Abstract
Objectives:
The purpose of this study was to examine the ability of CT to assess the relative difference of degree of bone mineralization (grey level) parameters in a human mandible.
Methods:
Ten mandibular sections from cadavers (81.5 ± 12.1 years) were scanned using micro-CT with 27.2 μm voxel size and cone beam CT (CBCT) with 200 μm, 300 μm, and 400 μm voxel sizes. In addition, 15 clinical CBCT images from young patients (mean age 18.9 ± 3.3 years) were identified. After segmentation of bone voxels, alveolar bone and basal cortical bone regions were digitally isolated. A histogram of grey level, which is equivalent to degree of bone mineralization, was obtained from each region of the CT images. Mean, standard deviation (SD), coefficient of variation (COV), fifth percentile low (Low5) and high (High5) of alveolar bone and basal cortical bone regions were obtained. Percentage differences of grey level parameters between alveolar and basal cortical bones were computed.
Results:
The alveolar bone region had significantly lower Mean, Low5 and High5 values but significantly higher SD and COV than the basal cortical bone region for all CT images (p < 0.05). All parameters were significantly lower for the old cadaver group than for the young patient group (p < 0.05).
Conclusions:
CBCT and micro-CT provide comparable results in the assessment of relative difference in grey level distribution between alveolar and basal cortical bone regions in the human mandible. The percentage difference relative to an internal reference (basal cortical bone) can be a reliable method when assessing the degree of bone mineralization using CBCT images for both cross-sectional and longitudinal comparisons.
Keywords: micro-computed tomography, cone beam computed tomography, bone mineralization, alveolar bone
Introduction
Masticatory force applied to teeth is directly transferred to the alveolar bone (AB) region stimulating more remodelling than other oral bone regions.1,2 The active modelling and remodelling of the AB region is also observed during tooth movement due to orthodontic treatment, bone healing after implantation and periodontal bone disease.3–6 Whereas bone modelling refers to the uncoupled resorption and formation of bone tissue, bone remodelling is the coupled process of bone formation following resorption.7–9 The resorption and formation of bone occurs at different points in time and involves bone tissue mineralization that progressively develops and changes over time.8 As such, the active modelling and remodelling at the AB region results in various tissue mineral distributions within the mandible.
As degree of bone mineralization (DBM) accounts for the amount of mineral content at the tissue level of bone, the distribution of DBM directly reflects biological activities (resorption and formation) in the process of bone remodelling.10,11 Although many studies have suggested possible tools to assess the DBM, destructive specimen preparation (histology, backscattered electron imaging and microradiography)12–14 and non-destructive but high radiation dose specimen preparation requiring micro-CT limited the usage of these methods to the laboratory.15,16 On the other hand, usage of clinical cone beam CT (CBCT) in the dental setting has rapidly increased over the past several years.17 Although many previous studies have indicated that CBCT can assess bone mineral using grey levels of CBCT image, its clinical applicability is still controversial.18–21
The objective of this study was to examine whether clinical CBCT can assess the relative difference in distribution of bone mineralization in a human mandible. Thus, the aims of this study were to determine if (1) clinical CBCT and higher resolution micro-CT provide comparable results to assess relative differences of DBM (grey level) parameters in the same CT image and (2) if the percentage difference relative to an internal reference can be a reliable method when assessing the DBM using CBCT images. Micro-CT and CBCT images of human cadaveric mandibles were used to address these aims. In addition, CBCT images obtained from patients were used to test applicability of the new DBM analysis methods clinically.
Materials and methods
Ten human mandibles were obtained from cadavers (seven males and three females, mean 81.5 ± 12.1 years) that were provided by the Division of Anatomy, College of Medicine of the Ohio State University, Columbus, OH. Individual teeth were randomly selected for analysis (four left canines, one right canine, two left premolars and three right premolars). All teeth with dental restorations were excluded to avoid X-ray artefacts. After removal of soft tissue, the mandibular bone was sectioned using a low-speed saw (Isomet®; Buehler, Lake Bluff, IL) with two parallel diamond blades under water irrigation. The 10 mm thick slice of specimen was made in a buccolingual direction and perpendicular to the occlusal plane (Figure 1). The specimens were stored at −21 °C until used.
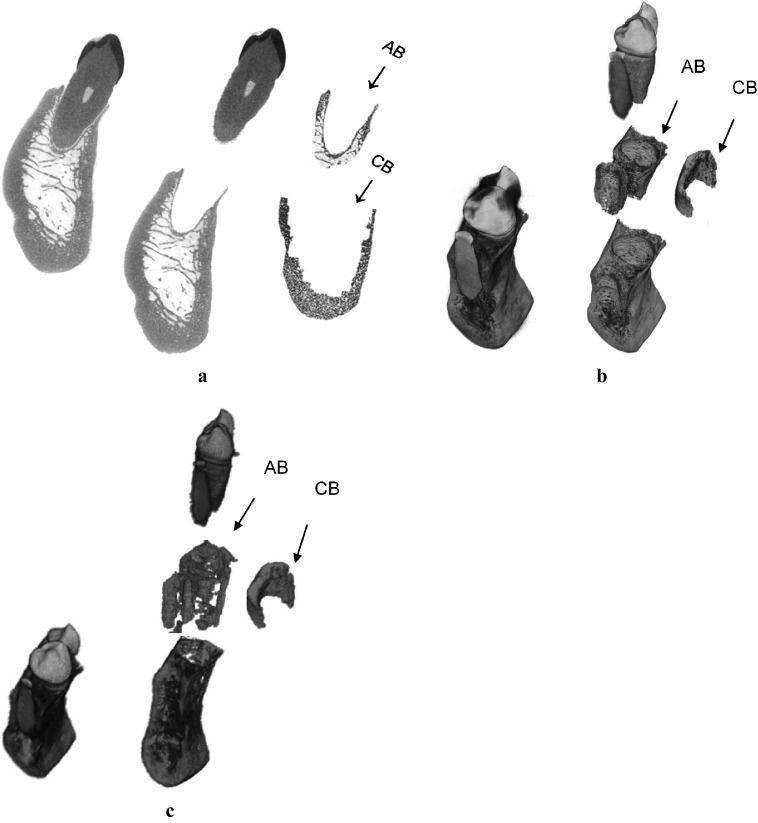
Figure 1.
(a) Alveolar bone (AB) and basal cortical bone (CB) regions of a human mandible on two-dimensional micro-CT image, (b) isolation of those regions using three-dimensional (3D) micro-CT image, and (c) using 3D cone beam CT image for the same specimen
After thawing at room temperature, specimens were scanned by Skyscan 1172 high-resolution micro-CT (Skyscan, Kontich, Belgium) with the scanning and reconstruction voxel sizes at 27.2 μm. The same scanning conditions (70 kV, 141 μA and 20 min scanning time) were applied for all specimens. These same specimens were also scanned by a CBCT scanner (i-CAT®; Imaging Science International, Hatfield, PA) at 200 μm, 300 μm and 400 μm voxel sizes under the same scanning energy (120 kV and 5 mA) but with different scanning times (26.9 s, 8.9 s and 8.9 s for images with 200 μm, 300 μm and 400 μm voxel sizes, respectively), which are the scanning ranges used in clinical practice. Bone voxels of those CT images were segmented from non-bone voxels using a heuristic algorithm as described in previous studies.2,22
After segmentation, mandibular bone regions were identified following the image process described in a previous study.2 The teeth in the three-dimensional (3D) micro-CT image were digitally separated from the mandible segment using imaging software (ImageJ; National Institutes of Health, Bethesda, MD) (Figure 1). Then, the isolated teeth images were three-dimensionally dilated by 39 voxels. The dilated teeth images were overlapped on the separated mandible segment providing a 3D AB region within 1 mm of the tooth roots (Figure 1a,b). A basal cortical bone (CB) region was determined by three-dimensionally eroding the separated mandible segment by 22 voxels (0.6 mm) from periosteal and endosteal surfaces of basal bone. This isolation process provided a core region of basal CB at which normal bone remodelling occurs, excluding the marginal basal bone at which a rapid bone turnover due to active bone formation is observed.23
As the resolution of a CBCT image is much coarser than that of a micro-CT image, the voxels of tooth and periodontal ligament in the CBCT image were not as clearly distinguishable as those in the micro-CT image. Thus, Livewire® (Institute of Computing, State University of Campinas, São Paulo, Brazil), a semi-automated segmentation software, was used to isolate the teeth in CBCT images following the procedure introduced in the previous study.24 After the removal of teeth, the 3D AB and basal CB regions were identified in the CBCT images corresponding to those regions in the micro-CT images (Figure 1c). The AB region was isolated within approximately 1 mm of the root surface, and the core region of basal CB region was located below the tooth root and approximately 0.6 mm away from the periosteal and endosteal borders of the mandibular bone base using ImageJ.
Following the Institutional Review Board approval at the Ohio State University (Protocol no. 2011H0128), 15 CBCT images were selected from a previously existing database of 350 CBCT scans taken during routine clinical practice at the College of Dentistry. These clinical CBCT images had been taken using the same CBCT scanner as the cadaveric specimens scanned in the current study. These young patients (seven males and eight females, mean 18.9 ± 3.3 years) were all healthy individuals without any medical complications or medications at the time of scanning. Mandibular left premolar tooth regions were identified from each CBCT image. Once again, restored teeth were excluded. The voxel size of the clinical CBCT images was 300 μm for 14 images and 400 μm for 1 image. The AB and basal CB regions in these clinical 3D CBCT images were identified following the same process used for the CBCT images of a cadaveric specimen.
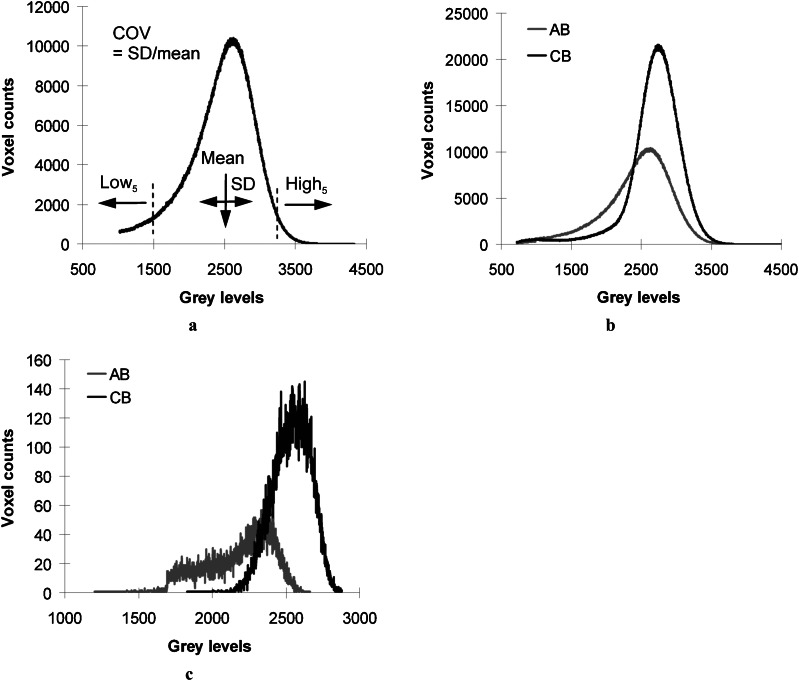
The grey level of each bone voxel, which is equivalent to the DBM, was obtained in the process of bone voxel segmentation. The grey level histograms of AB and basal CB regions were used to determine the mean value that was computed by dividing the sum of grey level values by the total voxel count in each region (Figure 2a). Standard deviation (SD) of the grey level histogram and the coefficient of variation (COV = SD/mean) were obtained to account for the variability of grey levels. Low and high grey levels (Low5 and High5) were determined at the 5th and the 95th percentiles of voxel counts in the histogram. Percentage difference (%) of the grey level parameters between AB and basal CB {(AB − CB)/[(AB + CB)/2] × 100} was then computed for each CBCT image. A paired t-test was used to compare the grey level parameters between AB and basal CB regions in the same individual image for all micro-CT images (27.2 μm voxel size) and CBCT images (the cadaveric images at 200 μm, 300 μm, 400 μm voxel sizes and the clinical images). Paired t-testing was also used to compare the percentage differences of grey level parameters between micro-CT and CBCT images of cadaver specimens. A linear regression was used to test correlations of the percentage differences of grey level parameters with voxel sizes of CBCT images of cadaver specimens. Finally, the percentage differences of grey level parameters based on the CBCT images (300 μm voxel size) of the old cadaver group were compared with those of a young patient group using analysis of variance. Significance was set at 0.05 or less.
Figure 2.
(a) Degree of bone mineralization parameters determined using a grey level histogram, (b) comparison of grey level histograms between alveolar bone (AB, grey line) and basal cortical bone (CB, black line) regions using three-dimensional (3D) micro-CT image, and (c) using a 3D cone beam CT image (200 μm voxel size) for the same specimen. COV, coefficient of variation; High5, grey level at the 95th percentile; Low5, grey level at the 5th percentile; SD, standard deviation
Results
The histograms of all grey level parameters were successfully obtained from the 3D micro-CT and CBCT images of the AB and the basal CB regions (Figures 1 and 2). The grey level histogram of micro-CT images resembled that of CBCT images (Figure 2b,c).
The AB region had significantly lower means of Mean, Low5 and High5 but significantly higher SD and COV than the basal CB region (p < 0.05) (Figure 2 and Table 1). These results were consistently observed for all micro-CT and CBCT images independent of the scanning voxel sizes (200 μm, 300 μm and 400 μm), and the old cadaver and the young patient groups. There was no gender effect evident (p > 0.05).
Table 1.
Measures of grey level parameters at each region using micro-CT and cone beam CT (200 μm, 300 μm and 400 μm voxel sizes) clinical images
| Mean |
SD |
COV |
Low5 | High5 | ||||||
| AB | CB | AB | CB | AB | CB | AB | CB | AB | CB | |
| Micro-CT | 2288.37 ± 289.42 | 2788.12 ± 213.36 | 561.96 ± 161.61 | 312.17 ± 59.05 | 0.25 ± 0.08 | 0.11 ± 0.02 | 1234.10 ± 503.88 | 2293.80 ± 235.37 | 3110.90 ± 307.92 | 3300.60 ± 268.88 |
| 200 μm | 2102.83 ± 117.56 | 2473.87 ± 59.00 | 291.98 ± 52.79 | 190.20 ± 46.55 | 0.14 ± 0.02 | 0.08 ± 0.02 | 1642.89 ± 108.57 | 2124.46 ± 118.33 | 2559.18 ± 144.03 | 2729.72 ± 79.68 |
| 300 μm | 2134.38 ± 104.31 | 2462.24 ± 175.00 | 234.79 ± 43.13 | 172.72 ± 40.75 | 0.11 ± 0.02 | 0.07 ± 0.02 | 1782.75 ± 88.12 | 2189.20 ± 257.86 | 2518.56 ± 117.61 | 2722.95 ± 170.86 |
| 400 μm | 2144.82 ± 129.60 | 2460.89 ± 105.85 | 229.08 ± 41.08 | 166.00 ± 51.03 | 0.11 ± 0.02 | 0.07 ± 0.02 | 1789.93 ± 106.50 | 2155.41 ± 194.49 | 2507.86 ± 145.49 | 2718.63 ± 95.92 |
| Clinical | 1732.39 ± 119.46 | 2519.79 ± 96.77 | 281.60 ± 33.85 | 82.79 ± 34.81 | 0.16 ± 0.03 | 0.03 ± 0.01 | 1283.53 ± 129.82 | 2370.53 ± 136.93 | 2200.53 ± 99.41 | 2631.87 ± 93.40 |
AB, alveolar bone; COV, coefficient of variation; CB, cortical bone; High5, grey level at the 95th percentile; Low5, grey level at the 5th percentile; SD, standard deviation.
All values were significantly different between AB and CB regions (p < 0.05).
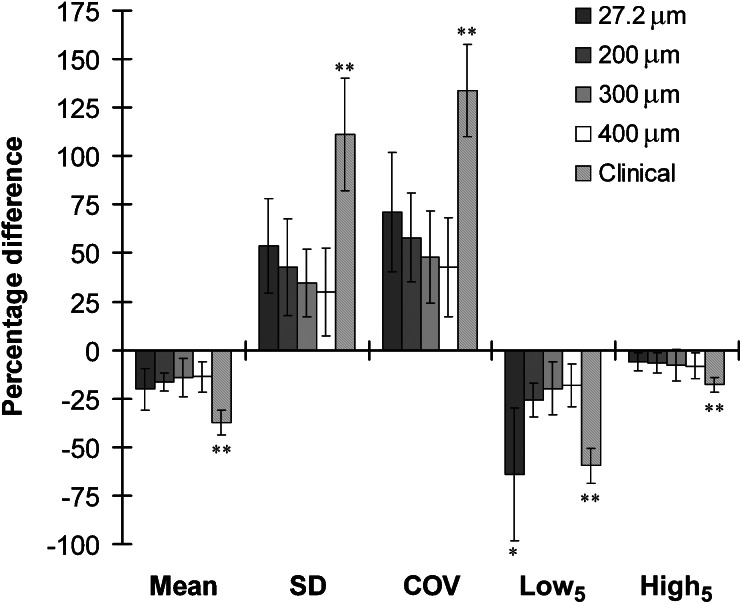
Means of the percentage differences of all grey level parameters between AB and basal CB regions were not significantly different between micro-CT and CBCT images (p > 0.05) except Low5, which had a significantly higher percentage difference for micro-CT images than for CBCT images (p < 0.05) (Figure 3). The percentage differences of SD, COV and Low5 decreased with increasing voxel sizes, having weak but significant correlations with voxel sizes (r2 < 0.425 and p < 0.05), whereas those of Mean and High5 had no significant correlations (p > 0.05). However, the means of the percentage differences of all grey level parameters were not significantly different between CBCT images at the range of voxel sizes examined here (p > 0.05).
Figure 3.
Percentage difference of grey level parameters between alveolar and basal cortical bones for micro-CT (27.2 μm) and cone beam CT (CBCT) images (200 μm, 300 μm, 400 μm voxel size images for old cadaver group and clinical images for young patient group). The negative values indicate that alveolar bone had lower Mean, Low5 and High5 than basal cortical bone. The error bars represent standard deviation of each parameter. The grey level parameters are not significantly different between CT images (p > 0.05) except *p < 0.05 for Low5 between micro-CT and other CBCT images for cadaver group and **p < 0.05 for all grey level parameters between clinical and other CBCT images for cadaver group. COV, coefficient of variation; High5, grey level at the 95th percentile; Low5 grey level at the 5th percentile; SD, standard deviation.
The clinical CBCT images of the young patient group had significantly higher means of the percentage differences of all grey level parameters than the 300 μm voxel size CBCT images of the old cadaver group (p < 0.05) (Figure 3). The negative values of percentage differences indicated that the AB had lower Mean, Low5 and High5 than the basal CB, although their absolute values were higher for the young patient group than for the old cadaver group.
Discussion
A significantly different distribution of the grey level parameters, which are equivalent to the DBM parameters, were found between AB and basal CB in the human mandible. This result was consistent between the in vitro micro-CT images and the CBCT images with different voxel sizes. This finding suggests that assessments for the relative values of DBM based on the clinical CBCT scanner are comparable with those based on the high-resolution micro-CT scanner. It is likely that a masticatory demand stimulated more active bone remodelling in the AB region resulting in the decrease in DBM (grey level Mean, Low5 and High5) and the increase in DBM variability (grey level SD and COV) when compared with those in the basal CB region where normal bone remodelling would occur. Values of the percentage difference of grey level parameters between AB and basal CB regions were not affected at the range of clinical voxel sizes (200 μm, 300 μm and 400 μm) of CBCT images. This result suggests an analysis of percentage differences is a possible method to compare the distribution of bone mineralization between CBCT images taken under different scanning conditions. The substantially lower percentage difference of grey level parameters found in the old cadaver group than in the young patient group possibly resulted from a decrease in bone-forming activities and an increase in tissue mineralization in the AB region with age. The current results support the use of non-invasive clinical CBCT to provide useful information to estimate changes in the degree of jaw bone tissue mineralization, which can account for the progress of dental treatments in a patient.
The DBM parameters have been used to estimate the alteration of bone tissue mineral distribution resulting from bone remodelling.8,25,26 The mineralization of bone at the tissue level correlates with the mechanical properties of bone at the macro level.13 As such, analysis of DBM parameters was proposed to aid the diagnosis of bone disease.10 The distribution of DBM was analysed using a thin section of bone specimen for microradiographs or quantitative backscattered electron imaging.12,13 Although the high-resolution images of bone surface allowed for accurate analysis of bone mineral distribution, these methods require a destructive process during specimen preparation, two-dimensional analysis on the surface of bone and a small local region of interest. Instead, some studies used micro-CT images for DBM analysis because it provided non-invasive 3D scanning of whole bone specimens.2,26 Although the micro-CT-based DBM parameters could provide a valid result in animal studies, its high radiation exposure limits its clinical application for scanning patients. The clinical CBCT image includes grey level of voxels while scanning patients with lower radiation. Thus, it was proposed to use the CBCT image for bone mineral density measurement.27,28 However, the reliability of CBCT in assessing the DBM had not been investigated. In the current study, we examined the regional variations of DBM parameters measured by micro-CT as the gold standard to evaluate those measured by CBCT, because micro-CT-based analysis was anticipated to provide more accurate results because of its much higher resolution (27.2 μm voxel size) than CBCT (greater than 200 μm voxel size). We found that micro-CT- and CBCT-based analyses provided consistent results for the relative difference of every grey level parameter in the human mandible. This finding suggested that micro-CT and CBCT-based analyses for the relative difference of bone mineralization are comparable at the hard tissue level of mandibular bone.
Other studies have demonstrated that CBCT can assess bone mineral density of the jaw bone.19,20,27,29 However, it was noted that the CBCT-based bone mineral density measures varied depending on scanning conditions.30,31 As such, caution should be used when comparing the bone mineral density values between different CBCT images. In the current study, we proposed two ways of analysis to obtain a better interpretation of results from CBCT-based bone mineralization measurement. First, the absolute values of DBM parameters in different oral regions can be compared only using the same image in which grey levels of bone tissue were attenuated under the same scanning and reconstruction conditions. Second, only the relative values of grey level, such as the percentage differences in the current study, can be compared between different CBCT images by using an internal reference, such as the basal CB region in the current study, which is included to compute the relative value.
A substantial amount of the masticatory force applied to teeth is transmitted to the AB through the periodontal ligament.32,33 This masticatory functional demand on the AB region stimulates active bone remodelling.1,2 More bone turnover due to bone remodelling increases newly forming bone regions and decreases pre-existing old bone regions.34,35 As the newly forming tissue has less mineral content than the pre-existing old tissue, the mean DBM is decreased in the actively remodelling region. Tissue mineralization is a long-term process and it takes years to complete full mineralization.8 Thus, the variability of DBM changes in the newly remodelled region depending on the status of mineralization after bone remodelling. The Low5 in the current study accounts for the DBM at the newly forming bone region and the High5 represents the DBM at the pre-existing old bone region. We hypothesize the current results that demonstrated lower Mean, Low5 and High5 but higher variability in the AB region than in the basal CB region reflects the active bone turnover in the AB region under the masticatory stimulus. As the current 3D micro-CT and CBCT image-based analysis allowed for identifying the 3D AB region completely surrounding the periodontal ligament that outlines the tooth structure, more comprehensive results could be obtained than the 2D histological and micro-radiography analysis. This is the first study analysing the DBM parameters using the 3D CT image of the human mandible. The DBM analysis technique was first verified using CBCT images for cadaver specimens and then applied to examine clinical CBCT images.
The percentage differences between AB and basal CB regions for grey level variability and Low5 decreased with increasing voxel sizes. This finding probably resulted from the larger voxel size and reduced image resolution which decreased the grey level contrast between voxels. In particular, the finding that the means of Low5 were significantly different between micro-CT images and other CBCT images indicated that high-resolution micro-CT can more precisely segment bone voxels from non-bone voxels than is possible with the lower resolution of CBCT. Owing to the proximity of the periodontal ligament, the AB region has a transition between bone and non-bone voxels whereas the region inside the basal CB does not have a similar demarcation of non-bone voxels. Thus, the Low5 of AB region has lower values because a higher resolution image can provide a more detailed gradient of the low grey levels than a lower resolution image which provides the averaged grey levels. On the other hand, the grey levels of the basal CB region with much less non-bone voxels would be less influenced by changing image resolution. In light of this aspect, it can be hypothesized that the significantly higher percentage difference of Low5 in the micro-CT image than in the CBCT images could arise from a decrease in the Low5 values of the AB region of the micro-CT image. Similarly, the percentage differences for Mean and High5 that were less affected by the non-bone voxels showed more consistent values between CT images independent of voxel sizes. Except for the Low5, the percentage differences of all other grey level parameters were comparable between the micro-CT and CBCT images with different voxel sizes for the same cadaver specimens. Most importantly, all of those parameters between CBCT images were not significantly different. This result indicates that the effect of imaging resolution on comparison of grey level parameters can be reduced when the relative grey levels are compared between different CBCT images scanned at the clinical range of imaging voxel sizes (200 μm, 300 μm and 400 μm), as examined in the current study.
The clinical patient CBCT images were obtained using the same CBCT scanner utilized for human cadaver specimens. The clinical patients and human cadaver mandible specimens represent young and elderly human groups, respectively. From the grey level histogram comparisons of AB and basal CB regions (Figure 2b,c), we hypothesize that the ageing process progressively transforms the grey level distribution of the AB region to be more similar to that of the basal CB region. While there is limited knowledge about the change in AB density with ageing, a previous clinical study showed that an abnormal increase in bone density due to sclerosis occurred at periapical lesions in elderly people.36 In particular, a diffuse type of sclerotic bone was more frequently observed in the mandible than in the maxilla. Sclerotic bone may increase the AB mineral content while decreasing its variability. The current findings supported this change in distribution of the DBM because all of the percentage differences of grey level parameters between AB and basal CB regions for the old cadaver specimens (81.5 years) were lower than those for young clinical patients (18.9 years).
One limitation of this study may be that correlations of the absolute values of DBM parameters between micro-CT and CBCT images were not examined. It was very difficult to exactly match the same regions of interest between the CT images of the mandibles obtained from the two different scanners owing to their more than sevenfold difference in voxel sizes. Additionally, not only the voxel size but also the scanning potential, current and duration were different between micro-CT and CBCT. These substantially different resolution and scanning conditions limited the direct comparison of absolute values between images obtained by the two CT scanners. The other limitation may be that clinical CBCT images of only young patients were analysed. The current study evaluated the feasibility of clinical CBCT images to assess the relative values of bone mineralization, which is the essential step before proceeding to a large cohort study. To extend the results from this study requires a large cohort of clinical CBCT images, which will permit examination of factors that can influence the variation of bone mineralization including age, gender, oral bone regions, status of oral bone complications and dental treatments etc.
In conclusion, CBCT can detect the relative difference in tissue mineral distribution between AB and basal CB regions in the jaw bone providing similar results to micro-CT. This finding indicated that alteration of tissue mineral distribution, which is a consequence of biological activities in jaw bone, can be detected using the individual clinical CBCT images. The percentage difference relative to an internal reference can be a reliable method when assessing the DBM using CBCT images for both cross-sectional and longitudinal comparisons. These CBCT-based analyses for grey level parameters further promote the validity of assessment of bone density changes that is required for dental processes, including orthodontic tooth movement, AB loss in periodontitis, jaw bone augmentation before implantation and bone regeneration after oral surgery.
Acknowledgments
We would like to thank Dr Amanda M. Agnew from the Division of Anatomy, College of Medicine, Ohio State University, Columbus, OH, for providing the human cadaver mandibles.
References
- 1.Ohtani S, Yamamoto T, Iimura A, Takahashi T, Kinoshita Y. Regional differences in D/L aspartic acid ratios in the human mandible as a possible indicator of the bone remodeling rate. Growth Dev Aging 2008; 71: 17–22 [PubMed] [Google Scholar]
- 2.Ames MS, Hong S, Lee HR, Fields HW, Johnston WM, Kim DG. Estrogen deficiency increases variability of tissue mineral density of alveolar bone surrounding teeth. Arch Oral Biol 2010; 55: 599–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meikle MC. The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur J Orthod 2006; 28: 221–240 [DOI] [PubMed] [Google Scholar]
- 4.Deguchi T, Takano-Yamamoto T, Yabuuchi T, Ando R, Roberts WE, Garetto LP. Histomorphometric evaluation of alveolar bone turnover between the maxilla and the mandible during experimental tooth movement in dogs. Am J Orthod Dentofacial Orthop 2008; 133: 889–897 [DOI] [PubMed] [Google Scholar]
- 5.Cavallaro J, Greenstein B, Greenstein G. Clinical methodologies for achieving primary dental implant stability: the effects of alveolar bone density. J Am Dent Assoc 2009; 140: 1366–1372 [DOI] [PubMed] [Google Scholar]
- 6.Nanci A, Bosshardt DD. Structure of periodontal tissues in health and disease. Periodontol 2000 2006; 40: 11–28 [DOI] [PubMed] [Google Scholar]
- 7.Martin BR, Burr DB, Sharkey NA. Skeletal tissue mechanics. New York, NY: Springer-Verlag; 1998. p 118 [Google Scholar]
- 8.Ruffoni D, Fratzl P, Roschger P, Klaushofer K, Weinkamer R. The bone mineralization density distribution as a fingerprint of the mineralization process. Bone 2007; 40: 1308–1319 [DOI] [PubMed] [Google Scholar]
- 9.Seeman E, Delmas PD. Bone quality–the material and structural basis of bone strength and fragility. N Engl J Med 2006; 354: 2250–2261 [DOI] [PubMed] [Google Scholar]
- 10.Roschger P, Paschalis EP, Fratzl P, Klaushofer K. Bone mineralization density distribution in health and disease. Bone 2008; 42: 456–466 [DOI] [PubMed] [Google Scholar]
- 11.Boivin G, Farlay D, Bala Y, Doublier A, Meunier PJ, Delmas PD. Influence of remodeling on the mineralization of bone tissue. Osteoporos Int 2009; 20: 1023–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roschger P, Fratzl P, Eschberger J, Klaushofer K. Validation of quantitative backscattered electron imaging for the measurement of mineral density distribution in human bone biopsies. Bone 1998; 23: 319–326 [DOI] [PubMed] [Google Scholar]
- 13.Follet H, Boivin G, Rumelhart C, Meunier PJ. The degree of mineralization is a determinant of bone strength: a study on human calcanei. Bone 2004; 34: 783–789 [DOI] [PubMed] [Google Scholar]
- 14.Boivin G, Meunier PJ. Methodological considerations in measurement of bone mineral content. Osteoporos Int 2003; 14: S22–S27 [DOI] [PubMed] [Google Scholar]
- 15.Ritman EL. Current status of developments and applications of micro-CT. Annu Rev Biomed Eng 2011; 13: 531–552 [DOI] [PubMed] [Google Scholar]
- 16.Burghardt AJ, Link TM, Majumdar S. High-resolution computed tomography for clinical imaging of bone microarchitecture. Clin Orthop Relat Res 2011; 469: 2179–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scarfe WC, Farman AG. What is cone-beam CT and how does it work? Dent Clin North Am 2008; 52: 707–730 [DOI] [PubMed] [Google Scholar]
- 18.Hua Y, Nackaerts O, Duyck J, Maes F, Jacobs R. Bone quality assessment based on cone beam computed tomography imaging. Clin Oral Implants Res 2009; 20: 767–771 [DOI] [PubMed] [Google Scholar]
- 19.Hsu JT, Chang HW, Huang HL, Yu JH, Li YF, Tu MG. Bone density changes around teeth during orthodontic treatment. Clin Oral Investig 2011; 15: 511–519 [DOI] [PubMed] [Google Scholar]
- 20.Naitoh M, Hirukawa A, Katsumata A, Ariji E. Evaluation of voxel values in mandibular cancellous bone: relationship between cone-beam computed tomography and multislice helical computed tomography. Clin Oral Implants Res 2009; 20: 503–506 [DOI] [PubMed] [Google Scholar]
- 21.Katsumata A, Hirukawa A, Okumura S, Naitoh M, Fujishita M, Ariji E, et al. Relationship between density variability and imaging volume size in cone-beam computerized tomographic scanning of the maxillofacial region: an in vitro study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 107: 420–425 [DOI] [PubMed] [Google Scholar]
- 22.Kim DG, Christopherson GT, Dong XN, Fyhrie DP, Yeni YN. The effect of microcomputed tomography scanning and reconstruction voxel-size on the accuracy of stereological measurements in human cancellous bone. Bone 2004; 35: 1375–1382 [DOI] [PubMed] [Google Scholar]
- 23.Huja SS, Beck FM. Bone remodeling in maxilla, mandible, and femur of young dogs. Anat Rec 2008; 291: 1–5 [DOI] [PubMed] [Google Scholar]
- 24.Agbaje JO, Jacobs R, Maes F, Michiels K, van Steenberghe D. Volumetric analysis of extraction sockets using cone beam computed tomography: a pilot study on ex vivo jaw bone. J Clin Periodontol 2007; 34: 985–990 [DOI] [PubMed] [Google Scholar]
- 25.Roschger P, Gupta HS, Berzlanovich A, Ittner G, Dempster DW, Fratzl P, et al. Constant mineralization density distribution in cancellous human bone. Bone 2003; 32: 316–323 [DOI] [PubMed] [Google Scholar]
- 26.Yao W, Cheng Z, Koester KJ, Ager JW, Balooch M, Pham A, et al. The degree of bone mineralization is maintained with single intravenous bisphosphonates in aged estrogen-deficient rats and is a strong predictor of bone strength. Bone 2007; 41: 804–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nomura Y, Watanabe H, Honda E, Kurabayashi T. Reliability of voxel values from cone-beam computed tomography for dental use in evaluating bone mineral density. Clin Oral Implants Res 2010; 21: 558–562 [DOI] [PubMed] [Google Scholar]
- 28.Naitoh M, Kurosu Y, Inagaki K, Katsumata A, Noguchi T, Ariji E. Assessment of mandibular buccal and lingual cortical bones in postmenopausal women. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 104: 545–550 [DOI] [PubMed] [Google Scholar]
- 29.Norton MR, Gamble C. Bone classification: an objective scale of bone density using the computerized tomography scan. Clin Oral Implants Res 2001; 12: 79–84 [DOI] [PubMed] [Google Scholar]
- 30.Kwong JC, Palomo JM, Landers MA, Figueroa A, Hans MG. Image quality produced by different cone-beam computed tomography settings. Am J Orthod Dentofacial Orthop 2008; 133: 317–327 [DOI] [PubMed] [Google Scholar]
- 31.Loubele M, Jacobs R, Maes F, Denis K, White S, Coudyzer W, et al. Image quality vs radiation dose of four cone beam computed tomography scanners. Dentomaxillofac Rad 2008; 37: 309–318 [DOI] [PubMed] [Google Scholar]
- 32.Poiate IA, de Vasconcellos AB, de Santana RB, Poiate E. Three-dimensional stress distribution in the human periodontal ligament in masticatory, parafunctional, and trauma loads: finite element analysis. J Periodontol 2009; 80: 1859–1867 [DOI] [PubMed] [Google Scholar]
- 33.Ona M, Wakabayashi N. Influence of alveolar support on stress in periodontal structures. J Dent Res 2006; 85: 1087–1091 [DOI] [PubMed] [Google Scholar]
- 34.Marcus R. Clinical review 76: The nature of osteoporosis. J Clin Endocrinol Metab 1996; 81: 1–5 [DOI] [PubMed] [Google Scholar]
- 35.Busse B, Hahn M, Soltau M, Zustin J, Puschel K, Duda GN, et al. Increased calcium content and inhomogeneity of mineralization render bone toughness in osteoporosis: mineralization, morphology and biomechanics of human single trabeculae. Bone 2009; 45: 1034–1043 [DOI] [PubMed] [Google Scholar]
- 36.Ohba T, Takata Y, Ansai T, Morimoto Y, Tanaka T, Kito S, et al. Evaluation of the relationship between periapical lesions/sclerotic bone and general bone density as a possible gauge of general health among 80-year-olds. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005; 99: 355–360 [DOI] [PubMed] [Google Scholar]