin was identified many years ago as a component of the bone extracellular matrix where it binds with a high affinity to hydroxyapatite (Hauschka et al., 1989, Lian et al., 1989, Price, 1989), however, it is also secreted in blood. It has long been assumed that, osteocalcin may play a role in mineralization. This possible role was ruled out by both loss and gain of function in vivo experiments that failed to detect any significant influence of osteocalcin on the mineralization of any extracellular matrices (Ducy et al., 1996, Murshed et al., 2004). Osteocalcin is initially synthesized as a pre-promolecule with three glutamic acid residues (Glu). During post-translational modifications, glutamic residues are gamma carboxylated into Gla residues by a gamma carboxylase, explaining the other name of Bone Gla Protein (BGP) for osteocalcin. Gla residues are responsible for the mineral high affinity of osteocalcin (Hauschka et al., 1989, Price, 1989). Intracellular cleavages produce the final mature osteocalcin to be secreted. Some observations suggest that anticoagulant decreasing carboxylation can cause a decrease in blood glucose (Hetzel et al., 2006). This clinical observation shed an interesting light on the fact that, unlike Esp -/- mice, Osteocalcin deficient mice display an increase in fat mass suggesting that osteocalcin could be the sought-after osteoblast-derived hormone regulating energy metabolism.
Thus we embarked on a thorough metabolic analysis of the Osteocalcin deficient mice (Ocn). In comparison to wild type littermate, Ocn -/- mice have higher basal blood glucose and a lower insulin level on a regular diet. Glucose handling of Ocn -/- mice was impaired in comparison to wild type littermates because of a decrease in insulin secretion, a decrease in β-cell mass and proliferation and a decrease in insulin sensitivity. Energy expenditure was also decreased. The Ocn -/- mice have increased serum triglycerides and fat pad weight. Thus regardless at what parameters we looked at, it appeared that the Osteocalcin deficient mice display a metabolic phenotype that is the mirror image of the one present in the Esp deficient mice (table 1) thus suggesting that Esp -/- mice were in fact a model of increased osteocalcin bioactivity. In order to genetically demonstrate that OST-PTP and osteocalcin belong to the same regulatory pathway, one allele of Osteocalcin was removed to Esp -/- mice. This genetic manipulation rescued the glucose metabolism phenotype of Esp -/- mice (Lee et al., 2007).
Table 1.
Mirror phenotype of Esp -/- and Ocn -/- mice.
| Strain | Esp -/- | Ocn -/- |
|---|---|---|
| Model | Gain of function of osteocalcin bioactivity | Reduced osteocalcin bioactivity |
| Sensivity | ↗ insulin secretion ↗ β cell proliferation |
↘ insulin secretion ↘ β cell proliferation |
| Resistance | ↘ insulin resistance | ↗ insulin resistance |
| Uncarboxylated OCN | 26% | 10% |
| Diabetes/ obesity | Protection | Susceptibility |
2.4 Osteocalcin bioactivity
That removing one allele of Osteocalcin is sufficient to rescue Esp -/- mice phenotype strongly verified that Esp -/- is a model of gain of function of osteocalcin but did not explain how OST-PTP affects osteocalcin bioactivity. This was an important issue since OST-PTP does not appear to regulate osteocalcin expression or synthesis. As it has been described for other proteins like coagulation factors (Furie et al., 1999, Furie and Furie, 1988) carboxylation offers a means to regulate the bioactivity of gla-containing proteins. In osteocalcin, three Glu residues can be carboxylated producing different status from uncarboxylated to fully carboxylated. Using the ability of Gla residues to bind hydroxyapatite, serum was incubated with hydroxyapatite in order to bind carboxylated osteocalcin. The remaining fraction corresponded to uncarboxylated osteocalcin and was assessed. In Esp -/- there was an increase in uncarboxylated osteocalcin in comparison to wild type mice, respectively 26% and 10% (Lee et al., 2007).
In an additional experiment, when wild type osteoblasts received warfarin to interrupt gamma carboxylation, fraction of uncarboxylated osteocalcin increased and osteoblasts induced a significantly higher Adiponectin expression. In contrast, carboxylated osteocalcin failed to induce Adiponectin in adipocytes and Insulin or Cyclin D1 in islets. This assay is still rudimentary but together with the biological and the clinical observations, it provided impetus to further study how OST-PTP could affect directly or not osteocalcin carboxylation
These findings confirm that at least in mice, osteoblast is an endocrine cell and acts on energy metabolism through a new hormone: osteocalcin. Uncarboxylated osteocalcin is the active circulating fraction on adipocytes and β-cells. The current suggested model is that the product of Esp, OST-PTP, stimulates carboxylation of osteocalcin and decreases osteocalcin bioactivity. The notion that osteocalcin exerts a metabolic function has since then received additional support from human studies (Pittas et al., 2008).
2.5 Osteocalcin pharmacological activity
If indeed Esp-/- mice are a model of osteocalcin gain of function then they provide a genetic tool to determine whether an excess of osteocalcin has beneficial consequences on energy metabolism. In the first experiment, chemical lesioning of VMH nuclei in hypothalamus interrupts appetite regulation and wild type mice become hyperphagic then obese and glucose intolerant in 3 months after injection. In the second experiment, mice are fed with a high fat diet during 6 weeks. In this case wild type mice keep a normal appetite but calorie intake increases and mice develop obesity and glucose intolerance. In these two experiments, Esp deficient mice remained lean, protected from obesity with a low fat pad mass and serum triglycerides. During metabolic tests, they do not display signs of decreased glucose tolerance and insulin sensitivity. In the third experiment, β-cell failure was induced by injection of streptozotocin. In wild type and Esp -/- mice serum insulin level markedly decreased. More than 40% of wild type mice died and the surviving ones became diabetic. In contrast, only 14% of Esp -/- mice died and the other ones did not develop diabetes. This set of experiments shows that Esp -/- mice that are a model of a gain of function of osteocalcin are protected from obesity and glucose intolerance.
The next critical issue is to show that the metabolic functions of osteocalcin could be observed in wild type animals. Mouse recombinant uncarboxylated osteocalcin was bacterially produced, purified and assayed in cell culture or infused in wild type mice (Ferron et al., 2008).
Based on uncarboxylated osteocalcin serum level observed in wild type mice at 7 ng/ml (Lee et al., 2007), the authors studied a broad range of concentrations from 20-fold lower (0.3 ng/ml) to 4-fold higher concentrations (30 ng/ml). Recombinant osteocalcin was able to trigger Insulin1 and Insulin2 expression at low dose. The largest effect was noticed with 0.3 ng/ml. Higher concentrations were progressively less efficient. The same dose response profile was observed with β-cell proliferation expression markers CyclinD2 and Cdk4. Experiments were performed with isolated islets and MIN6 cells, a mouse β-cell line retaining its glucose-insulin secretion capability (Miyazaki et al., 1990). The other identified target of osteocalcin is adipocytes. In white adipocytes, Adiponectin expression reflects regulation of insulin sensitivity (Kubota et al., 2002, Yamauchi et al., 2001). In brown adipocytes, Pgc1α and Ucp1 express energy expenditure (Uldry et al., 2006). In both cases, ex vivo osteocalcin treatment was not efficient below 1 ng/ml and the largest enhancement was for 10 and 30 ng/ml in a dose-dependant manner.
Considering the in vitro results, 8-weeks old wild type mice were implanted with osteocalcin pumps for 1 month. As expected, mice treated with low dose (0.3 ng/h) displayed lower blood glucose due to an increase in serum insulin level. No effect was observed with high dose (30 ng/h). During metabolic tests, GTT and GSIS were improved with the low dose but not the high dose. After sacrifice, an increase in β-cell proliferation was also observed at low dose. Insulin sensitivity was also improved dose dependently: low dose in comparison to vehicle and high dose compared to low dose. These first results were confirmed by molecular studies with insulin sensitivity expression markers: Mcad in muscles and Pparγ in white fat. Furthermore Adiponectin and adiponectin target genes such as AcylCoA, Ucp2, Pparα were up regulated in a dose dependent manner. Fat pad mass and serum triglycerides concentration underwent also a dose-dependent decrease. Food intake was not affected in these mice. To summarize, in vitro and in vivo data show that recombinant uncarboxylated osteocalcin affects β-cell proliferation and Insulin expression at low concentration (0.3 ng/ml) whereas osteocalcin affects insulin sensitivity and energy expenditure at higher concentrations (10 or 30 ng/ml) in a dose dependent manner (Ferron et al., 2008).
Finally the potential therapeutic effect of osteocalcin was tested in pathological conditions such as in a diet induced obesity model. In that case pellets delivering 3 ng/h of recombinant osteocalcin over 8 weeks significantly reduced body weight gain, improved insulin secretion and resistance and normalized serum triglycerides. There was also an increase in energy expenditure. A second model of obesity relies on chemical destruction by GTG of hypothalamic neurons controlling appetite. In this experiment, injected mice become hyperphagic and progressively obese and glucose intolerant. Two weeks after GTG injection, pumps of osteocalcin (3 ng/h) or vehicle were implanted in GTG-injected mice. After 8 weeks of treatment, osteocalcin-treated mice gained less weight, had a reduced fat mass and decreased serum triglycerides in comparison to mice receiving the vehicle only. Glucose tolerance and insulin sensitivity were also improved. These findings were consistent with the former results of high fat diet experiment (Ferron et al., 2008).
3 Integration of pathways: leptin inhibits insulin secretion partly through a bone relay
In the first part we described how leptin affects osteoblast function through a central relay and sympathetic nervous system (Karsenty, 2006). In the second part we described how osteoblasts affect energy metabolism through a new hormone osteocalcin. How do, if they do, these pathways intersect? Leptin has an inhibitory effect on β-cells and insulin secretion. A direct effect was already reported (Covey et al., 2006, Morioka et al., 2007, Shimabukuro et al., 1997). Nevertheless this effect was partial raising the testable hypothesis that, as it is the case for most of its other functions, leptin may use a neuronal indirect mechanism to regulate insulin secretion (Hinoi et al., 2008). In order to clearly separate insulin secretion from the insulin resistance caused by the obesity of the ob/ob mice, Hinoi et al. studied young ob/ob mice, before the onset of obesity and insulin resistance. Until 2 weeks of age ob/ob mice do not have any evidence of insulin resistance yet these mice have a hyper-insulinemia leading to a 30% decrease of blood glucose level after feeding in comparison to wild type littermates. The same findings were also present in newborn and 1-week old mice. Molecular analysis of these young ob/ob mice showed an up-regulation in Insulin expression, β-cell proliferation markers like Cdk4 expression, Ki67 immunostaining (Hinoi et al., 2008). These data verified that leptin inhibits insulin secretion in absence of insulin resistance (Hinoi et al., 2008).
To determine whether leptin uses, in part a neural pathway to regulate insulin secretion, the leptin receptor (Lepr) was specifically deleted in all neurons. Compared to wild type the Leprsyn -/- had an increase in serum insulin level and a decrease in blood glucose levels. These mutant mice also displayed a decrease in serum epinephrine, serum norepinephrine, and Ucp1 expression in brown fat reflecting a low sympathetic tone. Given the fact that the sympathetic tone regulates bone mass it was tempting to test whether leptin uses bone as a relay in its regulation of insulin secretion. To that end an osteoblast specific deletion (Adrβ2obs -/-) was engineered. As expected, serum insulin levels were higher in Adrβ2obs -/- compared to wild type mice in absence of any evidence of insulin resistance. Adrβ2obs -/- gene expression profile (Insulin, Glucokinase, Cdk4) was consistent with the one observed in ob/ob mice. The fact that glucose phenotype of double heterozygous mice (ob/+; Adrβ2obs +/−) display hyperinsulinemia and low blood glucose after feeding established genetically that it is under the control of leptin that sympathetic signalling in osteoblasts regulates insulin secretion. The sympathetic tone favours expression in osteoblasts of Esp, the gene which inhibits osteocalcin bioactivity. These data if true implied that inactivating Osteocalcin in ob/ob mice should rescue at least partially their hyperinsulinaemia. This is exactly what was observed indicating that indeed leptin regulates insulin secretion by decreasing osteocalcin bioactivity. These results also indicate that besides a cross regulation between bone and energy metabolisms, there is also a very tight functional relationship between fat cells and osteoblasts (Hinoi et al., 2008).
4. Model organisms and integrative physiology
Mouse genetics has been a powerful tool to reveal many new physiological loops. This became evident when it was used to show that the adipocyte is an endocrine cell type. The set of novel physiology did not stop at this cell type and for instance the use of cell specific gene deletion experiments revealed many unappreciated complexities in bone physiology. This approach to physiology based on a whole organism approach has allowed the bone field to make fundamental progress by showing how dynamic and complex skeleton physiology is. Within a decade it was possible through the use of this whole organism approach to demonstrate that indeed, based on genetic arguments in mice and humans, there is a cross talk between bone and energy metabolism and that the brain exerts a profound influence on bone remodelling (figure 2). It is clear that forwarding future this approach will re-awaken an aspect of biology that we are all inclined to forget in the molecular era and that is that not only cells are talking to each other but organs do the same. Beyond bone biology which should be viewed as a proof of paradigm, integrative physiology will certainly define novel and adapted therapies to many degenerative diseases
Figure 2.
Crosstalk physiological model between energy metabolism and bone.
Acknowledgments
G. Billard for her excellent technical assistance.
This work was supported by Philippe Foundation Inc, Société Française de Rhumatologie (SFR) and Association pour la Recherche sur le Cancer (ARC) (to C.B. Confavreux).
G. Karsenty is the founder of Escoublac, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Ahima RS. Body fat, leptin, and hypothalamic amenorrhea. N Engl J Med. 2004;351:959–62. doi: 10.1056/NEJMp048214. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21:263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- Ahn JD, Dubern B, Lubrano-Berthelier C, Clement K, Karsenty G. Cart overexpression is the only identifiable cause of high bone mass in melanocortin 4 receptor deficiency. Endocrinology. 2006;147:3196–202. doi: 10.1210/en.2006-0281. [DOI] [PubMed] [Google Scholar]
- Auwerx J, Staels B. Leptin. Lancet. 1998;351:737–42. doi: 10.1016/S0140-6736(97)06348-4. [DOI] [PubMed] [Google Scholar]
- Coleman DL, Hummel KP. Effects of parabiosis of normal with genetically diabetic mice. Am J Physiol. 1969;217:1298–304. doi: 10.1152/ajplegacy.1969.217.5.1298. [DOI] [PubMed] [Google Scholar]
- Covey SD, Wideman RD, Mcdonald C, Unniappan S, Huynh F, Asadi A, Speck M, Webber T, Chua SC, Kieffer TJ. The pancreatic beta cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metab. 2006;4:291–302. doi: 10.1016/j.cmet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Dacquin R, Mee PJ, Kawaguchi J, Olmsted-Davis EA, Gallagher JA, Nichols J, Lee K, Karsenty G, Smith A. Knock-in of nuclear localised beta-galactosidase reveals that the tyrosine phosphatase Ptprv is specifically expressed in cells of the bone collar. Dev Dyn. 2004;229:826–34. doi: 10.1002/dvdy.20003. [DOI] [PubMed] [Google Scholar]
- Dacquin R, Starbuck M, Schinke T, Karsenty G. Mouse alpha1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev Dyn. 2002;224:245–51. doi: 10.1002/dvdy.10100. [DOI] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–52. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–20. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Kim CA, Ogawa Y, Liu X, Ware SM, Craigen WJ, Robert JJ, Vinson C, Nakao K, Capeau J, Karsenty G. Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci U S A. 2004;101:3258–63. doi: 10.1073/pnas.0308744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–4. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A. 2008;105:5266–70. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friez L, Pere G, Breuillard P, Meignan S. Comparison of treatment with griseofulvin, beta blockers and calcitonin in 55 cases of post-traumatic algoneurodystrophies. Rev Rhum Mal Osteoartic. 1982;49:857–60. [PubMed] [Google Scholar]
- Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122:803–15. doi: 10.1016/j.cell.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Furie B, Bouchard BA, Furie BC. Vitamin K-dependent biosynthesis of gamma-carboxyglutamic acid. Blood. 1999;93:1798–808. [PubMed] [Google Scholar]
- Furie B, Furie BC. The molecular basis of blood coagulation. Cell. 1988;53:505–18. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- Gundberg CM, Markowitz ME, Mizruchi M, Rosen JF. Osteocalcin in human serum: a circadian rhythm. J Clin Endocrinol Metab. 1985;60:736–9. doi: 10.1210/jcem-60-4-736. [DOI] [PubMed] [Google Scholar]
- Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–55. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- Hetzel PG, Glanzmann R, Hasler PW, Ladewick A, Buhrer C. Coumarin embryopathy in an extremely low birth weight infant associated with neonatal hepatitis and ocular malformations. Eur J Pediatr. 2006;165:358–60. doi: 10.1007/s00431-005-0064-1. [DOI] [PubMed] [Google Scholar]
- Hinoi E, Gao N, Jung DY, Yadav V, Yoshizawa T, Myers MG, Jr, Chua SC, Jr, Kim JK, Kaestner KH, Karsenty G. The sympathetic tone mediates leptin's inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. 2008;183:1235–42. doi: 10.1083/jcb.200809113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Rodan SB, Rodan GA. Effects of retinoic acid on alkaline phosphatase messenger ribonucleic acid, catecholamine receptors, and G proteins in ROS 17/2.8 cells. Endocrinology. 1988;122:456–63. doi: 10.1210/endo-122-2-456. [DOI] [PubMed] [Google Scholar]
- Joseph F, Chan BY, Durham BH, Ahmad AM, Vinjamuri S, Gallagher JA, Vora JP, Fraser WD. The circadian rhythm of osteoprotegerin and its association with parathyroid hormone secretion. J Clin Endocrinol Metab. 2007;92:3230–8. doi: 10.1210/jc.2006-1832. [DOI] [PubMed] [Google Scholar]
- Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab. 2006;4:341–8. doi: 10.1016/j.cmet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest. 1995;96:2914–23. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–6. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- Lee NK, Karsenty G. Reciprocal regulation of bone and energy metabolism. Trends Endocrinol Metab. 2008;19:161–6. doi: 10.1016/j.tem.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, Mckee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levasseur R, Dargent-Molina P, Sabatier JP, Marcelli C, Breart G. Beta-blocker use, bone mineral density, and fracture risk in older women: results from the Epidemiologie de l'Osteoporose prospective study. J Am Geriatr Soc. 2005;53:550–2. doi: 10.1111/j.1532-5415.2005.53178_7.x. [DOI] [PubMed] [Google Scholar]
- Lewandoski M. Conditional control of gene expression in the mouse. Nat Rev Genet. 2001;2:743–55. doi: 10.1038/35093537. [DOI] [PubMed] [Google Scholar]
- Lian J, Stewart C, Puchacz E, Mackowiak S, Shalhoub V, Collart D, Zambetti G, Stein G. Structure of the rat osteocalcin gene and regulation of vitamin D-dependent expression. Proc Natl Acad Sci U S A. 1989;86:1143–7. doi: 10.1073/pnas.86.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, V SS, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–2. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest. 1984;74:2296–9. doi: 10.1172/JCI111658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro LJ, Olmsted EA, Skrobacz BM, Mourey RJ, Davis AR, Dixon JE. Identification of a hormonally regulated protein tyrosine phosphatase associated with bone and testicular differentiation. J Biol Chem. 1994;269:30659–67. [PubMed] [Google Scholar]
- Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, Oka Y, Yamamura K. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127:126–32. doi: 10.1210/endo-127-1-126. [DOI] [PubMed] [Google Scholar]
- Moore RE, Smith CK, 2nd, Bailey CS, Voelkel EF, Tashjian AH., Jr Characterization of beta-adrenergic receptors on rat and human osteoblast-like cells and demonstration that beta-receptor agonists can stimulate bone resorption in organ culture. Bone Miner. 1993;23:301–15. doi: 10.1016/s0169-6009(08)80105-5. [DOI] [PubMed] [Google Scholar]
- Morioka T, Asilmaz E, Hu J, Dishinger JF, Kurpad AJ, Elias CF, Li H, Elmquist JK, Kennedy RT, Kulkarni RN. Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. J Clin Invest. 2007;117:2860–8. doi: 10.1172/JCI30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshed M, Schinke T, Mckee MD, Karsenty G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol. 2004;165:625–30. doi: 10.1083/jcb.200402046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco JA, Henry MJ, Sanders KM, Kotowicz MA, Seeman E, Nicholson GC. Beta-adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong Osteoporosis Study. J Bone Miner Res. 2004;19:19–24. doi: 10.1359/JBMR.0301214. [DOI] [PubMed] [Google Scholar]
- Pierroz DD, Muzzin P, Glatt V, Bouxsein M, Rizzoli R, Ferrari S. Beta1 beta2- adrenergic receptor KO mice have decreased total body and cortical bone mass despite increased trabecular number. J Bone Miner Res. 2004;19:S32. [Google Scholar]
- Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab. 2008 doi: 10.1210/jc.2008-1422. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price PA. Gla-containing proteins of bone. Connect Tissue Res. 1989;21:51–7. doi: 10.3109/03008208909049995. discussion 57-60. [DOI] [PubMed] [Google Scholar]
- Raju B, Cryer PE. Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes: documentation of the intraislet insulin hypothesis in humans. Diabetes. 2005;54:757–64. doi: 10.2337/diabetes.54.3.757. [DOI] [PubMed] [Google Scholar]
- Reid IR, Gamble GD, Grey AB, Black DM, Ensrud KE, Browner WS, Bauer DC. beta-Blocker use, BMD, and fractures in the study of osteoporotic fractures. J Bone Miner Res. 2005a;20:613–8. doi: 10.1359/JBMR.041202. [DOI] [PubMed] [Google Scholar]
- Reid IR, Lucas J, Wattie D, Horne A, Bolland M, Gamble GD, Davidson JS, Grey AB. Effects of a beta-blocker on bone turnover in normal postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab. 2005b;90:5212–6. doi: 10.1210/jc.2005-0573. [DOI] [PubMed] [Google Scholar]
- Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–14. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- Rodan SB, Rodan GA. Dexamethasone effects on beta-adrenergic receptors and adenylate cyclase regulatory proteins Gs and Gi in ROS 17/2.8 cells. Endocrinology. 1986;118:2510–8. doi: 10.1210/endo-118-6-2510. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–53. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlienger RG, Kraenzlin ME, Jick SS, Meier CR. Use of beta-blockers and risk of fractures. Jama. 2004;292:1326–32. doi: 10.1001/jama.292.11.1326. [DOI] [PubMed] [Google Scholar]
- Shi Y, Yadav VK, Suda N, Liu XS, Guo XE, Myers MG, Jr, Karsenty G. Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc Natl Acad Sci U S A. 2008;105:20529–33. doi: 10.1073/pnas.0808701106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro M, Koyama K, Chen G, Wang MY, Trieu F, Lee Y, Newgard CB, Unger RH. Direct antidiabetic effect of leptin through triglyceride depletion of tissues. Proc Natl Acad Sci U S A. 1997;94:4637–41. doi: 10.1073/pnas.94.9.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simson G. Letter: Propranolol for causalgia and Sudeck's atrophy. Jama. 1974;227:327. doi: 10.1001/jama.227.3.327c. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Adipogenesis and obesity: rounding out the big picture. Cell. 1996;87:377–89. doi: 10.1016/s0092-8674(00)81359-8. [DOI] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–17. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–8. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- Turker S, Karatosun V, Gunal I. Beta-blockers increase bone mineral density. Clin Orthop Relat Res. 2006;443:73–4. doi: 10.1097/01.blo.0000200242.52802.6d. [DOI] [PubMed] [Google Scholar]
- Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006;3:333–41. doi: 10.1016/j.cmet.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Visitsunthorn U, Prete P. Reflex sympathetic dystrophy of the lower extremity: a complication of herpes zoster with dramatic response to propranolol. West J Med. 1981;135:62–6. [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]