Abstract
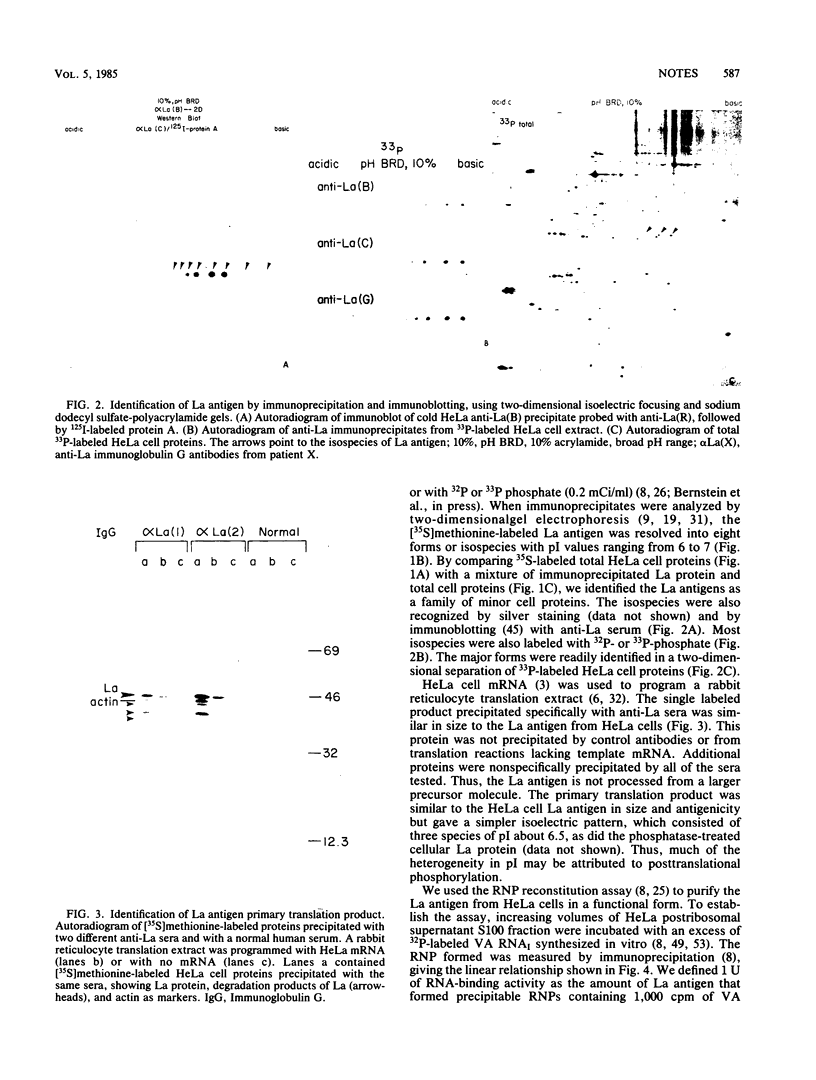
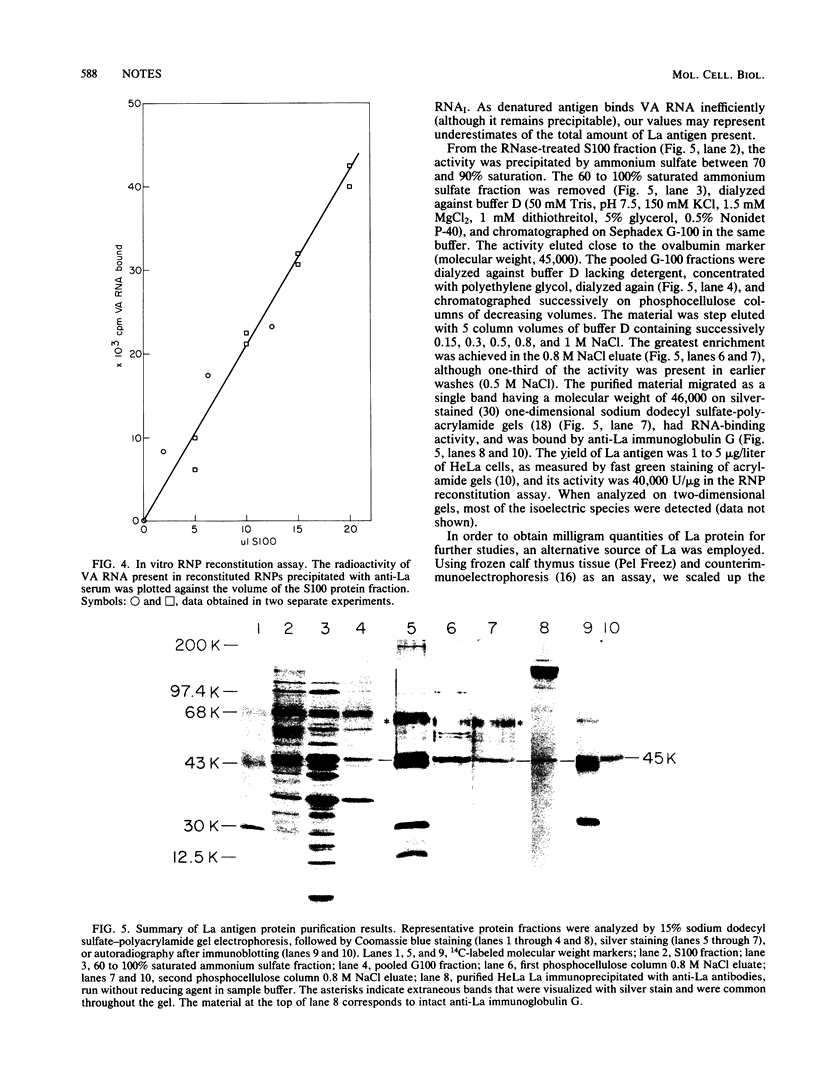
HeLa cell La antigen, an RNA-binding protein, was characterized by using two-dimensional gel electrophoresis. Eight isoelectric forms (pI 6 to 7) were observed, many containing phosphate. An in vitro translation product similar in size and antigenicity was identified. The HeLa cell protein purified by using an assay based on ribonucleoprotein reconstitution with adenovirus VA RNAI also comprised several isoelectric forms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akizuki M., Boehm-Truitt M. J., Kassan S. S., Steinberg A. D., Chused T. M. Purification of an acidic nuclear protein antigen and demonstration of its antibodies in subsets of patients with sicca syndrome. J Immunol. 1977 Sep;119(3):932–938. [PubMed] [Google Scholar]
- Akizuki M., Powers R., Holman H. R. A soluble acidic protein of the cell nucleus which reacts with serum from patients with systemic lupus erythermatosus and Sjögren's syndrome. J Clin Invest. 1977 Feb;59(2):264–272. doi: 10.1172/JCI108637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. W., Lewis J. B., Atkins J. F., Gesteland R. F. Cell-free synthesis of adenovirus 2 proteins programmed by fractionated messenger RNA: a comparison of polypeptide products and messenger RNA lengths. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2756–2760. doi: 10.1073/pnas.71.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J. C., Kurilla M. G., Keene J. D. Association between the 7 S RNA and the lupus La protein varies among cell types. J Biol Chem. 1983 Oct 10;258(19):11438–11441. [PubMed] [Google Scholar]
- Deng J. S., Takasaki Y., Tan E. M. Nonhistone nuclear antigens reactive with autoantibodies. Immunofluorescence studies on distribution in synchronized cells. J Cell Biol. 1981 Dec;91(3 Pt 1):654–660. doi: 10.1083/jcb.91.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon K. B., Culhane L. Partial immunochemical characterization of the Ro and La proteins using antibodies from patients with the sicca syndrome and lupus erythematosus. J Immunol. 1984 May;132(5):2350–2356. [PubMed] [Google Scholar]
- Francoeur A. M., Mathews M. B. Interaction between VA RNA and the lupus antigen La: formation of a ribonucleoprotein particle in vitro. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6772–6776. doi: 10.1073/pnas.79.22.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrels J. I. Two dimensional gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem. 1979 Aug 25;254(16):7961–7977. [PubMed] [Google Scholar]
- Gorovsky M. A., Carlson K., Rosenbaum J. L. Simple method for quantitive densitometry of polyacrylamide gels using fast green. Anal Biochem. 1970 Jun;35(2):359–370. doi: 10.1016/0003-2697(70)90196-x. [DOI] [PubMed] [Google Scholar]
- Gottesfeld J. M., Andrews D. L., Hoch S. O. Association of an RNA polymerase III transcription factor with a ribonucleoprotein complex recognized by autoimmune sera. Nucleic Acids Res. 1984 Apr 11;12(7):3185–3200. doi: 10.1093/nar/12.7.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets W. J., den Brok J. H., Boerbooms A. M., van de Putte L. B., van Venrooij W. J. Characterization of the SS-B (La) antigen in adenovirus-infected and uninfected HeLa cells. EMBO J. 1983;2(10):1625–1631. doi: 10.1002/j.1460-2075.1983.tb01636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto C., Steitz J. A. Sequential association of nucleolar 7-2 RNA with two different autoantigens. J Biol Chem. 1983 Feb 10;258(3):1379–1382. [PubMed] [Google Scholar]
- Hendrick J. P., Wolin S. L., Rinke J., Lerner M. R., Steitz J. A. Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol Cell Biol. 1981 Dec;1(12):1138–1149. doi: 10.1128/mcb.1.12.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik J. E., Huston M. M., Rose J. A. Locations of adenovirus genes required for the replication of adenovirus-associated virus. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1925–1929. doi: 10.1073/pnas.78.3.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurilla M. G., Keene J. D. The leader RNA of vesicular stomatitis virus is bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1983 Oct;34(3):837–845. doi: 10.1016/0092-8674(83)90541-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Andrews N. C., Miller G., Steitz J. A. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1981 Feb;78(2):805–809. doi: 10.1073/pnas.78.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Hardin J. A., Steitz J. A. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. 1981 Jan 23;211(4480):400–402. doi: 10.1126/science.6164096. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore S. J., Wieben E. D., Pederson T. Eukaryotic small ribonucleoproteins. Anti-La human autoantibodies react with U1 RNA-protein complexes. J Biol Chem. 1984 Feb 10;259(3):1929–1933. [PubMed] [Google Scholar]
- Mathews M. B. Binding of adenovirus VA RNA to mRNA: a possible role in splicing? Nature. 1980 Jun 19;285(5766):575–577. doi: 10.1038/285575a0. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Francoeur A. M. La antigen recognizes and binds to the 3'-oligouridylate tail of a small RNA. Mol Cell Biol. 1984 Jun;4(6):1134–1140. doi: 10.1128/mcb.4.6.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B., Grodzicker T. Virus-associated RNAs of naturally occurring strains and variants of group C adenoviruses. J Virol. 1981 Jun;38(3):849–862. doi: 10.1128/jvi.38.3.849-862.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter L., Schopfer K., Wilhelm J. A., Nyffenegger T., Parisot R. F., De Robertis E. M. Molecular characterization of ribonucleoprotein antigens bound by antinuclear antibodies. A diagnostic evaluation. Arthritis Rheum. 1982 Nov;25(11):1278–1283. doi: 10.1002/art.1780251102. [DOI] [PubMed] [Google Scholar]
- Moore C. L., Sharp P. A. Site-specific polyadenylation in a cell-free reaction. Cell. 1984 Mar;36(3):581–591. doi: 10.1016/0092-8674(84)90337-4. [DOI] [PubMed] [Google Scholar]
- Murray V., Holliday R. Mechanism for RNA splicing of gene transcripts. FEBS Lett. 1979 Oct 1;106(1):5–7. doi: 10.1016/0014-5793(79)80682-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pizer L. I., Deng J. S., Stenberg R. M., Tan E. M. Characterization of a phosphoprotein associated with the SS-B/La nuclear antigen in adenovirus-infected and uninfected KB cells. Mol Cell Biol. 1983 Jul;3(7):1235–1245. doi: 10.1128/mcb.3.7.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost T. T., Reichlin M. Antinuclear antibody-negative systemic lupus erythematosus. I. Anti-Ro(SSA) and anti-La(SSB) antibodies. J Am Acad Dermatol. 1981 Jan;4(1):84–89. doi: 10.1016/s0190-9622(81)70013-6. [DOI] [PubMed] [Google Scholar]
- Reddy R., Henning D., Tan E., Busch H. Identification of a La protein binding site in a RNA polymerase III transcript (4.5 I RNA). J Biol Chem. 1983 Jul 10;258(13):8352–8356. [PubMed] [Google Scholar]
- Reichlin M. Current perspectives on serological reactions in SLE patients. Clin Exp Immunol. 1981 Apr;44(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- Rinke J., Steitz J. A. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1982 May;29(1):149–159. doi: 10.1016/0092-8674(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Rosa M. D., Gottlieb E., Lerner M. R., Steitz J. A. Striking similarities are exhibited by two small Epstein-Barr virus-encoded ribonucleic acids and the adenovirus-associated ribonucleic acids VAI and VAII. Mol Cell Biol. 1981 Sep;1(9):785–796. doi: 10.1128/mcb.1.9.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. J., Weinberger C., Shenk T. Adenovirus VAI RNA facilitates the initiation of translation in virus-infected cells. Cell. 1984 May;37(1):291–298. doi: 10.1016/0092-8674(84)90325-8. [DOI] [PubMed] [Google Scholar]
- Shen C. K., Maniatis T. The organization, structure, and in vitro transcription of Alu family RNA polymerase III transcription units in the human alpha-like globin gene cluster: precipitation of in vitro transcripts by lupus anti-La antibodies. J Mol Appl Genet. 1982;1(4):343–360. [PubMed] [Google Scholar]
- Stefano J. E. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3' termini of RNA polymerase III transcripts. Cell. 1984 Jan;36(1):145–154. doi: 10.1016/0092-8674(84)90083-7. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Teppo A. M., Gripenberg M., Kurki P., Baklien K., Helve T., Wegelius O. Purification and characterization of a nuclear SS-B antigen. Scand J Immunol. 1982 Jan;15(1):1–7. doi: 10.1111/j.1365-3083.1982.tb00615.x. [DOI] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables P. J., Smith P. R., Maini R. N. Purification and characterization of the Sjögren's syndrome A and B antigens. Clin Exp Immunol. 1983 Dec;54(3):731–738. [PMC free article] [PubMed] [Google Scholar]
- Wasicek C. A., Reichlin M. Clinical and serological differences between systemic lupus erythematosus patients with antibodies to Ro versus patients with antibodies to Ro and La. J Clin Invest. 1982 Apr;69(4):835–843. doi: 10.1172/JCI110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil P. A., Segall J., Harris B., Ng S. Y., Roeder R. G. Faithful transcription of eukaryotic genes by RNA polymerase III in systems reconstituted with purified DNA templates. J Biol Chem. 1979 Jul 10;254(13):6163–6173. [PubMed] [Google Scholar]
- Williamson G. G., Boyle J. A. Antigenic relatedness of small ribonucleoprotein particles. Biochim Biophys Acta. 1984 Apr 10;798(2):149–155. doi: 10.1016/0304-4165(84)90297-6. [DOI] [PubMed] [Google Scholar]
- Wilusz J., Kurilla M. G., Keene J. D. A host protein (La) binds to a unique species of minus-sense leader RNA during replication of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5827–5831. doi: 10.1073/pnas.80.19.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. The Ro small cytoplasmic ribonucleoproteins: identification of the antigenic protein and its binding site on the Ro RNAs. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1996–2000. doi: 10.1073/pnas.81.7.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. J., Zubay G. Prolonged transcription in a cell-free system involving nuclei and cytoplasm. Proc Natl Acad Sci U S A. 1974 May;71(5):1803–1807. doi: 10.1073/pnas.71.5.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eekelen C., Buijtels H., Linné T., Ohlsson R., Philipson L., van Venrooij W. Detection of a cellular polypeptide associated with adenovirus-coded VA RNA using in vitro labeling of proteins cross-linked to RNA. Nucleic Acids Res. 1982 May 25;10(10):3039–3052. doi: 10.1093/nar/10.10.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]