Abstract
Objectives:
The purpose of this study was to determine the potential of high-resolution ultrasonography for the detection of temporomandibular joint (TMJ) changes in children with juvenile idiopathic arthritis (JIA).
Methods:
We investigated prospectively 20 children (17 female and 3 male; mean age 11.06 years, standard deviation 3.43 years) with TMJ disorders caused by JIA, over a period of 16 months. Using a 12 MHz array transducer, four images in each TMJ (160 images) were acquired. Each image was analysed with regard to five different aspects (condylar erosion, thickness of the condylar disc, synovial thickness, joint effusion and enlargement of the intra-articular space).
Results:
Diagnosis of JIA was ensured for every child and involvement of the TMJ was proven by MRI. Overall 287 changes (35.9%) were detected by using high-resolution ultrasonography. On 124 images (77.5%) condylar erosions were diagnosed; on 55 images (34.4%) synovial thickness was abnormal; on 48 images (30%) we could see higher thickness of the condylar disc; on 40 images (25%) irregularities of the bony surface were detected; and on 20 images (12.5%) we found joint effusion.
Conclusion:
High-resolution ultrasonography could be a sufficient diagnostic method, especially for the detection of condylar involvement in children with JIA, even if not all parts of the TMJ are visible for ultrasonography. High-resolution ultrasonography is a valuable tool in particular situations: (i) when MRI examination is not available; (ii) when children fear MRI examination; (iii) in more advanced stages of JIA; and (iv) for monitoring the progression of TMJ involvement and response of therapy.
Keywords: high-resolution ultrasonography, juvenile idiopathic arthritis, temporomandibular joint, condylar changes
Introduction
Juvenile idiopathic arthritis (JIA) is a disease belonging to a heterogeneous group of diseases of unknown aetiology. Typical signs for JIA are chronic inflammation of one or more joints, with an onset before the age of 16 years and a minimum duration of 6 weeks.1,2 JIA is considered to be an autoimmune disease with a genetic component. The annual incidence ranges from 8 to 22.6 per 100 000 children. The prevalence of 7–401 per 100 000 children depends on different regions.3–5 JIA is the most common chronic systemic autoimmune disease of childhood and adolescence.6 Differential diagnoses showing similar symptoms should include purulent arthritis, haemophilia and rheumatic fever as well as rare diseases such as leukaemia or lupus erythematosus disseminatus.7 According to the current International League of Associations for Rheumatology (ILAR) classification, JIA is divided into seven subtypes (Table 1). Involvement of four or fewer joints in the first 6 months correlates with an oligoarthritis, while involvement of more than four joints correlates with a polyarthritis.1,3,6 Initial symptoms for JIA are fatigue, concentration weakness and reduced general condition.7 Severe forms of the disease may include retardation of growth and development, delayed sexual maturation, and in some cases even an arrest of growth.
Table 1.
ILAR classification of JIA, in agreement with the ILAR conference of 2001 in Edmonton1
| No. | Subtypes of JIA | Extra-articular manifestations | Disqualifying criteria |
| 1 | Systemic arthritis (Still's syndrome) | Fever, exanthema, pleuritis, hepatosplenomegaly, pericarditis, dystrophia, lymphadenopathia, vasculitis, short stature | a,b,c,d |
| ICD-10: M08.2 | |||
| 2 | Seronegative polyarthritis (RF−) | Subfebrile temperatures, tenosynovitis, uveitis, vasculitis | a,b,c,d,e |
| ICD-10: M08.3 | |||
| 3 | Seropositive polyarthritis (RF+) | Subfebrile temperatures, tenosynovitis, rheumatic nodes | a,b,c,d |
| ICD-10: M08.0 | |||
| 4 | Oligoarthritis | Chronic uveitis | a,b,c,d,e |
| ICD-10: M08.4 | |||
| 5 | Psoriatic arthritis | Psoriasis | b,c |
| ICD-10: L40.5/M09. | |||
| 6 | Enthesitis-associated arthritis | Arthritis and enthesitis, acute uveitis, sacroiliitis, Reiter syndrome, ankylosing spondylitis | a,d,e |
| ICD-10: M08.1 | |||
| 7 | Undifferentiated arthritis | ||
| ICD-10: M08.8 |
ICD, (World Health Organization) International Classification of Diseases; ILAR, International League of Associations for Rheumatology; JIA, juvenile idiopathic arthritis.
Disqualifying criteria: a, psoriasis in patients or psoriasis of relatives in first degree; b, human leukocyte antigen B27 positive form, male gender or older than 6 years; c, ankylosing spondylitis, enthesitis associated arthritis, sacroiliitis with chronic and inflammatory intestinal diseases, Reiter syndrome in patients or relatives in first degree; d, rheuma factor repeatedly provable within an interval of at least 3 months; e, systemic signs of disease.
Source: Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004; 31: 390–392.
Temporomandibular joint (TMJ) disorders in children are very rare. The reported prevalence for TMJ involvement in children with JIA varies widely, ranging from 17% to 87%, depending on the different subtypes of JIA, methods used for diagnosis and the population studied.8–13 Among children with JIA, females dominate, with a distribution of 3:2.14 The risk of TMJ involvement in JIA is elevated in children with the polyarticular-onset form or the antinuclear antibody (ANA)-positive form, but also affects those with other subtypes of JIA. A lower risk is related to children with human leukocyte antigen B27 (HLA-B27)-positive form.10,13,15 A profound consequence of TMJ involvement in JIA could be a growth deficit of the mandible due to inflammatory processes located in the condylar growth zone.15,16
Radiological investigation methods play a significant role in the diagnosis of JIA and contribute decisively to early detection of the disease, and thus allow initiation of different therapies as early as possible. Conventional X-rays have been the leading procedure for imaging arthritic joints. In recent years, X-rays have been gradually replaced by newer methods such as MRI and high-resolution ultrasonography (HR-US).17,18 Imaging diagnostic procedures such as panoramic view, MRI and HR-US could detect destructive changes in early stages of the disease, since clinical symptoms of TMJ involvement in JIA commonly appear very late. According to the literature, results for HR-US are unverifiable and therefore this method is still not established.19
The purpose of this study was to determine the potential of HR-US for the detection of condylar erosions and further TMJ changes in children with JIA.
Materials and methods
Over a period of 16 months (December 2008 to March 2010) 236 young patients, comprising 161 females (68%) and 75 males (32%), were seen in the rheumatologic consultation hour of the Department of Orthodontics at the University Medical Center Hamburg Eppendorf (head: Professor Dr B. Kahl-Niecke) with the diagnosis of JIA. Informed consent and ethical approval from the local ethical committee were obtained (PV 3077). Indications of HR-US in patients with TMJ arthritis for this study were made by fulfilling the following inclusion criteria: ICD-10 diagnosis of JIA, female or male gender and children aged 0–18 years. Exclusion criteria were as follows: other rheumatic diseases (e.g. rheumatoid arthritis, ankylosing spondylitis etc.), serious somatic diseases, unsafe, or not yet confirmed, diagnosis, pregnancy and lactation, patients who received medical or surgical treatment for their disease, cardiac pacemakers, artificial limbs, metal implants and joint replacements.
Among these children we found 38 patients (16.1%) under the age of 18, who were referred to our centre with a new diagnosis of JIA to clarify the possibility of TMJ involvement, comprising 27 females (71.1%) and 11 males (28.9%). Overall we detected TMJ involvement in 28 patients (73.7%)—21 female (75%) and 8 male (25%). Out of this group it was possible for only 20 children to participate in this prospective study. With a mean age of 11.06 years (standard deviation ±3.43 years), we included 17 female patients (85%, mean age 10.9 years) and 3 male (15%, mean age 12 years).
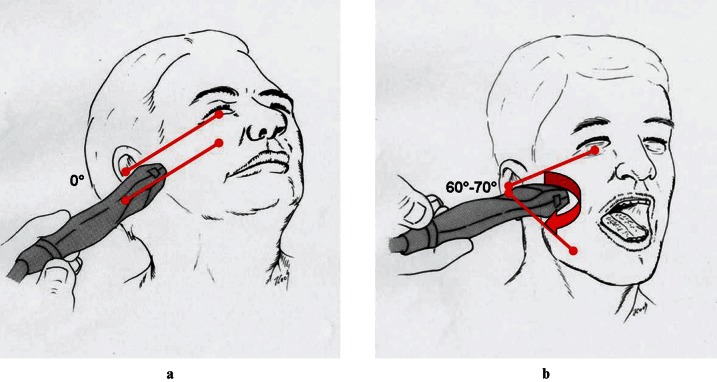
All children and parents were informed about the study procedure and written consent was received. In all cases, TMJ involvement was detected by MRI investigation and clinical examination. Laboratory analysis was performed with regard to the detection of increased laboratory values (e.g. rheumatic factor, HLA-B27 or Borrelia antibodies). For clinical examination the following signs of joint involvement were checked: deviation of the chin, abnormality of the mandibular angle, dental midline shift, crossbite situation, deviation at mouth opening, reduced maximum mouth opening of less than 30 mm, reduced protrusion and laterotrusion of less than 5 mm, joint clicking, crepitation, and pain on palpation and TMJ lock. HR-US was performed 14 days at the latest after MRI (mean duration 8.85 days, standard deviation 2.98 days). To perform the study single-blind, the HR-US operator, as well as the radiologist evaluating the MRIs, independently established the presence or absence of TMJ involvement in JIA. A direct metrical comparison of MRI and HR-US was not done, as MRI was used only to verify the diagnosis of TMJ involvement. All 20 patients underwent HR-US on both sides, so that a total of 40 TMJs were investigated. On each TMJ a total of four ultrasonographic recordings were made. The transducer was positioned against the patient's face overlying the zygomatic arch and TMJ at a 60–70° angle, as well as parallel to the Frankfurt horizontal plane, once with the mouth closed and once with an open mouth (Figure 1). HR-US was performed with a real-time 12 MHz linear-array transducer made by General Electric (LOGIQ P6; GE Healthcare, Fairfield, CT). HR-US was performed by a radiologist experienced in US of the head and neck (CRH). All HR-US investigations were performed in a darkened room on a special examination table, with the children lying on their side.To avoid errors, all HR-US scan-series were performed bilaterally, using a standardized protocol in the same order. Starting with the left side, the transducer was positioned parallel to the Frankfurt horizontal plane. The first scan was taken at closed mouth position, and the second was taken at maximum mouth-opening position. The transducer position was then changed to an angle of 60–70° to the Frankfurt horizontal plane. Two scans were taken, the first with a closed mouth and the second at maximum mouth-opening position.
Figure 1.
Ultrasonographic investigation of the temporomandibular joint with the transducer positioned (a) against the patient's face parallel to the Frankfort horizontal plane and (b) overlying the zygomatic arch and the temporomandibular joint at a 60–70° angle
The images were interpreted by two different investigators (ATA and CRH) independently, and checked against each other. Evaluation of the HR-US images was performed by using OsiriX v. 3.7.1 imaging software for Mac OS10.5 or higher (Fondation OsiriX, Geneva, Switzerland). All images were evaluated with respect to TMJ changes caused by JIA. These were pathological changes such as condylar erosion, thickness of the condylar disc, synovial thickness, joint effusion and enlargement of the intra-articular space. Figures 2 and 3 demonstrate typical HR-US scans with and without the marked anatomical structures (Figures 2 and 3).
Figure 2.
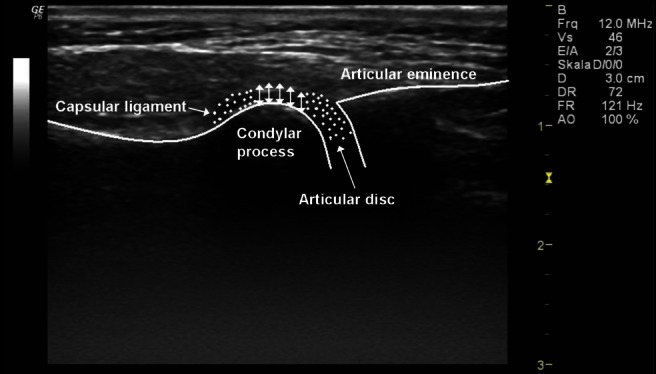
High-resolution ultrasonographic scan of the left temporomandibular joint in transversal/axial slice of an infant without pathological changes (typical anatomical structures with marked anatomical structures)
Figure 3.
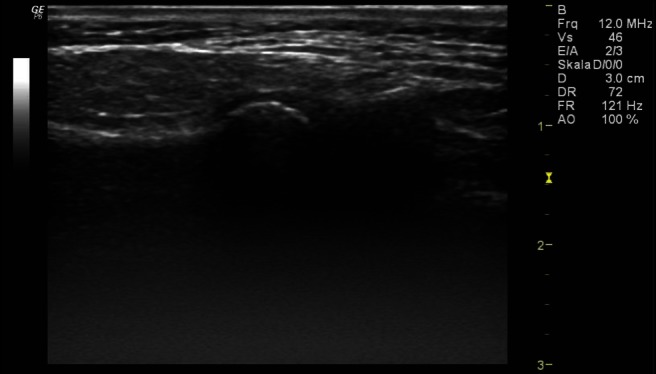
High-resolution ultrasonographic scan of the left temporomandibular joint in transversal/axial slice of an infant without pathological changes (typical anatomical structures without marked anatomical structures)
As demonstrated in Table 2, condylar changes have been evaluated with regard to five different aspects. First, we measured the synovial width to evaluate a possible synovial thickening resulting from TMJ involvement in JIA. This has been measured in millimetres based on the width of the synovia with a standard value of 1.56 mm (range 1.76–1.89 mm, standard deviation 0.2 mm; Table 2).
Table 2.
Standard values/average values of the different parameters measured with HR-US (approximated with an accuracy of two digits after the decimal point)
| Parameter | Synovial width | Effusion in the joint space | Width of the articular disc | Condylar erosion—hyperechoic reflection | Visible irregularities on the bony surface |
| Transversal cmp | 1.89 mm ± 0.2 mm | Yes/no | 1.81 mm ± 0.2 mm | 0.59 mm ± 0.14 mm (sv < 0.45 mm) | Yes/no |
| Coronal cmp | 1.88 mm ± 0.2 mm | Yes/no | 1.81 mm ± 0.2 mm | 0.59 mm ± 0.15 mm (sv < 0.44 mm) | Yes/no |
| Transversal mmop | 1.77 mm ± 0.2 mm | Yes/no | 1.69 mm ± 0.2 mm | 0.59 mm ± 0.15 mm (sv < 0.45 mm) | Yes/no |
| Coronal mmop | 1.76 mm ± 0.2 mm | Yes/no | 1.67 mm ± 0.2 mm | 0.59 mm ± 0.14 mm (sv < 0.45 mm) | Yes/no |
cmp, closed-mouth position; HR-US, high-resolution ultrasonography; mmop, maximum mouth-open position; sv, standard value.
The second parameter was the evaluation of joint effusion resulting from a current inflammatory activity in the affected joint. This parameter has been evaluated by sonographically visible fluid accumulation within the articular joint.
As a third parameter we measured the thickness of the condylar disc to evaluate a narrowed or thickened disc resulting from reactive arthritic or traumatic changes. Measuring the thickness of the condylar disc can simultaneously provide information about the height of the anterior joint space. The disc was measured in millimetres, with a standard value of 1.57 mm (average value 1.67–1.81 mm, standard deviation 0.2 mm; Table 2).
The fourth parameter was to diagnose condylar erosions, which become visible by an increased hyperechoic reflection resulting from arthritic inflammatory activity on the condylar surface. The width of the hyperechoic reflection was measured in millimetres, with a standard value lower than 0.44 mm (average value 0.59 mm, standard deviation 0.14 mm; Table 2).
The fifth parameter to detect TMJ involvement in JIA has been analysed by the presence of irregularities of the condylar surface. Visible irregularities argue for already advanced destruction of cartilaginous and osseous structures (Table 2).
Results
20 children were investigated by HR-US, 17 female (85%) and 3 male (15%). The mean age of all children at the time of examination was 11.06 years (10.9 years in females, 12 years in males). The mean age at onset of JIA was 6.9 years (6.5 years in females, 9 years in males). The mean duration of the disease at time of TMJ manifestation was 4.19 years (4.4 years in females and 3 years in males; Table 3). The children included in this study showed the following JIA subtypes: oligoarthritis, 10 children (50%); psoriatic arthritis, 5 children (25%); polyarthritis, 2 children (10%); enthesitis-related arthritis, 2 children (10%); and systemic arthritis, 1 child (5%; Table 3). Depending on the subtype of JIA the shortest period between disease onset and first presentation in our department was in the children with enthesitis-related arthritis (mean duration 0 years), while the longest period was in children with oligoarthritis (mean duration 5.85 years; Table 3).
Table 3.
Characteristics of the study population
| Characteristics | Number (%) | Average age at disease onset (years) | Average age at time of MRI and HR-US (years) | Disease duration at time of first orthodontic presentation (years) |
| Gender | ||||
| Male | 3 (15%) | 9 | 12.0 | 3 |
| Female | 17 (85%) | 6.5 | 10.9 | 4.4 |
| JIA-subtype | ||||
| Oligoarthritis | 10 (50%) | 4.25 | 10.1 | 5.85 |
| Polyarthritis | 2 (10%) | 5.5 | 8.5 | 3 |
| Psoriatic arthritis | 5 (25%) | 10 | 13 | 3 |
| Systemic arthritis | 1 (5%) | 10 | 15 | 5 |
| Enthesitis related | 2 (10%) | 11.5 | 11.5 | 0 |
| Serological results | ||||
| RF | 5 (25%) | 3.9 | 10 | 6.1 |
| ANA | 11 (55%) | 6.6 | 10.9 | 4.3 |
| AST | 5 (25%) | 3.6 | 9.6 | 6 |
| HLA-B27 | 1 (5%) | 11 | 11 | 0 |
| Borrelia AB | 2 (10%) | 7 | 9 | 2 |
| No proof | 3 (15%) | 4.5 | 6.5 | 2.5 |
AB, antibodies; ANA, antinuclear antibodies; AST, antistreptolysin titre; HLA-B27, human leukocyte antigen B27; HR-US, high-resolution ultrasonography; RF, rheumatoid factor.
In addition to TMJ involvement in JIA, children in this study most frequently exhibited an inflammatory involvement of the knees (70%) and the ankles (55%). An organic manifestation of JIA was described in three cases (15%). These was a spina bifida occulta in one child (5%) and an inflammatory involvement of the intestine in two children (10%).
Referring to extra- and intraoral asymmetries, we found 15 children with a deviation of the chin to the middle of the face (75%), 8 children with a different amount of the mandibular angle (40%), 2 children with a dental midline shift (10%) and 2 more children with a crossbite situation in the molar region (10%). As part of the functional analysis we could see a deviation at mouth opening in 14 children (70%), reduced maximum mouth opening of less than 30 mm in 8 children (40%), and reduced protrusion in 1 child (5%). However, we found reduced laterotrusion of less than 5 mm in none of the children. The most common pathological finding by examination was joint clicking during mouth opening in 7 children (35%) followed by joint clicking during mouth closing in 1 child (5%) and crepitation in the TMJ in 1 child (5%). We found pain on palpation of the TMJ in two children (10%). A case of TMJ lock in the closed- or open-mouth position was not observed.
By analysis of the rheuma specific values, we measured an increased value of rheumatic factor (RF) in 5 children (25%), ANA in 11 children (55%) and antistreptolysin titer (AST) in 5 children (25%). We found an increased value of HLA-B 27 in 1 child (5%) and in 2 children an increased value of Borrelia antibodies (10%; Table 3).
Among the 20 children in this study population with the diagnosis of JIA, we made and evaluated a total of 160 HR-US images of the TMJ. The results of the investigation by HR-US are shown in Table 4. In the HR-US images we most frequently saw a hyperechoic cortical inflammation in surface structure of the mandibular condyle. Overall we found a total of 124 positive results in 160 images (77.5%), 68 on the right side (42.5%) and 56 on the left side (35%; Figures 4 and 5). We found a total number of 55 positive results (34.4%) out of 160 images for an abnormal width of the synovia also demonstrating a narrowed joint space in the TMJ. Of these 55 positive results 34 were on the right side (21.3%) and 21 were on the left side (13.1%). An abnormal width of the articular disc could be detected on 48 images (30%), with 26 positive results on the right side (16.25%) and 22 on the left side (13.75%). We found irregularities of the condylar surface in 40 images (25%), with 20 positive results (12.5%) on each side. The least common findings on HR-US images were an effusion in the TMJ space with 20 positive results (12.5%), 8 on the right side (5%) and 12 on the left side (7.5%). In total, among these 20 children with JIA of the TMJ, we collected 200 findings by recording five different diagnostic criteria out of four images per side. In summary, we found 182 matches of the diagnostic findings by HR-US with a specificity of 91% (confidence interval 95%) and 18 non-matches (9%).
Table 4.
Sonographically visible changes caused by arthritic inflammation in the TMJ
| Findings by high-resolution ultrasonography | Right TMJ no. (%) | Left TMJ no. (%) | Total no. (%) |
| Synovial width (width of the joint space) | 34 (21.3%) | 21 (13.1%) | 55 (34.4%) |
| Effusion in the joint space | 8 (5%) | 12 (7.5%) | 20 (12.5%) |
| Width of the articular disc | 26 (16.25%) | 22 (13.75%) | 48 (30%) |
| Condylar erosion—hyperechoic reflection | 68 (42.5%) | 56 (35%) | 124 (77.5%) |
| Visible irregularities on the bony surface | 20 (12.5%) | 20 (12.5%) | 40 (25%) |
| Total | 156 (19.5%) | 131 (16.4%) | 287 (35.9%) |
TMJ, temporomandibular joint.
The table data are based on 20 children, with four different images of each TMJ, every image receiving five different result parameters—hence 400 possible parameters in all children per side, 800 possible finding parameters in total.
Figure 4.
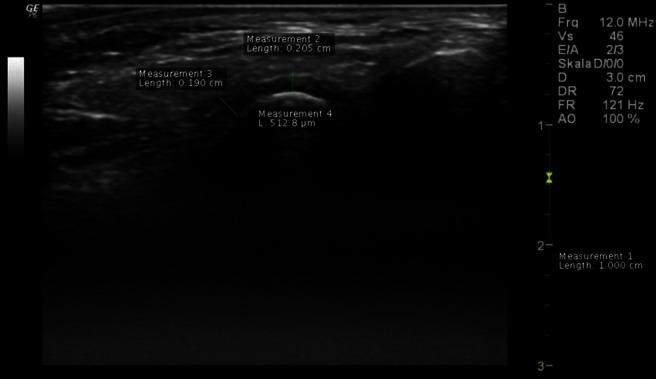
High-resolution ultrasonographic of the left temporomandibular joint in transversal/axial slice at closed-mouth position—increased width of the synovia (measurement 2), thickened articular disc (measurement 3), hyperechoic cortical bone (measurement 4) and inconspicuous bone surface of the articular condyle without joint effusion
Figure 5.
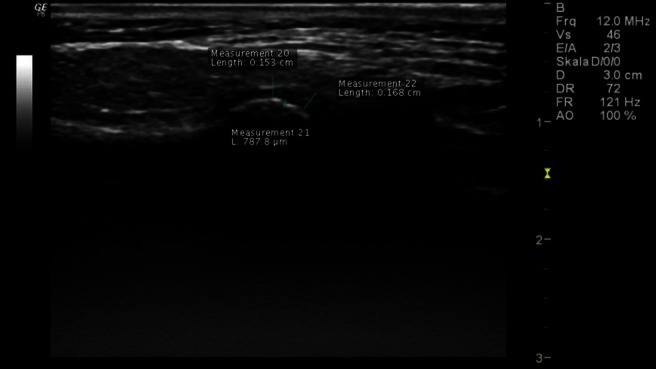
High-resolution ultrasonographic of the right temporomandibular joint in transversal/axial slice at maximum-mouth-open position—clearly visible hyperechoic cortical inflammation in the area of the condyle (measurement 21), reduced width of the synovia with narrowed joint space (measurement 20), visible joint effusion with normal width of the articular disc (measurement 22)
Interexaminer agreement, measured in terms of Cohen's kappa coefficient, was 0.88 for abnormal width of the synovia, 0.81 for abnormal width of the articular disc, 0.91 irregularities of the condylar surface, 0.93 for effusion in the TMJ space and 0.95 for hyperechoic cortical inflammation in the surface structure of the mandibular condyle. The values of interclass correlation coefficient between both examiners show substantial agreement (0.62–0.80), and almost perfect agreement (0.81–1.00).
Discussion
In this prospective study of paediatric patients with all subtypes of JIA, according to the Edmonton criteria (Table 1), we tried to evaluate the advantages of HR-US in the diagnostic investigation of TMJ involvement in JIA. Principal characteristics of JIA are inflammatory processes in the synovia of all joints. In paediatric patients, these characteristics occur in large joints, such as the hip and knee joints, unlike in adult patients with rheumatoid arthritis.1,20,21 The classification of different subtypes of JIA was based on the current ILAR classification containing seven subgroups of JIA (Table 1). Separation between oligo- and polyarthritis is done by a number of affected joints.1,3,6,22 Arthritic changes are reparative or destructive, and can occur in every condyle, as well as in the TMJ. Furthermore, they can appear as inflammatory erosions with joint effusion, sclerosis and flattening of the articular condyle, as well as destructive changes of the articular disc and synovial structures. The prevalence of TMJ involvement in children with JIA is declared, depending on the study, with an incidence of 17–87%.8–13,23 Compared with these results we detected TMJ involvement in 28 of 38 children with JIA (73.7%). The mean age of the children, as well as mean duration between age at onset of JIA and TMJ manifestation, depending on different subtypes, is in accordance to findings in the published literature.2,12,23–25 Among all 20 children who participated in this study, the most common subtype of JIA was the oligoarticular-onset form (50%), which is also in agreement with the results in the literature.6,12,23,26 Pain is a rare symptom in children with TMJ involvement in JIA. Attention should therefore be focused on preventing mandibular growth disturbances, which could precede malocclusion and jaw dysfunction. During clinical examination all children described or demonstrated some type of complaint in their affected TMJ region. Extraoral investigation most frequently revealed a chin deviation, evident in 15 children (75%), which is similar to the results of Jank et al23 and Harper et al.27 Intraoral signs of asymmetry were rare. A dental midline shift and a crossbite situation were found in only 2 children (10%), which is in accordance with the results of Meyer et al28, but less frequent than the results reported by Marini et al.29 By detailed consideration of our results of the functional analysis concerning interincisor distance, we agree with studies evaluating maximal mouth opening in healthy children vs those with a TMJ disease. Those studies have shown that restricted mouth opening to less than 3.5–4.0 cm (interincisor distance) is indicative of a restrained function of the TMJ.6,30 In children affected with JIA, protrusion of the mandible is often limited, ranging from 3.5 mm to 8 mm, which is in accordance with our results.11,31 Furthermore, the frequency of inflammation- or pain-related restricted laterotrusion of less than 5 mm varies from 5% to 45%. This does not match our results, in which we could not detect one case of restricted laterotrusion.11,31 In addition to functional analysis of TMJ involvement in JIA, bilateral clicking and crepitus, as well as asymmetrical mouth opening and lack of translational movement in the TMJ, have been proven to be important predictors, with high specificity but low sensitivity.11,29,32 These symptoms were rare in our study group and correlate only in some cases, as for 9 children (45%) with an abnormal clicking or crepitus in the affected TMJ.
Referring to increased laboratory values, our results are in accordance with the results of Guillaume et al26, Ravelli et al33 and Jordan and McDonagh6, which showed an increased ANA in connection with an oligoarticular or a psoriatic subtype of JIA. The detection of RF can exclude an oligoarthritis as a rule. With regard to the polyarthritis the detection of RF allows a distinction between RF-positive and RF-negative polyarthritis.6,34 In addition, sometimes there is a positive detection of HLA-B27 in the enthesitis-related arthritis, as it was found also in 1 child (5%) in our study population.6,14
Various radiological examination techniques, such as conventional X-ray or CT scans, allow an accurate assessment of osseous structures in the TMJ, but offer less assistance in the analysis of soft tissue and articular disc changes.2,6,35 The evaluation of inflammatory joint processes in rheumatic diseases is done exclusively by MRI, which has gradually replaced conventional X-rays, and thus can be called the gold standard.25,36,37
The majority of clinical studies using HR-US to detect TMJ involvement in JIA are from European university medical centres, which are well experienced in using this technique.35–39 HR-US is less frequently used in the USA, except for identification of synovial thickening and effusions in joints other than the TMJ.25As described in previous studies, the bony anatomy and the small size of the TMJ makes it difficult to adequately visualize effusions and synovial thickening.25,37
A few years ago, ultrasonography with transducers using a frequency ranging from 5 MHz to 7.5 MHz was more common.17 Today, ultrasonography units contain transducers with a standard frequency of 10–12.5 MHz.25,35–39 In our opinion, HR-US using 10 MHz or 12.5 MHz transducers is much more effective for the evaluation of TMJ involvement in JIA, owing to lower penetration into soft tissues and significantly higher resolution on structures located near the surface. Compared with conventional X-rays, the application of HR-US additionally allows the detection of skeletal changes, synovial changes, joint effusion and cartilage thickening, as well as cystic changes.40 The approach to TMJ investigation using HR-US is comparable with those of other investigators, and corresponds with the study design in similar studies.19,36–38 Crucial for the detection of inflammatory involvement of the TMJ in JIA are, as described by several authors, mandibular condyle changes, joint space widening or narrowing, changes in the articular disc, and TMJ or synovial effusion.35,37–39,41
As described by Emshoff et al,38 inflammatory changes of cortical structures can be clearly identified by ultrasonography. Brandlmaier et al36 showed that interpretation of HR-US images using a 12 MHz transducer revealed TMJ osteoarthritis in 79% of cases. Compared with these results, we present almost equal results for the detection of arthritic changes with a frequency of 77.5% (31 TMJs). These are displayed as hyperechoic cortical inflammation (Figures 4 and 5).
Manfredini et al39 described effusion-related changes in the TMJ space in their study population with an incidence of 48.5%. Almost equally, we could show an inflammatory thickened or narrowed reaction of the synovia due to an effusion with a frequency of 34.4% (14 TMJs) by using HR-US.
Further properties of a typical inflammatory affected TMJ in children with JIA could be articular disc changes. Those changes can be depicted as a heightened discus articularis, resulting from joint effusion, as well as a hypoechoic inhomogeneity in the range of an involved discus articularis.35,39,42 On HR-US images we showed such changes in 30% of cases (12 TMJs).
The evaluation of clearly visible irregularities of the condylar surface is a standard procedure in any assessment of MRI scans or conventional panoramic radiographs of the TMJ.11,15,19,32,38 Such an evaluation, in our opinion, can also be done on HR-US images. The assessment of the condylar surface concerning deformities can still be performed, despite imaging of the mandibular condyle on HR-US images only being possible in two planes, without access to the medial portions. In our study group we detected irregularities of the condylar surface in 25% of cases (10 TMJ).
Regarding the detection of inflammatory changes of the TMJ in JIA (e.g. joint or capsular effusion), we share the opinion of Arabshahi and Cron13 that HR-US in addition to MRI is currently the only efficient method of investigation. Thus, HR-US is clearly superior to X-ray examination in the early stages of JIA of the TMJ. Melchiorre et al35 determined TMJ effusion in 65% of cases in a prospective study including 33 children, which was pioneering for imaging TMJ involvement, and thus an indication for early stages of JIA. In our study group, TMJ effusion was detected in 12.5% of cases (5 TMJs), which is in accordance with the results of Jank et al.37 In all cases, TMJ effusion was a reliable marker for an acute stage of JIA.37
One weakness of our study is that there was no possibility of including a control group of healthy children. This is explained by the fact that the local ethical board did not allow inclusion of a healthy control group, as, according to German regulations, unjustified investigations in healthy children are not tolerable. Therefore, we could investigate only children who really suffered from JIA and who showed any symptoms in their TMJ. TMJ involvement in children with JIA was verified by the medical assessment of an experienced paediatric rheumatologist, the clinical examination results at the department of orthodontics and the MRI examination results of TMJ.
Attributable to this fact, it seems that all our findings were not comparable with normal ranges. We attempted to carry out a similar approach by comparing the ultrasonographic with the clinical findings, as demonstrated by Manfredini et al.43 Additionally, we could find only one publication comparable with our work, by Elias et al, demonstrating normal range values of the TMJ by using HR-US.44 One major shortcoming of this publication is that normal range values were analysed only in adults. For that reason, a verifiable and realistic comparison was not achievable. To date, we have been unable to find any publication that works on measurements of the TMJ by HR-US in healthy children.
In our study, all children were known to have TMJ involvement in JIA and were included in the study for this reason. In consideration of the fact that it was previously known whether or not the TMJ was affected, a coincidence between the diagnosis and the visibility of destructive changes or the absence of destructive changes in the analysed HR-US image of each TMJ could be found in 91% of cases.
In conclusion, HR-US can be applied to detect distinctions in synovial width, TMJ space effusion, articular cartilage width, cortical inflammation and condylar surface irregularities. We are confident that HR-US could become one of the leading instruments for the evaluation of TMJ involvement in JIA, owing to lower costs, the less invasive nature of this technique and its better acceptance of the young patients.
Further multicentre investigations, involving a control group and a larger sample size, will be needed in order to more accurately evaluate the effectiveness of HR-US for the evaluation of TMJ involvement in children suffering from JIA.
References
- 1.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004; 31: 390–392 [PubMed] [Google Scholar]
- 2.Huntjens E, Kiss G, Wouters C, Carels C. Condylar asymmetry in children with juvenile idiopathic arthritis assessed by cone-beam computed tomography. Eur J Orthodont 2008; 30: 545–551 [DOI] [PubMed] [Google Scholar]
- 3.Petty RE. Growing pains: the ILAR classification of juvenile idiopathic arthritis. J Rheumatol 2001; 28: 927–928 [PubMed] [Google Scholar]
- 4.Manners PJ, Bower C. Worldwide prevalence of juvenile arthritis why does it vary so much? J Rheumatol 2002; 29: 1520–1530 [PubMed] [Google Scholar]
- 5.Minden K. Epidemiologie der juvenilen idiopathischen arthritis. : Wagner N, Dannecker G. (eds). Pädiatrische rheumatologie. 1st edn. Heidelberg, Germany: Springer Medizin Verlag; 2007. pp. 179–181 [Google Scholar]
- 6.Jordan A, McDonagh JE. Juvenile idiopathic arthritis: the paediatric perspective. Pediatr Radiol 2006; 36: 734–742 [DOI] [PubMed] [Google Scholar]
- 7.Ganser G, Zepp F, Wagner N. Juvenile rheumatoide arthritis. : Lentze MJ, Schulte FJ, Schaub J, Spanger J. (eds). Pädiatrie – grundlagen und praxis. 2nd edn. Heidelberg, Germany: Springer-Verlag; 2003. pp. 633–647 [Google Scholar]
- 8.Mericle PM, Wilson VK, Moore TL, Hanna VE, Osborn TG, Rotskoff KS, et al. Effects of polyarticular and oligoarticular onset juvenile rheumatoid arthritis on facial and mandibular growth. J Rheumatol 1996; 23: 159–165 [PubMed] [Google Scholar]
- 9.Pearson MH, Ronning O. Lesions of the mandibular condyle in juvenile chronic arthritis. Br J Orthod 1996; 23: 49–56 [DOI] [PubMed] [Google Scholar]
- 10.Martini G, Bacciliero U, Tregnaghi A, Montesco MC, Zulian F. Isolated temporomandibular synovitis as unique presentation of juvenile idiopathic arthritis. J Rheumatol 2001; 28: 1689–1692 [PubMed] [Google Scholar]
- 11.Küseler A, Pedersen TK, Gelineck J, Herlin T. A 2 year followup study of enhanced magnetic resonance imaging and clinical examination of the temporomandibular joint in children with juvenile idiopathic arthritis. J Rheumatol 2005; 32: 162–169 [PubMed] [Google Scholar]
- 12.Twilt M, Schulten AJM, Nicolaas P, Dulger A, van Suijlekom-Smit LW. Facioskeletal changes in children with juvenile idiopathic arthritis. Ann Rheum Dis 2006; 65: 823–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arabshahi B, Cron RQ. Temporomandibular joint arthritis in juvenile idiopathic arthritis: the forgotten joint. Curr Opin Rheumatol 2006; 18: 490–495 [DOI] [PubMed] [Google Scholar]
- 14.Kjellberg H. Juvenile chronic arthritis: dentofacial morphology, growth, mandibular function and orthodontic treatment [review]. Swed Dent J Suppl 1995; 109: 1–56 [PubMed] [Google Scholar]
- 15.Twilt M, Mobers SM, Arends LR, Cate R, van Suijlekom-Smit L. Temporomandibular involvement in juvenile idiopathic arthritis. J Rheumatol 2004; 31: 1418–1422 [PubMed] [Google Scholar]
- 16.Ronchezel MV, Hilario MO, Goldenberg J, Lederman HM, Faltin K, Jr, de Azevedo MF, et al. Temporomandibular joint and mandibular growth alterations in patients with juvenile rheumatoid arthritis. J Rheumatol 1995; 22: 1956–1961 [PubMed] [Google Scholar]
- 17.Lamer S, Sebag GH. MRI and ultrasound in children with juvenile chronic arthritis. Eur J Radiol 2000; 33: 85–93 [DOI] [PubMed] [Google Scholar]
- 18.Johnson K, Gardner-Medwin J. Childhood arthritis: classification and radiology. Clin Radiol 2002; 57: 47–58 [DOI] [PubMed] [Google Scholar]
- 19.Hayashi T, Ito J, Koyama J, Yamada K. The accuracy of sonography for evaluation of internal derangement of the temporomandibular joint in asymptomatic elementary school children: comparison with MR and CT. Am J Neuroradiol 2001; 22: 728–734 [PMC free article] [PubMed] [Google Scholar]
- 20.Dannecker G. Juvenile idiopathische arthritis. Polyartikuläre verlaufsformen. : Wagner N, Dannecker G. (eds). Pädiatrische rheumatologie. Heidelberg, Germany: Springer Medizin Verlag; 2007. pp. 211–230 [Google Scholar]
- 21.Ganser G, Minden K. Juvenile idiopathische arthritis. Oligoartikuläre verlaufsform. In: Wagner N, Dannecker G, (eds). Pädiatrische rheumatologie. Heidelberg, Germany: Springer Medizin Verlag; 2007. pp. 179–181 [Google Scholar]
- 22.Petty RE, Southwood TR, Baum J, Bhettay E, Glass DN, Manners P, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol 1998; 25: 1991–1994 [PubMed] [Google Scholar]
- 23.Jank S, Schroder D, Haase S, Laimer K, Emshoff R, Michels H, et al. Temporomandibular disorders in juvenile patients with rheumatic diseases. Mund Kiefer GesichtsChir 2003; 7: 214–219 [DOI] [PubMed] [Google Scholar]
- 24.Bellintani C, Ghiringhelli P, Gerloni V, Gattinara M, Farronato G, Fantini F. Trattamento con un dispositivo ortodontico per l'impegno temporomandibulare nell'artrite idiopatica giovanile. Osservazioni su 72 casi. Reumatismo 2005; 57: 201–207 [DOI] [PubMed] [Google Scholar]
- 25.Weiss PF, Arabshahi B, Johnson A, Bilaniuk LT, Zarnow D, Cahill AM, et al. High prevalence of temporomandibular joint arthritis at disease onset in children with juvenile idiopathic arthritis as detected by magnetic resonance imaging but not by ultrasound. Arthritis Rheum 2008; 58: 1189–1196 [DOI] [PubMed] [Google Scholar]
- 26.Guillaume S, Prieur AM, Coste J, Job-Deslandre C. Long-term outcome and prognosis in oligoarticular-onset juvenile idiopathic arthritis. Arthritis Rheum 2000; 43: 1858–1865 [DOI] [PubMed] [Google Scholar]
- 27.Harper RP, Brown CM, Triplett M, Villasenor A, Gatchel RJ. Masticatory function in patients with juvenile rheumatoid arthritis. Pediatr Dentr 2000; 22: 200–206 [PubMed] [Google Scholar]
- 28.Meyer K, Foeldvari I, Küster RM, Huck L, Kahl-Nieke B. Das Kiefergelenk bei juveniler idiopathischer Arthritis. ZWR 2003; 112: 64–74 [Google Scholar]
- 29.Marini I, Vecchiet F, Spiazzi L, Capurso U. Stomatognathic function in juvenile rheumatoid arthritis and in developmental open-bite subjects. J Dent Child 1999; 66: 305–312 [PubMed] [Google Scholar]
- 30.Svensson B, Larsson A, Adell R. The mandibular condyle in juvenile chronic arthritis patients with mandibular hypoplasia: a clinical and histological study. Int J Oral Maxillofac Surg 2001; 30: 300–305 [DOI] [PubMed] [Google Scholar]
- 31.Savioli C, Silva CA, Ching LH, Campos LM, Prado EF, Siqueira JT. Dental and facial characteristics of patients with juvenile idiopathic arthritis. Rev Hosp Clin Fac Med Sao Paulo 2004; 59: 93–98 [DOI] [PubMed] [Google Scholar]
- 32.Biliau AD, Hu Y, Verdonck A, Carels C, Wouters C. Temporomandibular joint arthritis in juvenile idiopathic arthritis: Prevalence, clinical and radiological signs, an relation to dentofacial morphology. J Rheumatol 2007; 34: 1925–1933 [PubMed] [Google Scholar]
- 33.Ravelli A, Felici E, Magni-Manzoni S, Pistorio A, Novarini C, Bozzola E, et al. Patients with antinuclear antibody-positive juvenile idiopathic arthritis constitute a homogeneous subgroup irrespective of the course of joint disease. Arthritis Rheum 2005; 52: 826–832 [DOI] [PubMed] [Google Scholar]
- 34.Hofer M, Southwood TR. Classification of childhood arthritis. Best practice and research. Clin Rheumatol 2002; 16: 379–396 [PubMed] [Google Scholar]
- 35.Melchiorre D, Calderazzi A, Maddali Bongi S, Cristofani R, Bazzichi L, Eligi C, et al. A comparison of ultrasonography and magnetic resonance imaging in the evaluation of temporomandibular joint involvement in rheumatoid arthritis and psoriatic arthritis. Rheumatol 2003; 44: 413–416 [DOI] [PubMed] [Google Scholar]
- 36.Brandlmaier I, Bertram S, Rudisch A, Bodner G, Emshoff R. Temporomandibular joint osteoarthrosis diagnosed with high resolution ultrasonography versus magnetic resonance imaging: how reliable is high resolution ultrasonography? J Oral Rehabil 2003; 30: 812–817 [DOI] [PubMed] [Google Scholar]
- 37.Jank S, Haase S, Strobl H, Michels H, Häfner R, Missmann M, et al. Sonographic investigation of the temporomandibular joint in patients with juvenile idiopathic arthritis: A pilot study. Arthritis Rheum 2007; 57: 213–218 [DOI] [PubMed] [Google Scholar]
- 38.Emshoff R, Brandlmaier I, Bodner G, Rudisch A. Condylar erosion and disc displacement: detection with high-resolution ultrasonography. J Oral Maxillofac Surg 2003; 61: 877–881 [DOI] [PubMed] [Google Scholar]
- 39.Manfredini D, Tognini F, Melchiorre D, Bazzichi L, Bosco M. Ultrasonography of the temporomandibular joint: comparison of findings in patients with rheumatic diseases and temporomandibular disorders. A preliminary report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005; 100: 481–485 [DOI] [PubMed] [Google Scholar]
- 40.El-Miedany YM, Housny IH, Mansour HM, Mourad HG, Mehanna AM, Megeed MA. Ultrasound versus MRI in the evaluation of juvenile idiopathic arthritis of the knee. Joint Bone Spine 2001; 68: 222–230 [DOI] [PubMed] [Google Scholar]
- 41.Rudisch A, Emshoff R, Maurer H, Kovacs P, Bodner G. Pathologic-sonographic correlation in temporomandibular joint pathology. Eur Radiol 2006; 16: 1750–1756 [DOI] [PubMed] [Google Scholar]
- 42.Landes CA, Goral W, Mack MG, Sader R. 3-D Sonography for diagnosis of osteoarthrosis and disk degeneration of the temporomandibular joint, compared with MRI. Ultrasound Med Biol 2006; 32: 627–632 [DOI] [PubMed] [Google Scholar]
- 43.Manfredini D, Tognini F, Melchiorre D, Cantini E, Bosco M. The role of ultrasonography in the diagnosis of temporomandibular joint disc displacement and intra-articular effusion. Minerva Stomatol 2003; 52: 93–100 [PubMed] [Google Scholar]
- 44.Elias FM, Birman EG, Matsuda CK, Oliveira IR, Jorge WA. Ultrasonographic findings in normal temporomandibular joints. Braz Oral Res 2006; 20: 25–32 [DOI] [PubMed] [Google Scholar]