This review describes the neuroprotective effects of stem cells with special emphasis on the current translational research using stem cells to treat neuropathic pain. Recent studies using stem cells in animal models have demonstrated promising results and provide encouraging groundwork on which to continue research in the pursuit of optimal cellular therapies for the treatment of neuropathic pain.
Keywords: Adult stem cells, Cellular therapy, Neuropathy, Stem cell-microenvironment interactions
Abstract
Neuropathic pain is a chronic condition that is heterogeneous in nature and has different causes. Different from and more burdensome than nociceptive pain, neuropathic pain more severely affects people's quality of life. Understanding the various mechanisms of the onset and progression of neuropathic pain is important in the development of an effective treatment. Research is being done to replace current pharmacological treatments with cellular therapies that will have longer lasting effects. Stem cells present an exciting potential therapy for neuropathic pain. In this review, we describe the neuroprotective effects of stem cells along with special emphasis on the current translational research using stem cells to treat neuropathic pain.
Deciphering the Code: Defining Neuropathic Pain
In 2008, the International Association for the Study of Pain formed a special interest group to redefine neuropathic pain as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system” [1]. Neuropathy is heterogeneous in nature; however, neuropathic lesions may be characterized into four broad categories: focal or multifocal lesions of the peripheral nervous system, generalized lesions of the peripheral nervous system (polyneuropathies), lesions of the central nervous system, and complex neuropathic disorders (Table 1) [2].
Table 1.
Categories of neuropathic lesions and examples

Abbreviation: CNS, central nervous system.
From [1] with permission.
Although categorized as chronic pain, neuropathic pain is regarded as more severe than other types of chronic pain. This is due to the increased disruption of both physical and mental quality of life when compared with other chronic pain syndromes. People with chronic neuropathic pain report a higher severity of pain and significantly worse scores for all interference items of the Brief Pain Inventory than non-neuropathic chronic pain patients. Also, those with chronic neuropathic pain report mean scores for the Neuropathic Pain Scale significantly higher than those with non-neuropathic chronic pain, even after adjusting for pain severity, age, and sex [3].
Types of Pain
Neuropathic pain presents itself in many different forms. Spontaneous sensations include paroxysmal pain (shooting pain that lasts several seconds) and superficial pain (an ongoing, burning sensation). Evoked pain includes mechanical allodynia (pain caused by normally nonpainful pressure), heat or cold allodynia (pain caused by normally nonpainful hot/cold stimuli), hyperalgesia (increased sensitivity to a normally painful stimulus), and temporal summation (increasing pain sensation from repetitive application of identical stimuli) [4].
Neuropathic pain differs from nociceptive pain in that nociceptive pain is caused by tissue damage, whereas neuropathic pain is produced by nerve damage. In particular, pain signaling areas of the peripheral or central nervous system are injured, causing neuropathic pain. In nociceptive pain, tissue damage causes the generation of prostaglandins that cause vasodilation, increased blood flow, inflammatory exudates, and the sensitization of nociceptive nerve endings. In neuropathic pain, signals are generated by the injured nerve, sent to the brain, and interpreted as pain. Nociceptive pain is proportional to the intensity of the stimulus; neuropathic pain is not—a small stimulus may provoke increased sensations of pain [5].
Peripheral Sensitization
C fibers (unmyelinated) and Aδ fibers (thinly myelinated) are responsible for eliciting pain sensations in response to noxious stimuli. Spontaneous ectopic firing, however, increases abnormally after peripheral nerve injury. The firing increase is due to sensitization brought on by molecular and cellular changes at the primary afferent nociceptor, such as increased expression of voltage-gated sodium channel RNA (particularly Nav1.3, an embryonic channel), increased expression of a cold/menthol-sensitive channel (TRPM8), and mutations in the encoding of sodium channels (an example is the SCN9A gene of the Nav1.7 channel). Changes also occur in the regulation of membrane-bound receptors; TRPV1, a vanilloid receptor typically found on afferent nociceptive fibers, has been shown to increase in medium to large injured dorsal root ganglion cells. Additionally, the TRPV1 receptor has also been shown to be downregulated in damaged afferents and upregulated in noninjured C and Aδ fibers. There is also upregulation of TRPV4, which seems to play a role in taxol-induced mechanical hyperalgesia [2]. The abnormal changes in the structure and function of the pain-eliciting fibers after peripheral nerve injury increase the probability of neuropathic pain.
Central Sensitization
Hypersensitization in the peripheral nerves causes changes in the spinal cord dorsal horn. Presynaptically, sensitization is initiated by the injured C fibers, which release both glutamate (acting on the N-methyl-d-aspartate receptors) and the neuropeptide substance P through voltage-gated N-calcium channels [2]. Additionally, peripheral nerve injury decreases the amount of GABA-releasing interneurons in the superficial dorsal horn of the spinal cord. This decrease is caused by a reduction in the levels of the GABA synthesizing enzyme glutamic acid decarboxylase [6]. Postsynaptically, voltage-gated sodium channel expression increases (particularly Nav1.3). Consequently, the potential of low-threshold mechanoreceptors (Aδ and Aβ) to signal pain responses from normally innocuous stimuli increases. After peripheral nerve injury, the general excitability of multireceptive spinal cord neurons increases, causing an increase in neuronal activity in response to noxious stimuli. Additionally, the neuronal receptive fields expand and spread the hyperexcitability to other spinal segments [2]. The increase in excitability of both presynaptic and postsynaptic membranes following injury provides abnormal excitation levels, which leads to an increase in the probability for neuropathic pain.
Another mechanism for disinhibition of the intraspinal pathway has alternatively been proposed. A study has shown the reduction in the expression of a potassium-chloride exporter (KCC2) in lamina 1 neurons, disrupting homeostasis of the anions. This causes the normally inhibitory actions of GABA to become excitatory in the lamina 1 neurons [7].
Other Mechanisms
Although injured neurons play a large role in the development of neuropathic pain, recent research has demonstrated that glial cells have the ability to modify the function of nociceptive networks. One type of glial cells in particular, microglia, extend their processes to sites of neural damage and release specific factors, contributing to the pathologies caused by disease or injury. The mechanism behind the activation of microglia is ATP, which is an endogenous ligand of the P2 receptor family. Several P2 receptor subtypes are expressed by microglia; of these receptors, the P2X4 receptor subtype is now known to be key in the microglia-neuron signaling pathway. Activation of the P2X4 receptor signals the release of brain-derived nerve factor (BDNF), which causes disinhibition of the intraspinal pathway—in particular the spinal lamina 1 neurons [8]. As stated above, this causes the normally inhibitory actions of GABA to become excitatory in the lamina 1 neurons.
Stem Cell Therapy
Stem Cells: Why the Hype?
Uninjured fibers that intermingle with degenerating nerve fibers participate in pain signaling. Spontaneous activity in the uninjured nerve fiber produces sensitization in the area of the central nervous system responsible for development of pain. Additionally, products associated with Wallerian degeneration released near uninjured nerves might trigger changes in channel and receptor expression in the uninjured nerve, contributing to neuropathic pain [9]. It is important, therefore, that the environment surrounding uninjured nerve fibers should protect them from degenerating and exacerbating neuropathic pain.
Critical in providing a protective microenvironment, neurotrophic factors are growth factors known to promote neuron development and survival. They also maintain functional integrity, promote regeneration, regulate neuronal plasticity, and aid in the repairing of damaged nerves [10]. Neurotrophic factors include various types of protective factors. The neurotrophins are: nerve growth factor (NGF), BDNF, neurotrophin 3 (NT-3), and NT-4/5. Other well-characterized neurotrophic factors include: insulin-like growth factor I and II (IGF-I and IGF-II), glial cell line-derived neurotrophic factor (GDNF), neurturin, and persephin. Ciliary neurotrophic factor (CNTF) has also been studied and shown to act upon neural crest-derived sensory nerves, as well as parasympathetic and motor nerves [11]. The various neurotrophic factors affect different cell populations within the peripheral and central nervous system (Table 2).
Table 2.
Neurotrophic factors and the peripheral nerve populations they affect

Abbreviations: BDNF, brain-derived nerve factor; GDNF, glial cell line-derived neurotrophic factor; IGF, insulin-like growth factor; NGF, nerve growth factor; NT, neurotrophin.
From [11] with permission.
Presently, neurotrophic factors are secreted by the various populations of stem cells discovered in the human body. Human mesenchymal stem/stromal cells (hMSCs) produce a large variety of trophic factors; 84 trophic factors have been found in hMSC-conditioned medium and/or cell lysates versus basal medium. Neurotrophic factors found in both conditioned medium and cell lysates were epidermal growth factor, BDNF, NT-3, CNTF, basic fibroblast growth factor (bFGF/FGF-2), hepatocyte growth factor, and vascular endothelial growth factor (VEGF) [12]. Human umbilical cord-derived MSCs (hUC-MSCs) have also been found to secrete neurotrophic factors: VEGF, GDNF, and BDNF. Secretion of neurotrophic factors was demonstrated before, during, and after neuronal differentiation. The results of this study indicated higher production of neurotrophic factors in hUC-MSC versus bone marrow-derived stem cells; however, both cell types had measurable amounts of secreted neurotrophic factors. This study was done in vitro, however, and in vivo tests did not confirm the secretion of neurotrophic factors and the antiapoptotic effects seen in vitro [13]. Dental pulp stem cells express various neurotrophic factors, including BDNF, NGF, and GDNF [14]. Similarly, adipose-derived stem cells differentiated to glial-like cells also express a range of neurotrophic factors, namely NGF, BDNF, GDNF, and NT-4 [15]. The secretion of neurotrophic factors by different populations of stem cells suggests that no matter the source of the stem cell, there is a possible use for it in treating neuropathic pain.
The secretion of neurotrophic factors by stem cells provides neuroprotection and neuroregenerative effects. When transplanted into an animal model of Parkinson's disease, hMSCs support sustained endogenous proliferation and maturation of cells in the subventricular zone of rats. Additionally, hMSCs exert a neuroprotective effect, decreasing the loss of dopaminergic neurons and increasing the levels of dopamine in animal models of Parkinson's disease [12, 16]. These effects were possibly accomplished through decreased caspase-3 activity. Adhesion removal tests also indicate a tendency toward lower removal times in hMSC-treated mice versus mice injected with proteasome inhibitors and no hMSC transplantation [16]. It is interesting to note that transplanted hMSCs did not differentiate into a neural phenotype, indicating that the mechanism of action was mediated through paracrine signaling rather than through the differentiation of the stem cells [12]. When injected into the same location as a forced quinolinic acid (QA)-induced cerebellar lesion site, the negative impacts of QA on rotarod learning and beam walking speed were ameliorated. Also, hMSC transplantation protected against Purkinje cell loss [17]. Similarly, in a rat model of ischemic stroke, hUC-MSC transplantation provided a significant increase in neurobehavioral function (neurobehavioral tests included consciousness, gait, limb tone, and pain reflex), and a significant decrease in the final infarct volume relative to the control group [13]. These studies demonstrate that MSCs not only protect against nerve damage but also help regenerate damaged nerves and restore them to their preinjured state.
Neuroprotective effects have also been noticed with different populations of stem cells. One such population is dental pulp stem cells. When mesencephalic neurons in culture were exposed to 1-methyl-4-phenylpyridinium (MPP+) or rotenone (two neurotoxins commonly used to induce Parkinson's in animal models), a statistically significant neuroprotective effect was seen in cocultures of the neurons with dental pulp stem cells. In particular, mesencephalic neurons challenged with MPP+ demonstrated a reduction of [3H] dopamine uptake, demonstrating a loss in dopaminergic cells. When cocultured with dental pulp stem cells, however, the decrease of [3H] dopamine uptake was significantly less. Mesencephalic neurons challenged with rotenone demonstrated similar results; those cocultured with dental pulp stem cells demonstrated an increase in [3H] dopamine uptake, further demonstrating the neuroprotective effect of the dental pulp stem cells [14]. Another stem cell population is derived from adipose tissue. When transplanted into an animal model of nerve injury, differentiated adipose-derived stem cells were shown to decrease the expression of the proapoptotic genes caspase-3 and Bax as well as significantly increase the ratio of Bcl-2:Bax (a ratio that is seemingly vital to the survival of neurons) in the dorsal root ganglia [15]. This seems to demonstrate, therefore, that stem cells, regardless of source, possess the potential to be used in regenerative and therapeutic applications for neuropathy and the resulting neuropathic pain.
Do stem cells need to be in direct contact with the lesioned nerve to provide neuroprotection? Several studies have demonstrated that direct contact is not necessary to provide neuroprotection. When bone marrow stem cells (BMSCs) were cocultured with “ischemic” injured tissue (where BMSCs were not in direct contact with the ischemic tissue), the neuroprotective effects provided by the BMSC were found to be equal to the effects of direct transplantation onto injured tissue. The amount of cell death in the cornu ammonis of the brain significantly decreased. When assessed for the occurrence of apoptotic cells, both cytochrome c expression and activated caspase-3-positive cells were reduced in oxygen-glucose deprivation-treated hippocampal organotypic slices that were cocultured with BMSCs. Additionally, oxygen-glucose deprivation-treated hippocampal organotypic slices that were cocultured with serum-deprived BMSCs demonstrated an increase in the secretion of neurotrophic factors; significantly IGF-1, bFGF, and NGF [18]. In the study by Nesti et al. described above [14], dental pulp stem cells were not in direct contact with mesencephalic neurons when cultured in vitro and were still found to provide neuroprotection from harmful toxins. It was highlighted by the researchers, however, that further in vivo studies were required to clarify whether the transplanted stem cells still retained the ability to produce neurotrophic factors after transplantation. In another study, cortical neurons cultured with MSC conditioned medium better survived trophic deprivation and nitric oxide (NO) exposure than those cultured in regular medium. The cortical neurons were not cocultured with MSCs; medium from the MSC culture was applied to cortical neurons in vitro, and a protective effect was observed. This effect was shown to depend on an intact phosphatidylinositol 3-kinase/Akt signaling pathway, which was activated upon application of MSC conditioned medium to cortical cultures exposed to NO. BDNF secretion by the cultured MSCs is believed to be the cause of the increased activation of this pathway [19]. These studies, although not completely negating the need for direct cell-to-cell contact, provide for the potential to treat nerve lesions and disorders in locations where direct cell-to-cell contact would be impossible or difficult to achieve.
The neuroprotective effects of stem cells described thus far demonstrate a potential use in different pathological states. Although the above-mentioned studies focus mainly on diseases in which neuropathic pain is of major concern, other equally crippling neurodegenerative diseases, such as Alzheimer's and Huntington's diseases, may benefit from the neuroprotective effects of stem cells. It is worth looking into the potential for stem cell therapies to create a neurotrophic environment conducive to the amelioration or reversal of the neurodegeneration that leads to these debilitating diseases, as well as the prevention of degeneration and degradation of uninjured nerve fibers. Additionally, the above-mentioned studies have shown that stem cells have the ability to decrease and ameliorate the negative effects on injured nerve fibers, improving the function of the injured nerve. The release of key neurotrophic factors, along with the neuroprotective and neuroregenerative effects of stem cells, make them ideal candidates for arresting and possibly reversing the incapacitating effects of neuropathic pain.
Potential Shortcomings
Although stem cell therapies have been shown to protect from neurodegeneration and promote neuroregeneration, there are several issues that need to be addressed. The optimal dose for stem cell transplantation is still unknown and needs further characterization prior to being introduced into clinical trials. One of the studies demonstrated that hMSCs transplanted into their animal model generated different grafts depending on the cell dosing: low numbers of transplanted hMSCs generated Nestin-containing grafts, whereas higher numbers of transplanted hMSCs generated considerable amounts of grafts with astroglial markers [12]. The number of cells transplanted also raises questions about cell survival; one of the aforementioned studies indicated that only 1.7% of total injected hMSCs survived [16]. What happened to the remaining 98.3% of cells? Did the body discard the unneeded cells? Did the cells die? Are the cells that survive the only ones required to produce the desired effect? In another, unrelated study, following 4 weeks of stem cell transplantation, no cells were detected in the animal model. It was stated that the reasons were unknown; some possible suggestions were death or loss of fluorescence [20]. There are other unknown variables in the use of stem cells to treat neuropathic disease. According to the aforementioned studies, despite all of their benefits, much research still needs to be done to understand the homing capabilities of stem cells [16] and the mechanism of action [19]. These and other questions may provide a real and formidable challenge prior to moving stem cell therapies from bench to bedside.
Current Uses in Neuropathic Pain
Aside from the trophically rich environment produced by the transplantation of various types of stem cells into different animal models, continuing work is being done to determine the potential use of stem cells for the debilitating disease of neuropathic pain. Transplantation of hMSCs into a mouse model of spared nerve injury has been found to significantly reduce both mechanical allodynia and thermal hyperalgesia, as measured with paw withdrawal latency and paw withdrawal threshold tests. The mechanisms believed to be responsible for the reduction in allodynia and hyperalgesia are several: the decrease of the proinflammatory interleukin (IL)-1β and IL-17, an increase in protein expression of the anti-inflammatory IL-10, and a reduction in the overactivation of β-galactosidase. It was also found that motor coordination was not affected by stem cell transplantation [21]. Similar results have been achieved through both intrabrain injection of hMSCs and injection into the tail vein of spared nerve injury mice, indicating that the effects of hMSCs may also be achieved through a minimally invasive procedure, because of the homing effect of the cells [22].
Another area in which stem cell therapies are currently being explored is the treatment of diabetic peripheral neuropathy, a subset of neuropathy that affects people with diabetes. In rats that have streptozotocin-induced diabetes, bone marrow-derived mesenchymal stem cell transplantation produced a significant increase in mRNA expression and protein expression of both bFGF and VEGF compared with rats receiving saline injections. Sciatic nerve blood flow was significantly ameliorated compared with diabetes-induced rats treated with saline. Additionally, the decreases in the capillary-to-muscle ratio and the neurofilament content were prevented in the stem cell-transplanted group [23]. Transplantation of bone marrow-derived MSCs has improved motor nerve conduction velocity in diabetic animals compared with animals simply receiving saline injections [23, 24]. Despite these benefits, however, motor nerve conduction velocity and the increase in the levels of NGF and NT-3 were found to last for only 4 weeks [24]. Interestingly, when it comes to neurotrophic factors, these two studies contradict each other. In the study by Kim et al. [24], levels of neurotrophic factors, such as NGF and NT-3, but not VEGF or bFGF, were found to increase in the animals that received bone marrow-derived mesenchymal stem cell transplantation. In the study by Shibata et al. [23], however, VEGF and bFGF, but not NGF and NT-3, were found to increase in the animals that received stem cell transplantation. As can be clearly seen, many more studies need to be performed to understand the effects of stem cells in these diseases.
In addition to uses in neuropathic pain with peripheral nerve injury, promising results have been seen in spinal cord injuries. In a study by Ichim et al. [25], a patient with an incomplete spinal cord injury at the T12–L1 level and a crush fracture in the L1 vertebral body (described as a type A in the ASIA scale) was administered several doses of MSC and CD34+ cells. Prior to the allogeneic transplantation of the MSCs, this patient reported neuropathic pain at a level of 10/10. After several cycles of MSC and CD34+ cell transplantation, he reported a marked decrease of neuropathic pain, from daily 10/10 to once a week 3/10. No adverse effects were noted following transplantation. Muscle strength improved, various dermatome sensation increased, and urological and sexual function was recovered. Although these results are positive, the study is a case report of only one patient. Therefore, caution must be taken in the interpretation of these results, particularly with regards to efficacy [25]. Table 3 summarizes recent studies using stem cells to treat neuropathic pain.
Table 3.
Summary of recent studies using stem cells to treat neuropathic pain
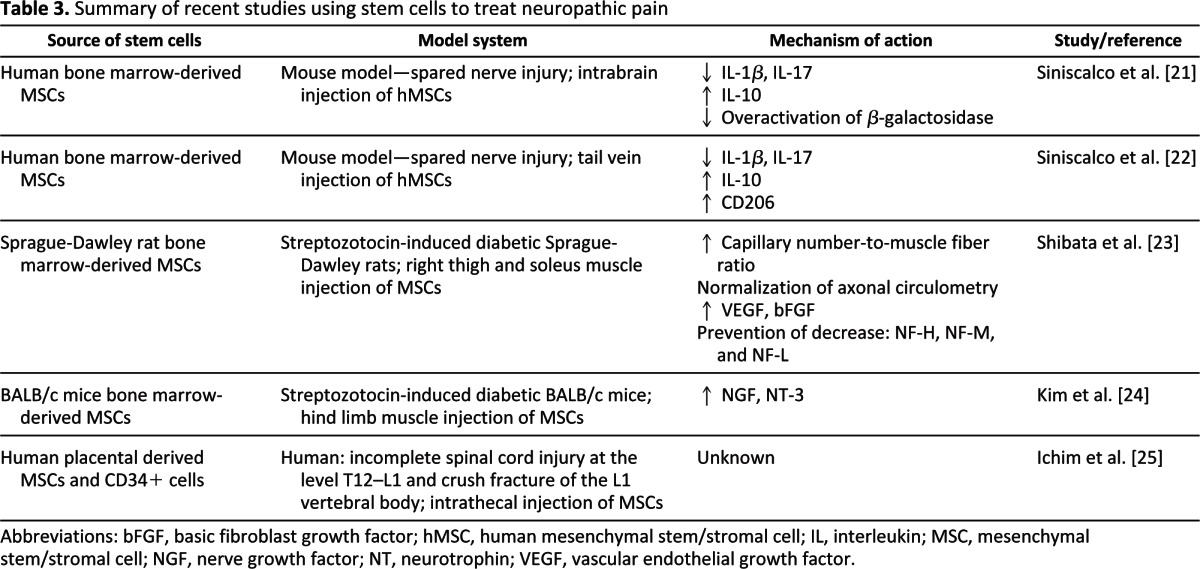
Abbreviations: bFGF, basic fibroblast growth factor; hMSC, human mesenchymal stem/stromal cell; IL, interleukin; MSC, mesenchymal stem/stromal cell; NGF, nerve growth factor; NT, neurotrophin; VEGF, vascular endothelial growth factor.
Conclusion
Neuropathic pain is a chronic, heterogeneous condition that presently has no long-term treatments. Current pharmacological treatments are mainly palliative, and although they temporarily relieve the pain, they do not address the underlying mechanisms of the pain. Stem cells pose an exciting therapeutic potential to deal with neuropathic pain. Although the mechanism of action of stem cells is not completely understood, the aforementioned studies demonstrate that stem cells have the potential to arrest degenerative processes, inhibit apoptotic pathways, and augment the survival/recovery pathways of injured and uninjured nerves. Coupled with the neurotrophic factor-releasing nature of stem cells (including newly discovered populations), the ability of stem cells to modify cellular processes provides for a protective and restorative microenvironment that can potentially fully reverse the causes behind the onset of neuropathic pain. Nevertheless, as with any cellular therapy approach, challenges that will have to be addressed before reaching the full therapeutic potential of stem cells still remain. Sourcing of stem cells, considerations on autologous versus allogeneic transplants, precommitment to neuronal lineages, characterization of neurotrophic factor release, and dosing requirements, among other concerns, will have to be addressed by scientists wanting to use stem cells for the treatment of neuropathic pain. Recent studies using stem cells in animal models, however, have demonstrated promising results and provide encouraging groundwork on which to continue research in the pursuit of optimal cellular therapies for the treatment of neuropathic pain.
Author Contributions
V.R.F.: conception and design, research and analysis of articles, manuscript writing; D.P.: topic refinement, manuscript review; H.S.C.: conception and design, final approval of manuscript, financial support.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Treede R, Jensen T, Campbell J, et al. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 2.Baron R. Mechanisms of disease: Neuropathic pain: A clinical perspective. Nat Clin Pract Neurol. 2006;2:95–106. doi: 10.1038/ncpneuro0113. [DOI] [PubMed] [Google Scholar]
- 3.Smith B, Torrance N, Bennett M, et al. Health and quality of life associated with chronic pain of predominantly neuropathic origin in the community. Clin J Pain. 2007;23:143–149. doi: 10.1097/01.ajp.0000210956.31997.89. [DOI] [PubMed] [Google Scholar]
- 4.Baron R, Binder A, Wasner G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 5.Urgellés-Lorié L. Nociceptive vs. neuropathic pain: Physiological regulating medicine (PRM)/new approach for its control. Dolor, Clinica y Terapia. 2008:17–22. [Google Scholar]
- 6.Moore K, Kohno T, Karchewski L, et al. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coull J, Boudreau D, Bachand K, et al. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 8.Trang T, Beggs S, Salter MW. Brain-derived neurotrophic factor from microglia: A molecular substrate for neuropathic pain. Neuron Glia Biol. 2011;7:99–108. doi: 10.1017/S1740925X12000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu G, Ringkamp M, Hartke T, et al. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci. 2001;21:RC140. doi: 10.1523/JNEUROSCI.21-08-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ossipov M. Growth factors and neuropathic pain. Curr Pain Headache Rep. 2011;15:185–192. doi: 10.1007/s11916-011-0183-5. [DOI] [PubMed] [Google Scholar]
- 11.Apfel S. Neurotrophic factors in peripheral neuropathies: Therapeutic implications. Brain Pathol. 1999;9:393–413. doi: 10.1111/j.1750-3639.1999.tb00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cova L, Armentero M, Zennaro E, et al. Multiple neurogenic and neurorescue effects of human mesenchymal stem cell after transplantation in an experimental model of Parkinson's disease. Brain Res. 2010;1311:12–27. doi: 10.1016/j.brainres.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 13.Koh S, Kim K, Choi M, et al. Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res. 2008;1229:233–248. doi: 10.1016/j.brainres.2008.06.087. [DOI] [PubMed] [Google Scholar]
- 14.Nesti C, Pardini C, Barachini S, et al. Human dental pulp stem cells protect mouse dopaminergic neurons against MPP+ or rotenone. Brain Res. 2011;1367:94–102. doi: 10.1016/j.brainres.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 15.Reid A, Sun M, Wiberg M, et al. Nerve repair with adipose-derived stem cells protects dorsal root ganglia neurons from apoptosis. Neuroscience. 2011;199:515–522. doi: 10.1016/j.neuroscience.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 16.Park H, Lee P, Bang O, et al. Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson's disease. J Neurochem. 2008;107:141–151. doi: 10.1111/j.1471-4159.2008.05589.x. [DOI] [PubMed] [Google Scholar]
- 17.Edalatmanesh M, Bahrami A, Hosseini E, et al. Neuroprotective effects of mesenchymal stem cell transplantation in animal model of cerebellar degeneration. Neurol Res. 2011;33:913–920. doi: 10.1179/1743132811Y.0000000036. [DOI] [PubMed] [Google Scholar]
- 18.Sarnowska A, Braun H, Sauerzweig S, et al. The neuroprotective effect of bone marrow stem cells is not dependent on direct cell contact with hypoxic injured tissue. Exp Neurol. 2009;215:317–327. doi: 10.1016/j.expneurol.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 19.Wilkins A, Kemp K, Ginty M, et al. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009;3:63–70. doi: 10.1016/j.scr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Wang F, Yasuhara T, Shingo T, et al. Intravenous administration of mesenchymal stem cells exerts therapeutic effects on parkinsonian model of rats: Focusing on neuroprotective effects of stromal cell-derived factor-1alpha. BMC Neurosci. 2010;11:52. doi: 10.1186/1471-2202-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siniscalco D, Giordano C, Galderisi U, et al. Intra-brain microinjection of human mesenchymal stem cells decreases allodynia in neuropathic mice. Cell Mol Life Sci. 2010;67:655–669. doi: 10.1007/s00018-009-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siniscalco D, Giordano C, Galderisi U, et al. Long-lasting effects of human mesenchymal stem cell systemic administration on pain-like behaviors, cellular, and biomolecular modifications in neuropathic mice. Front Integr Neurosci. 2011;5:79. doi: 10.3389/fnint.2011.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shibata T, Naruse K, Kamiya H, et al. Transplantation of bone marrow-derived mesenchymal stem cells improves diabetic polyneuropathy in rats. Diabetes. 2008;57:3099–3107. doi: 10.2337/db08-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim B, Jin H, Bae J. Bone marrow-derived mesenchymal stem cells improve the functioning of neurotrophic factors in a mouse model of diabetic neuropathy. Lab Anim Res. 2011;27:171–176. doi: 10.5625/lar.2011.27.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichim T, Solano F, Lara F, et al. Feasibility of combination allogeneic stem cell therapy for spinal cord injury: A case report. Int Arch Med. 2010;3:30. doi: 10.1186/1755-7682-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]


