This study examines the therapeutic potential of stem cells derived from a variety of sources for the cure of type 1 diabetes mellitus, for example, embryonic stem cells, induced pluripotent stem cells, bone marrow-derived hematopoietic stem cells, and multipotent mesenchymal stromal cells derived from bone marrow, umbilical cord blood, and adipose tissue. The benefits of combinatorial approaches designed to ensure the successful clinical translation of stem cell therapeutic strategies, such as approaches combining effective stem cell strategies with islet transplantation, immunomodulatory drug regimens, and/or novel bioengineering techniques, are also discussed.
Summary
Type 1 diabetes mellitus (T1D) is a chronic, multifactorial autoimmune disease that involves the progressive destruction of pancreatic β-cells, ultimately resulting in the loss of insulin production and secretion. The goal of clinical intervention is to prevent or arrest the onset and progression of autoimmunity, reverse β-cell destruction, and restore glycometabolic and immune homeostasis. Despite promising outcomes observed with islet transplantation and advancements in immunomodulatory therapies, the need for an effective cell replacement strategy for curing T1D still persists. Stem cell therapy offers a solution to the cited challenges of islet transplantation. While the regenerative potential of stem cells can be harnessed to make available a self-replenishing supply of glucose-responsive insulin-producing cells, their immunomodulatory properties may potentially be used to prevent, arrest, or reverse autoimmunity, ameliorate innate/alloimmune graft rejection, and prevent recurrence of the disease. Herein, we discuss the therapeutic potential of stem cells derived from a variety of sources for the cure of T1D, for example, embryonic stem cells, induced pluripotent stem cells, bone marrow-derived hematopoietic stem cells, and multipotent mesenchymal stromal cells derived from bone marrow, umbilical cord blood, and adipose tissue. The benefits of combinatorial approaches designed to ensure the successful clinical translation of stem cell therapeutic strategies, such as approaches combining effective stem cell strategies with islet transplantation, immunomodulatory drug regimens, and/or novel bioengineering techniques, are also discussed. To conclude, the application of stem cell therapy in the cure for T1D appears extremely promising.
Introduction
Type 1 diabetes mellitus (T1D) is a chronic, multifactorial autoimmune disease that involves the progressive destruction of pancreatic β-cells, ultimately resulting in the loss of insulin production and secretion [1]. The ideal goal of clinical intervention would be to prevent or arrest the onset and progression of autoimmunity, reverse β-cell destruction, and restore glycometabolic control and immune homeostasis. Since 70%–90% of β-cells have been destroyed at the time of diagnosis, the impact of strategies that aim at preserving β-cell mass is limited [2–4]. Although significant advancement in our understanding of T1D immunopathogenesis has occurred since the efficacy of cyclosporine in reducing insulin requirement was reported more than 25 years ago, immunomodulatory therapies since then have not met with expected clinical success [5]. Failure of interventional therapies in preventing autoimmune β-cell destruction can be attributed to a number of issues such as the transient nature of immune protection that often results in the recurrence of autoimmunity upon drug withdrawal and the failure to induce a tolerant state. Therefore, understanding the immunopathogenesis of T1D is crucial for designing effective β-cell replacement and immunomodulatory strategies. This review will focus on the role of stem cells in diabetes cell therapy, with emphasis on bone marrow-derived hematopoietic stem cells (BM-HSCs) and multipotent mesenchymal stromal cells (MSCs).
Immunopathogenesis of T1D
A combination of environmental risk factors, genetic predisposition, and autoimmune-mediated processes contribute to T1D etiology [1, 6, 7]. Autoantibodies against islet antigens are a hallmark of disease development [8]. Antigen-presenting cells such as macrophages and dendritic cells (DCs) are the first to infiltrate islets followed by CD4 and CD8 T lymphocytes, natural killer (NK) cells, and B lymphocytes [9, 10]. Studies indicate that interleukin (IL)-12 secreted by macrophages may activate Th1-type CD4 T cells [10]. IL-2 and proinflammatory cytokines released by activated CD4 T cells (e.g., interferon-γ [IFN-γ], tumor necrosis factor [TNF-α], and IL-1β) maximize the activation of cytotoxic CD8 T cells, the final effectors of β-cell death via apoptosis. IFN-γ may also activate macrophages to release proinflammatory cytokines and reactive oxygen species (ROS). Proinflammatory cytokines further induce signal transducer and activator of transcription 1, nuclear factor κB, and interferon regulatory factor 3 in β-cells, contributing to the maintenance and amplification of the immune processes [11]. Ultimately, T-cell-mediated β-cell destruction is effected by the interplay between receptor-mediated interactions (e.g., Fas-Fas ligand, CD40-CD40 ligand, and TNF-TNF receptor), secretion of proinflammatory cytokines and ROS, as well as the release of granzymes and perforin from cytotoxic effector T cells. Th17 cells may also contribute to immunopathogenesis [12], while regulatory T cells (Treg cells) play a crucial role in determining the fate of the disease process [13].
Therapeutic Interventions to Treat T1D
Currently, standard treatment for T1D consists of lifelong, exogenous insulin administration by either insulin pump or multiple daily injections. Although advances in insulin delivery methods and glucose monitoring have succeeded in improving glycometabolic control and patient survival, daily insulin therapy does not represent a cure and is often associated with debilitating hypoglycemic episodes and unawareness, as well as the devastating complications of retinopathy, nephropathy, and neuropathy. To date, pancreas or islet transplantation remains the most reliable clinical approach to cure T1D [14, 15]. Unfortunately, the requisite use of immunosuppressants, corticosteroids, and anti-inflammatory agents accompanying transplantation is often associated with deleterious diabetogenic and nephrotoxic side effects and an increased risk of infections and tumors. Also, despite promising results observed in clinical islet transplantation, widespread application is hampered by an inadequate supply of cadaveric donor tissue and innate and alloimmune graft rejection, as well as the recurrence of autoimmunity [16]. Nevertheless, islet transplantation has provided proof of concept that independence from exogenous insulin treatment can be achieved through replacement of β-cells and has paved the way for the use of cellular therapy as a cure for T1D.
Therapeutic interventions to cure T1D focus on (a) preservation of residual β-cells; (b) restoration of glucose-responsive, insulin-producing β-cells using replacement or regeneration strategies; (c) protection of replaced β-cells from allo- or autoimmune destruction; and/or (d) restoration of β-cell-specific unresponsiveness in the absence of chronic immunosuppression. β-Cell restoration consists of β-cell replacement and regenerative strategies. The latter include among others, replication of pre-existing β-cells, neogenesis from ductal and non-β-cell progenitors, transdifferentiation of fully differentiated acinar cells, transdetermination of liver progenitor cells, and the directed differentiation of stem cells/β-cell progenitors [17–19]. β-Cell-immune protection strategies include a wide variety of antigen-specific or broad-based immunosuppressive, immunomodulatory, and tolerance-inducing therapies and immunoisolation techniques such as cell encapsulation using bioscaffolds supplemented with angiogenic factors and anti-inflammatory drugs [9, 17]. However, as we have discovered over the last decade, no single intervention standing alone has succeeded in curing this multifactorial, multistage disease. Therefore, the principal focus of ongoing research is the development of combinatorial therapeutic strategies designed to target different aspects of the disease in order to additively bring about a cure.
Stem Cell-Based Strategies for β-Cell Regeneration and Immunomodulation
Obtaining a large source of β-cells for cellular therapy is a major challenge. Stem cell-based strategies represent significant therapeutic potential owing to both the intrinsic regenerative capacity and the immunomodulatory potential of stem cells (Fig. 1). While the capacity of stem cells to self-renew and to differentiate into specialized cell types can be harnessed to make available a self-replenishing supply of glucose-responsive insulin-producing cells for transplantation, the immunomodulatory properties of stem cells, such as MSCs and HSCs, can be used to help arrest β-cell destruction, preserve residual β-cell mass, facilitate endogenous β-cell regeneration, and ameliorate islet graft rejection [20–23]. Thus, stem cells with immunomodulatory properties can potentially be used, both alone and in combination with β-cell replacement strategies, to reverse hyperglycemia in T1D [24–26]. Stem cells obtained from a variety of sources have been tested for their β-cell-regenerative potential and for their ability to restore immune homeostasis or promote longitudinal islet graft survival. These include, among others, embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), BM-HSCs and BM-MSCs, umbilical cord blood-derived MSCs (UCB-MSCs), adipose tissue-derived MSCs (ADSCs), and pancreas-derived multipotent precursor cells, as well as pancreatic β-cell progenitors that reside in the ductal epithelium, exocrine tissue, and within the islet proper; neural progenitor cells; and facultative β-cell progenitors from spleen, liver, and the endometrium.
Figure 1.
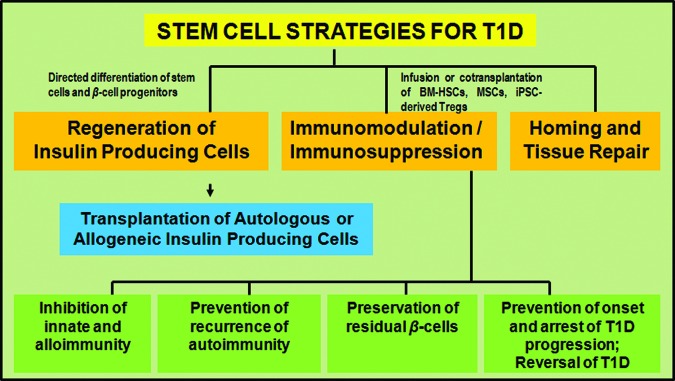
Stem cell strategies for the cure of T1D. Stem cell-based strategies to restore glycometabolic and immune homeostasis are based on the intrinsic regenerative capacity as well as the immunomodulatory potential of stem cells. The regenerative capacity can be harnessed to make available a self-replenishing supply of glucose-responsive insulin-producing cells for transplantation. The immunomodulatory properties can potentially be harnessed to arrest β-cell destruction, preserve residual β-cell mass, facilitate endogenous β-cell regeneration, ameliorate innate/alloimmune graft rejection, and prevent the recurrence of autoimmunity. Abbreviations: BM-HSC, bone marrow-derived hematopoietic stem cell; iPSC, induced pluripotent stem cell; MSC, mesenchymal stromal cell; T1D, type 1 diabetes mellitus; Treg, regulatory T cell.
ESCs
The significant therapeutic potential of ESCs to generate large quantities of cells with an insulin-expressing phenotype for β-cell replacement therapy is apparent when comparing stem cells obtained from a variety of sources [18, 27–30]. Kroon et al. [29] provided definitive evidence that human ESC (hESC)-derived pancreatic endoderm matured into functional mature β-cells in vivo and protected against streptozotocin (STZ)-induced hyperglycemia a few months following implantation. However, the use of hESC-derived insulin-producing cells in clinical trials is likely to remain a “nonstarter” because of a number of ethical and scientific considerations. Although limitations such as incomplete induction of the insulin-secreting phenotype, inadequate glucose sensing, low-efficiency/high-cost differentiation methods for large-scale generation, and allo- and autoimmune destruction encountered following transplantation continue to be addressed and may eventually be overcome, the teratogenic/tumorigenic potential of hESCs remains a major concern [29, 31–34]. Recent studies have demonstrated the possibility of selecting ESC-derived endodermal cells using cell surface markers [31–35]. For instance, Kelly et al. [35] enriched the pancreatic endodermal cell fraction using anti-CD142 antibodies, which upon transplantation differentiated into all the pancreatic lineages including functional insulin-producing cells. Similarly, a 14-day, four-stage differentiation protocol has now been developed to differentiate commercially available hESCs into a highly enriched Pdx1+ pancreatic progenitor cell population in vitro. Transplantation into diabetic mice resulted in functional maturation and restoration of normoglycemia [36]. Currently, a tractable manufacturing process for the generation of functional pancreatic progenitors from hESC on a scale amenable to clinical entry is under development [37]. However, the necessity to ensure complete elimination of undifferentiated cells still remains, along with the uncertainty that the differentiated products might revert to a pluripotent state. Currently, technologies such as cell encapsulation are in development that could theoretically enable the transplanted encapsulated insulin-producing cells to survive and function without the need for chronic immunosuppressive therapy and ensure prevention of tumor dissemination in the event that malignant degeneration occurred [31, 38, 39]. Despite these challenges, it is important to bear in mind that many groundbreaking advances in the treatment of previously considered incurable diseases has been elucidated through hESC research [40], providing proof of the tremendous potential of cellular therapy in the cure of T1D.
iPSCs
iPSCs may also be considered an appropriate source for the generation of large quantities of β-cells from an autologous nonembryonic source [41, 42], providing an answer to the cited limitations of islet transplantation, that is, tissue supply and chronic immunosuppression. Reversal of hyperglycemia using induced pluripotent stem-derived pancreatic β-like cells has been demonstrated in both type 1 and 2 diabetes mouse models [43]. iPSCs obtained by reprogramming nonobese diabetic (NOD) mouse pancreas-derived epithelial cells were shown to differentiate into insulin-producing cells that expressed diverse pancreatic β-cell markers and normalized hyperglycemia upon transplantation into diabetic mice [44]. iPSCs have also been obtained by reprogramming dermal fibroblasts from patients with T1D [45, 46]. Despite “proof of concept” that iPSCs may be an appropriate source for generating autologous β-cells, their actual clinical utility is hampered by factors such as incomplete maturation of differentiated cells, chromosomal aberrations, and tumorigenic/oncogenic potential [41, 47]. Currently, several cell lineages are being considered as viable sources of iPSCs, while issues of poor efficiency/high cost and slow kinetics of the reprogramming process are being addressed by improving and/or developing novel cell-penetrating protein methodologies and the use of small-molecule gene inducers [18, 20, 42]. However, like hESCs, their clinical utility is hampered by the theoretical risk of mutagenesis and teratoma/tumor formation that prevail in the use of oncogenes and viral transduction [41]. A number of strategies are being tested to circumvent the use of viral vectors. These include among others, the use of nonintegrating or excisable vector systems, such as synthetic modified mRNA for Klf4, c-Myc, Oct4, and Sox2; miR302/367 microRNAs; nonintegrating episomal constructs; DNA minicircles; transducible proteins; and even small chemical agents to enhance reprogramming and functionally replace some reprogramming factors [48–58]. However, even if these strategies succeed, the caveat remains that autologous iPSCs would still be subject to the autoimmune response upon transplantation. A recent study with significant clinical potential has described a novel approach for inducing iPSCs to generate transforming growth factor-β and IL-10-secreting Tregs that are capable of significantly suppressing host immune responses upon adoptive transfer into mice [59]. Currently, a phase I clinical trial to test the safety of ex vivo expanded human autologous polyclonal Treg adoptive immunotherapy for the treatment of T1D is under way (ClinicalTrials.gov identifier NCT01210664). The success of this trial would highlight the translational potential of iPSCs in generating Tregs on a scale amenable to clinical entry.
BM-HSCs and BM-MSCs
The most extensively studied adult stem cells thus far, have been BM-HSCs and multipotent BM-MSCs. Clinical trials have demonstrated the achievement of exogenous insulin independence following nonmyeloablative, autologous HSC transplantation in new-onset T1D patients [60, 61] and in type 1 diabetic adolescents with diabetic ketoacidosis at diagnosis [62]. In the latter study, the mean age of patients was 17.6 ± 3.7 years, and the mean duration of time from symptoms of hyperglycemia to the transplantation (beginning of stem cell mobilization) was 12 ± 4.7 weeks. Young children are generally excluded in HSC transplantation clinical trials because of potential health risks, such as growth arrest/delay, endocrine dysfunctions (e.g., hypogonadism and autoimmune hypothyroidism), and potential infertility, associated with the conditioning regimens [63, 64]. Inclusion criteria broadly comprise a diagnosis of T1D by clinical/metabolic parameters along with the presence of positive antiglutamic acid decarboxylase antibodies. However, additional criteria for selection include the presence of diabetes-associated complications and comorbidities, poor glucose control despite intensive insulin therapy, and symptoms such as severe hypoglycemic episodes and hypoglycemia unawareness that significantly incapacitate the patient and impact their quality of life. Although not appearing to differentiate into insulin-producing cells themselves, autologous HSCs may aid in the preservation of residual β-cells and promote increase in β-cell mass by enhancing neovascularization, decreasing apoptosis, and/or stimulating proliferation. Although the mechanisms of action have yet to be elucidated, a possible achievement of immunological tolerance via clonal exhaustion, cytokine effects, and alterations in immune cell repertoires has been suggested [65]. Interestingly, although allogeneic HSC transplantation is able to eliminate insulitis, augment residual β-cell proliferation, and reverse hyperglycemia in new-onset diabetic mice, only hematopoietic chimerism without graft versus host disease (GvHD) has been observed in overtly diabetic animals [66]. Interestingly, a pilot clinical trial combining BM-HSC allotransplantation with islet transplantation under “Edmonton-like” immunosuppression without ablative conditioning did not lead to stable hematopoietic chimerism and graft tolerance as expected [67]. Therefore, preclinical studies are currently developing combinatorial strategies to induce mixed chimerism with an aim to “immunologically reset” destructive immunity with a reconstituted immune system in which the balance is shifted towards immunological tolerance. For example, allogeneic bone marrow transplantation combined with an induction therapy consisting of low-intensity nonmyeloablative conditioning with anti-CD154 plus rapamycin was able to induce stable mixed chimeras that tolerized both innate and adaptive T- and B-cell immune responses in mice [68]. Similarly, mixed chimerism induced using a radiation-free nontoxic anti-CD3/CD8 conditioning regimen combined with administration of gastrin and epidermal growth factor augmented both β-cell neogenesis and replication, as well as reversed late-stage T1D in NOD mice, without any signs of GvHD [69]. This combinatorial therapeutic strategy, although delicate to reproduce, theoretically has clinical translational potential as a long-term curative therapy for T1D. Recently, a novel stem cell therapeutic approach involving a bioengineered mobilized cellular product enriched for BM-HSCs and tolerogenic graft “facilitating cells” combined with nonmyeloablative conditioning resulted in engraftment, durable chimerism, and tolerance induction without GvHD or engraftment syndrome in recipients of highly human leukocyte antigen (HLA)-mismatched related and unrelated kidney donors [70]. The recipients received pretransplant conditioning with fludarabine, total body irradiation, and cyclophosphamide that was well tolerated, followed by post-transplant immunosuppression with tacrolimus and mycophenolate mofetil. This exciting study using “tolerogenic graft facilitating cells” has paved the way for reapproaching nonmyeloablative, allogeneic HSC and islet transplantation for the induction of durable hematopoietic chimerism to restore tolerance and halt the underlying autoimmune process without the use of chronic immunosuppressive therapy.
Although proof of concept has been provided that high-dose immunosuppression coupled with autologous HSC transplantation can act synergistically to downregulate autoreactive cells, reset the immune system to a more tolerant phenotype, and improve immune regulatory networks, several clinical limitations hamper widespread application of the procedure. These include, among others, the questionable potential of HSCs to directly differentiate in vivo into large numbers of β-cells, the potentially harmful conditioning regimens used, an effectiveness restricted to the early-onset, newly diagnosed stage of the disease prior to complete destruction of the β-cell compartment, an expensive, labor-intensive and complex procedure performed in specialized bone-marrow transplantation facilities, and the potential for life-threatening complications and higher rates of disease reactivation. The procedure also needs to be extensively standardized in other patient subgroups as well, such as patients with diabetic ketoacidosis, those with longer duration of the disease, and young children. Follow-up studies confirming the duration of insulin independence and delineating the mechanisms of action are vital, as are randomized controlled trials that confirm and evaluate the therapeutic potential of HSCs in the treatment of T1D. In contrast, allogeneic HSC transplants, although bearing the risk of GvHD, may induce longer remission, not only because the infusion of autoreactive lymphocytes is avoided, but also because of a possible graft versus autoimmunity effect wherein circulating donor lymphocytes may eliminate autoreactive recipient lymphocytes that have survived therapy. Taken together, although several challenges persist, the establishment of a durable mixed chimerism provided by autologous nonmyeloablative HSC transplantation in combination with immunomodulatory and regenerative β-cell therapies may eventually prove to be an important approach to halt the autoimmune process and induce permanent tolerance.
The potential therapeutic promise of hypoimmunogenic MSCs is also under study following the demonstrated ability of MSCs to differentiate into insulin-producing cells as well as ameliorate immune injury through immunomodulation [71–75]. Although differentiation of MSCs into physiologically competent, glucose-responsive β-cells upon infusion in vivo has yielded contradictory results, both promising and disappointing, their significant immunomodulatory and proangiogenic properties make them ideal candidates for combination therapies. For instance, they have been shown to inhibit the proliferation and function of major immune cell populations such as T, B, and NK cells, induce CD4/CD8 Foxp3 Tregs, and modulate the activities of DCs, both in vivo and in vitro [20, 76]. They also release immunosuppressive, anti-inflammatory, and tolerogenic cytokines/chemokines, express a number of proangiogenic growth factors, and form cell-to-cell inhibitory interactions. These properties potentially enable BM-MSCs to abrogate immune injury, enhance β-cell repair/regeneration, promote longitudinal islet graft survival, and counteract autoimmunity [17, 77–79]. For example, administration of in vitro expanded syngeneic BM-MSCs into STZ-induced diabetic rats was able to induce sustained normoglycemia, alter T-cell cytokine pattern toward IL-10/IL-13 production, and preserve Tregs in the periphery, establishing a tissue microenvironment that supported β-cell activation/survival in the pancreas [80]. Although significant homing and tissue repair potential of human BM-MSCs has been demonstrated in the pancreas [81], engraftment might not even be a necessary prerequisite, because MSCs embolized in the lung were shown to improve myocardial infarction by secreting anti-inflammatory factors [82]. Additionally, MSCs express a serine protease SP16 that may allow them to escape the host immune response [83]. Beyond their role in β-cell regeneration, the potent immunomodulatory and proangiogenic properties of MSCs have significantly improved islet transplantation outcomes in preclinical MSC-islet cotransplantation studies [84–87]. Improved graft survival and glycometabolic control might in turn reduce the dose of the requisite immunosuppression from lifelong administration to low-dose or transient administration. The safety and efficacy of MSC-islet cotransplantation is currently being evaluated in T1D patients (ClinicalTrials.gov identifier NCT00646724). Prochymal, an intravenous infusion of ex vivo cultured human adult MSCs, is also currently under investigation in new-onset diabetics to determine whether the drug can halt the progression of autoimmunity and restore glycometabolic homeostasis (ClinicalTrials.gov identifier NCT00690066). Prochymal is also in phase III clinical trials for GvHD and Crohn's disease and currently is the only stem cell therapeutic designated by the Food and Drug Administration as both an orphan drug and a fast track product.
Although MSCs have tremendous therapeutic potential, limitations such as lack of a standardized protocol to produce MSCs, poor engraftment, limited differentiation under in vivo conditions, malignant transformation, and unwanted release of cytokines need to be overcome to enhance their clinical utility. An important factor to take into consideration in clinical studies that involve MSC therapy is the risk of tumor induction, either through spontaneous malignant transformation of MSCs themselves [88–91] or by the promotion of tumor development and growth [92]. Regarding the former, although spontaneous transformation has not been noted in clinical trials using human MSCs to date, several studies have demonstrated the effect of long-term in vitro expansion of MSCs in increasing the risk of accumulating mutations and consequently enhancing their potential of tumor development in vivo [90, 91]. Malignant transformation has been attributed to factors such as chromosome instability, loss of contact inhibition, aberrant expression of c-Myc, and elevated telomerase activity [91]. In contrast, the significant inhibitory effects that MSCs exert on the proliferation and function of major immune cells populations may also play an important role in favoring cancer development and/or progression, as well as in helping pre-existing non-MSC-derived tumors escape immune surveillance [92–94]. In vivo imaging has confirmed the targeting of microscopic tumors by MSCs and their contribution to the development of tumor stroma [95]. Tumor cells transplanted with MSCs displayed an elevated capacity for proliferation, a highly metastatic ability, and the presence of rich angiogenesis within the tumor tissues [96, 97]. These studies highlight the necessity for a greater understanding of MSC biology in order to establish a safe criterion for their use. Also, although the purported immunoprivilege of MSCs should prevent their rejection in an allotransplantation setting, reports of loss of immune privilege upon differentiation [98] and rejection of HLA-matched MSCs [99] will likely require immunological interventions or immunoisolation to avoid the recurrence of autoimmunity. Clinically applicable assays that monitor the genetic and phenotypic stability of transplanted cells and cells before transplantation, as well as the safety of recipients, need to be developed.
The requirement for invasive harvesting procedures, as well as age and health-dependent differences in yield and cell proliferation ability, are also major limitations. For instance, although BM-MSCs from diabetic patients respond to differentiation cues in a manner comparable to cells from healthy individuals [100], the diabetic microenvironment does have significant influence on the differentiation of BM-MSCs in vivo and in vitro [101]. BM-MSCs obtained from diabetic animals have been shown to exhibit a state of disease memory depicted by an upregulated expression of inflammatory markers (IL-6 or TNFα), impaired proliferation rates, increased mature adipocyte formation, and insulin-like cellular aggregates nonresponsive to high glucose [102]. A reduction in the number of endogenous BM-MSCs, as well as impaired proliferation, survival, and homing ability to the site of injury, has also been observed in diabetic mice [103]. Advanced glycation end products, which accumulate on long-lived proteins of various tissues in advanced age and diabetes mellitus, have also been shown to inhibit the differentiation potential of BM-MSCs [104]. BM-MSCs obtained from diabetic patients exclusively produce proinsulin and very little mature insulin and also do not significantly contribute to insulin production in vivo [101]. Whether these observations preclude the use of BM-MSCs from diabetic patients for autologous stem cell therapies has yet to be determined.
ADSCs and UCB-MSCs
In comparison with BM-MSCs, the clinical feasibility of autologous, hypoimmunogenic human ADSCs (hADSCs) and UCB-MSCs (hUCB-MSCs) is far more appealing, owing to their comparative similarity to BM-MSCs in morphology and immunologic phenotype, their proven preclinical ability to differentiate into glucose-responsive insulin-producing cells, an abundant availability of human donor tissue, and minimal patient discomfort accompanying harvesting procedures [105–110]. Also, high stem cell yield and proliferation capacity, as well as the ability to secrete angiogenic, antiapoptotic, anti-inflammatory, and antifibrotic factors and promote wound healing and tissue repair, significantly increase their suitability for clinical use in T1D [105–110]. Ex vivo generation of glucose-sensitive insulin-secreting MSCs from human adipose tissue and the ability of ADSC therapy to ameliorate autoimmune diabetes in early-onset diabetic mice has been successfully achieved [111, 112]. An open-labeled clinical trial has also demonstrated “cotransplantation of hADSCs and cultured bone marrow” as a means of “insulin replacement therapy” in T1D patients [113–115]. Currently, the safety and efficacy of intravenous administration of autologous activated hADSCs is being tested in overtly diabetic patients (ClinicalTrials.gov identifier NCT00703599). Successful conclusion of these clinical trials would redefine hADSCs as a serious contender in stem cell therapies for curing T1D.
In recent years, hUCB-SCs have gained importance in regenerative medicine because of a number of features, including safe collection, substantial naïveté, considerable regenerative potential, lower rates of GvHD, zero risk to the donor, and less stringent HLA matching requirements with easy access to rare haplotypes. More than 20,000 UCB transplants have been performed worldwide, and ∼460,000 UCB units are being stored in >47 UCB banks worldwide [116]. Not only has the generation of glucose-responsive insulin-producing cells from hUCB-MSCs been demonstrated [117], in vitro differentiation of insulin-producing cells from banked “cryopreserved” hUCB-MSCs has also been achieved [118]. Most compelling, however, has been a recent open-label, phase I/phase II study using a technique known as “stem cell educator therapy” that has definitively demonstrated the efficacy of hUCB-SCs in simultaneously reversing autoimmunity via systemic and local immunomodulation and promoting islet β-cell regeneration [109]. Currently, a number of active clinical trials are under way to determine the safety and efficacy of autologous hUCB-MSCs in the treatment of recently diagnosed and overtly diabetic patients. Although long-term follow-up is necessary to positively establish their therapeutic potential, for now hUCB-MSCs appear to be the most promising candidate in stem cell therapeutics for curing T1D. Another rich, noninvasive and abundant source of MSC is the umbilical cord matrix, a convenient alternative to hUCB because of the ease of harvesting considerably large numbers of MSCs from this source for routine clinical use [119, 120].
Conclusion
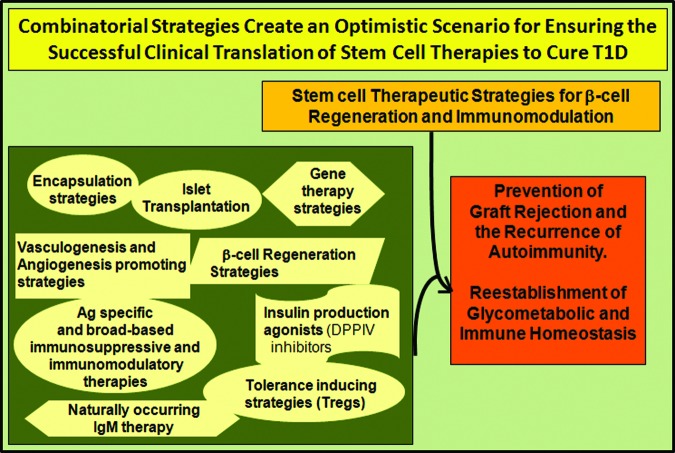
The need for an effective cell replacement and immunomodulatory therapeutic strategy for diabetes persists. Advancements during the last decade in the fields of regenerative medicine, tissue engineering, immunomodulatory therapy, and gene therapy have drawn us a step closer to making the application of stem cell therapy a feasible reality in the cure of T1D. However, a combinatorial approach that can combine safe and effective stem cell strategies with reliable existing therapies such as islet transplantation, as well as the latest immunosuppressive and immunomodulatory drug regimens and/or novel bioengineering techniques, would ensure an optimistic scenario for successful translation of stem cell therapy in the cure of T1D (Fig. 2). In short, the application of stem cell therapy in the cure for T1D appears extremely promising, with bona fide hope for a permanent cure.
Figure 2.
Combinatorial strategies for treatment of T1D. Combining safe and effective stem cell strategies with reliable existing therapies such as islet transplantation, as well as the latest immunosuppressive and immunomodulatory drug regimens and/or novel bioengineering techniques and/or gene therapies, would ensure an optimistic scenario for successful translation of stem cell therapy in the cure of T1D. Abbreviations: Ag, antigen; DPPIV, dipeptidyl peptidase IV; IgM, immunoglobulin M; T1D, type 1 diabetes mellitus; Treg, regulatory T cell.
Author Contributions
P.C.: conception and design, manuscript writing; K.L.B.: conception and design, financial support, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: Etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91:79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 2.Eisenbarth GS. Type I diabetes mellitus: A chronic autoimmune disease. N Engl J Med. 1986;314:1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 3.Sherry NA, Tsai EB, Herold KC. Natural history of beta-cell function in type 1 diabetes. Diabetes. 2005;54:S32–S39. doi: 10.2337/diabetes.54.suppl_2.s32. [DOI] [PubMed] [Google Scholar]
- 4.Tsai EB, Sherry NA, Palmer JP, et al. The rise and fall of insulin secretion in type 1 diabetes mellitus. Diabetologia. 2006;49:261–270. doi: 10.1007/s00125-005-0100-8. [DOI] [PubMed] [Google Scholar]
- 5.Tooley JE, Waldron-Lynch F, Herold KC. New and future immunomodulatory therapy in type 1 diabetes. Trends Mol Med. 2012;18:173–181. doi: 10.1016/j.molmed.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atkinson MA, Bluestone JA, Eisenbarth GS, et al. How does type 1 diabetes develop?: The notion of homicide or β-cell suicide revisited. Diabetes. 2011;60:1370–1379. doi: 10.2337/db10-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chhabra P, Brayman KL. Current status of immunomodulatory and cellular therapies in preclinical and clinical islet transplantation. J Transplant. 2011;2011:637692. doi: 10.1155/2011/637692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenbarth GS. Prevention of type 1A diabetes. Endocr Pract. 2012;18:745–749. doi: 10.4158/EP12080.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waldron-Lynch F, Herold KC. Immunomodulatory therapy to preserve pancreatic β-cell function in type 1 diabetes. Nat Rev Drug Discov. 2011;10:439–452. doi: 10.1038/nrd3402. [DOI] [PubMed] [Google Scholar]
- 10.Yoon JW, Jun HS, Santamaria P. Cellular and molecular mechanisms for the initiation and progression of beta cell destruction resulting from the collaboration between macrophages and T cells. Autoimmunity. 1998;27:109–122. doi: 10.3109/08916939809008041. [DOI] [PubMed] [Google Scholar]
- 11.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and β-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5:219–226. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- 12.Honkanen J, Nieminen JK, Gao R, et al. IL-17 immunity in human type 1 diabetes. J Immunol. 2010;185:1959–1967. doi: 10.4049/jimmunol.1000788. [DOI] [PubMed] [Google Scholar]
- 13.Cabrera SM, Rigby MR, Mirmira RG. Targeting regulatory T cells in the treatment of type 1 diabetes mellitus. Curr Mol Med. 2012;12:1261–1272. doi: 10.2174/156652412803833634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruessner AC, Sutherland DE, Gruessner RW. Long-term outcome after pancreas transplantation. Curr Opin Organ Transplant. 2012;17:100–105. doi: 10.1097/MOT.0b013e32834ee700. [DOI] [PubMed] [Google Scholar]
- 15.Jamiolkowski RM, Guo LY, Li YR, et al. Islet transplantation in type I diabetes mellitus. Yale J Biol Med. 2012;85:37–43. [PMC free article] [PubMed] [Google Scholar]
- 16.Huang X, Moore DJ, Ketchum RJ, et al. Resolving the conundrum of islet transplantation by linking metabolic dysregulation, inflammation, and immune regulation. Endocr Rev. 2008;29:603–630. doi: 10.1210/er.2008-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chhabra P, Kensinger CD, Moore DJ, et al. Present accomplishments and future prospects of cell-based therapies for type 1 diabetes mellitus. In: Wagner D, editor. Type 1 Diabetes: Pathogenesis, Genetics and Immunotherapy. Rijeka, Croatia: In Tech Open Access; 2011. pp. 295–336. [Google Scholar]
- 18.Weir GC, Cavelti-Weder C, Bonner-Weir S. Stem cell approaches for diabetes: Towards beta cell replacement. Genome Med. 2011;3:61. doi: 10.1186/gm277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granger A, Kushner JA. Cellular origins of beta-cell regeneration: A legacy view of historical controversies. J Intern Med. 2009;266:325–338. doi: 10.1111/j.1365-2796.2009.02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiorina P, Voltarelli J, Zavazava N. Immunological applications of stem cells in type 1 diabetes. Endocr Rev. 2011;32:725–754. doi: 10.1210/er.2011-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barcala Tabarrozzi AE, Castro CN, Dewey RA. Cell-based interventions to halt autoimmunity in type 1 diabetes mellitus. Clin Exp Immunol. 2013;171:135–146. doi: 10.1111/cei.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fändrich F, Ungefroren H. Customized cell-based treatment options to combat autoimmunity and restore beta-cell function in type 1 diabetes mellitus: Current protocols and future perspectives. Adv Exp Med Biol. 2010;654:641–665. doi: 10.1007/978-90-481-3271-3_28. [DOI] [PubMed] [Google Scholar]
- 23.Sims E, Evans-Molina C. Stem cells as a tool to improve outcomes of islet transplantation. J Transplant. 2012;2012:736491. doi: 10.1155/2012/736491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madec AM, Mallone R, Afonso G, et al. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia. 2009;52:1391–1399. doi: 10.1007/s00125-009-1374-z. [DOI] [PubMed] [Google Scholar]
- 25.Jurewicz M, Yang S, Augello A, et al. Congenic mesenchymal stem cell therapy reverses hyperglycemia in experimental type 1 diabetes. Diabetes. 2010;59:3139–3147. doi: 10.2337/db10-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rackham CL, Chagastelles PC, Nardi NB, et al. Co-transplantation of mesenchymal stem cells maintains islet organisation and morphology in mice. Diabetologia. 2011;54:1127–1135. doi: 10.1007/s00125-011-2053-4. [DOI] [PubMed] [Google Scholar]
- 27.Furth ME, Atala A. Stem cell sources to treat diabetes. J Cell Biochem. 2009;106:507–511. doi: 10.1002/jcb.22000. [DOI] [PubMed] [Google Scholar]
- 28.Mfopou JK, Chen B, Sui L, et al. Recent advances and prospects in the differentiation of pancreatic cells from human embryonic stem cells. Diabetes. 2010;59:2094–2101. doi: 10.2337/db10-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 30.Bose B, Shenoy PS, Konda S, et al. Human embryonic stem cell differentiation into insulin secreting β-cells for diabetes. Cell Biol Int. 2012;36:1013–1020. doi: 10.1042/CBI20120210. [DOI] [PubMed] [Google Scholar]
- 31.Lysy PA, Weir GC, Bonner-Weir S. Concise review: Pancreas regeneration: Recent advances and perspectives. Stem Cells Translational Medicine. 2012;1:150–159. doi: 10.5966/sctm.2011-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang P, Rodriguez RT, Wang J, et al. Targeting SOX17 in human embryonic stem cells creates unique strategies for isolating and analyzing developing endoderm. Cell Stem Cell. 2011;8:335–346. doi: 10.1016/j.stem.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahan B, Magliocca J, Merriam F, et al. Elimination of tumorigenic stem cells from differentiated progeny and selection of definitive endoderm reveals a Pdx1+ foregut endoderm stem cell lineage. Stem Cell Res. 2011;6:143–157. doi: 10.1016/j.scr.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang W, Sui X, Zhang D, et al. CD24: A novel surface marker for PDX1-positive pancreatic progenitors derived from human embryonic stem cells. Stem Cells. 2011;29:609–617. doi: 10.1002/stem.608. [DOI] [PubMed] [Google Scholar]
- 35.Kelly OG, Chan MY, Martinson LA, et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol. 2011;29:750–756. doi: 10.1038/nbt.1931. [DOI] [PubMed] [Google Scholar]
- 36.Rezania A, Bruin JE, Riedel MJ, et al. Maturation of human embryonic stem cell derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61:2016–2029. doi: 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulz TC, Young HY, Agulnick AD, et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS One. 2012;7:e37004. doi: 10.1371/journal.pone.0037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Sullivan ES, Vegas A, Anderson DG, et al. Islets transplanted in immunoisolation devices: A review of the progress and the challenges that remain. Endocr Rev. 2011;32:827–844. doi: 10.1210/er.2010-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuch BE, Hughes TC, Evans MD. Encapsulated pancreatic progenitors derived from human embryonic stem cells as a therapy for insulin-dependent diabetes. Diabetes Metab Res Rev. 2011;27:928–932. doi: 10.1002/dmrr.1274. [DOI] [PubMed] [Google Scholar]
- 40.Atala A. Human embryonic stem cells: Early hints on safety and efficacy. Lancet. 2012;379:689–690. doi: 10.1016/S0140-6736(12)60118-4. [DOI] [PubMed] [Google Scholar]
- 41.Maehr R. iPS cells in type 1 diabetes research and treatment. Clin Pharmacol Ther. 2011;89:750–753. doi: 10.1038/clpt.2011.1. [DOI] [PubMed] [Google Scholar]
- 42.Hosoya M. Preparation of pancreatic β-cells from human iPS cells with small molecules. Islets. 2012;4:249–252. doi: 10.4161/isl.20856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alipio Z, Liao W, Roemer EJ, et al. Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic beta-like cells. Proc Natl Acad Sci USA. 2010;107:13426–13431. doi: 10.1073/pnas.1007884107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeon K, Lim H, Kim JH, et al. Differentiation and transplantation of functional pancreatic beta cells generated from induced pluripotent stem cells derived from a type 1 diabetes mouse model. Stem Cells Dev. 2012;21:2642–2655. doi: 10.1089/scd.2011.0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maehr R, Chen S, Snitow M, et al. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci USA. 2009;106:15768–15773. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jang J, Yoo JE, Lee JA, et al. Disease-specific induced pluripotent stem cells: A platform for human disease modeling and drug discovery. Exp Mol Med. 2012;44:202–213. doi: 10.3858/emm.2012.44.3.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayshar Y, Ben-David U, Lavon N, et al. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell. 2010;7:521–531. doi: 10.1016/j.stem.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 48.Stadtfeld M, Nagaya M, Utikal J, et al. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hiratsuka M, Uno N, Ueda K. Integration-free iPS cells engineered using human artificial chromosome vectors. PLoS One. 2011;6:e25961. doi: 10.1371/journal.pone.0025961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaji K, Norrby K, Paca A, et al. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anokye-Danso F, Trivedi CM, Juhr D, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8:376–438. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jia F, Wilson KD, Sun N, et al. A nonviral minicircle vector for deriving human iPS cells. Nat Methods. 2010;7:197–199. doi: 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou H, Wu S, Joo JY, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nie B, Wang H, Laurent T, et al. Cellular reprogramming: A small molecule perspective. Curr Opin Cell Biol. 2012;24:784–792. doi: 10.1016/j.ceb.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng B, Ng JH, Heng JC, et al. Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell Stem Cell. 2009;4:301–312. doi: 10.1016/j.stem.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 58.Lu J, Kong X, Luo C, et al. Application of epigenome-modifying small molecules in induced pluripotent stem cells. Med Res Rev. 2012 doi: 10.1002/med.21265. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 59.Haque R, Lei F, Xiong X, et al. Programming of regulatory T cells from pluripotent stem cells and prevention of autoimmunity. J Immunol. 2012;189:1228–1236. doi: 10.4049/jimmunol.1200633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Couri CE, Oliveira MC, Stracieri AB, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009;301:1573–1579. doi: 10.1001/jama.2009.470. [DOI] [PubMed] [Google Scholar]
- 61.Snarski E, Milczarczyk A, Torosian T, et al. Independence of exogenous insulin following immunoablation and stem cell reconstitution in newly diagnosed diabetes type I. Bone Marrow Transplant. 2011;46:562–566. doi: 10.1038/bmt.2010.147. [DOI] [PubMed] [Google Scholar]
- 62.Gu W, Hu J, Wang W, et al. Diabetic ketoacidosis at diagnosis influences complete remission after treatment with hematopoietic stem cell transplantation in adolescents with type 1 diabetes. Diabetes Care. 2012;35:1413–1419. doi: 10.2337/dc11-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Socié G, Salooja N, Cohen A, et al. Nonmalignant late effects after allogeneic stem cell transplantation. Blood. 2003;101:3373–3385. doi: 10.1182/blood-2002-07-2231. [DOI] [PubMed] [Google Scholar]
- 64.Couri CE, de Oliveira MC, Simões BP. Risks, benefits, and therapeutic potential of hematopoietic stem cell transplantation for autoimmune diabetes. Curr Diab Rep. 2012;12:604–611. doi: 10.1007/s11892-012-0309-0. [DOI] [PubMed] [Google Scholar]
- 65.Voltarelli JC, Couri CE, Stracieri AB, et al. Autologous hematopoietic stem cell transplantation for type 1 diabetes. Ann NY Acad Sci. 2008;1150:220–229. doi: 10.1196/annals.1447.048. [DOI] [PubMed] [Google Scholar]
- 66.Zhang C, Todorov I, Lin CL, et al. Elimination of insulitis and augmentation of islet beta cell regeneration via induction of chimerism in overtly diabetic NOD mice. Proc Natl Acad Sci USA. 2007;104:2337–2342. doi: 10.1073/pnas.0611101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mineo D, Ricordi C, Xu X, et al. Combined islet and hematopoietic stem cell allotransplantation: A clinical pilot trial to induce chimerism and graft tolerance. Am J Transplant. 2008;8:1262–1274. doi: 10.1111/j.1600-6143.2008.02230.x. [DOI] [PubMed] [Google Scholar]
- 68.Xu H, Zhu Z, Huang Y, et al. Innate and adaptive immune responses are tolerized in chimeras prepared with nonmyeloablative conditioning. Transplantation. 2012;93:469–476. doi: 10.1097/TP.0b013e318242bddf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang M, Racine JJ, Song X, et al. Mixed chimerism and growth factors augment β cell regeneration and reverse late-stage type 1 diabetes. Sci Transl Med. 2012;4:133ra59. doi: 10.1126/scitranslmed.3003835. [DOI] [PubMed] [Google Scholar]
- 70.Leventhal J, Abecassis M, Miller J, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4:124ra28. doi: 10.1126/scitranslmed.3003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gabr MM, Zakaria MM, Refaie AF, et al. Insulin-producing cells from adult human bone marrow mesenchymal stem cells control streptozotocin-induced diabetes in nude mice. Cell Transplant. 2012 doi: 10.3727/096368912X647162. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Domínguez-Bendala J, Lanzoni G, Inverardi L, et al. Concise review: Mesenchymal stem cells for diabetes. Stem Cells Translational Medicine. 2012;1:59–63. doi: 10.5966/sctm.2011-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Volarevic V, Arsenijevic N, Lukic ML, et al. Concise review: Mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells. 2011;29:5–10. doi: 10.1002/stem.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chhabra P, Mirmira RG, Brayman KL. Regenerative medicine and tissue engineering: Contribution of stem cells in organ transplantation. Curr Opin Organ Transplant. 2009;14:46–50. doi: 10.1097/MOT.0b013e328322f989. [DOI] [PubMed] [Google Scholar]
- 75.Ho JH, Tseng TC, Ma WH, et al. Multiple intravenous transplantations of mesenchymal stem cells effectively restore long-term blood glucose homeostasis by hepatic engraftment and β-cell differentiation in streptozocin-induced diabetic mice. Cell Transplant. 2012;21:997–1009. doi: 10.3727/096368911X603611. [DOI] [PubMed] [Google Scholar]
- 76.Abdi R, Fiorina P, Adra CN, et al. Immunomodulation by mesenchymal stem cells: A potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57:1759–1567. doi: 10.2337/db08-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davis NE, Hamilton D, Fontaine MJ. Harnessing the immunomodulatory and tissue repair properties of mesenchymal stem cells to restore β cell function. Curr Diab Rep. 2012;12:612–622. doi: 10.1007/s11892-012-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ezquer FE, Ezquer ME, Parrau DB, et al. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant. 2008;14:631–640. doi: 10.1016/j.bbmt.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 79.Bell GI, Broughton HC, Levac KD, et al. Transplanted human bone marrow progenitor subtypes stimulate endogenous islet regeneration and revascularization. Stem Cells Dev. 2012;21:97–109. doi: 10.1089/scd.2010.0583. [DOI] [PubMed] [Google Scholar]
- 80.Boumaza I, Srinivasan S, Witt WT, et al. Autologous bone marrow-derived rat mesenchymal stem cells promote PDX-1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. J Autoimmun. 2009;32:33–42. doi: 10.1016/j.jaut.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 81.Lee RH, Seo MJ, Reger RL, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA. 2006;103:17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.El Haddad N, Heathcote D, Moore R, et al. Mesenchymal stem cells express serine protease inhibitor to evade the host immune response. Blood. 2011;117:1176–1183. doi: 10.1182/blood-2010-06-287979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yeung TY, Seeberger KL, Kin T, et al. Human mesenchymal stem cells protect human islets from pro-inflammatory cytokines. PLoS One. 2012;7:e38189. doi: 10.1371/journal.pone.0038189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Figliuzzi M, Cornolti R, Perico N, et al. Bone marrow-derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplant Proc. 2009;41:1797–1800. doi: 10.1016/j.transproceed.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 86.Sakata N, Goto M, Yoshimatsu G, et al. Utility of co-transplanting mesenchymal stem cells in islet transplantation. World J Gastroenterol. 2011;17:5150–5155. doi: 10.3748/wjg.v17.i47.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ito T, Itakura S, Todorov I, et al. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation. 2010;89:1438–1445. doi: 10.1097/tp.0b013e3181db09c4. [DOI] [PubMed] [Google Scholar]
- 88.Fiorina P, Jurewicz M, Augello A, et al. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol. 2009;183:993–1004. doi: 10.4049/jimmunol.0900803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rubio D, Garcia-Castro J, Martin MC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 90.Miura M, Miura Y, Hesed M, et al. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–1103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- 91.Tolar J, Nauta AJ, Osborn MJ, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 92.Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 93.De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, et al. Immunosuppressive properties of mesenchymal stem cells: Advances and applications. Curr Mol Med. 2012;12:574–591. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]
- 94.Han Z, Jing Y, Zhang S. The role of immunosuppression of mesenchymal stem cells in tissue repair and tumor growth. Cell Biosci. 2012;2:8. doi: 10.1186/2045-3701-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hung SC, Deng WP, Yang WK, et al. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin Cancer Res. 2005;11:7749–7756. doi: 10.1158/1078-0432.CCR-05-0876. [DOI] [PubMed] [Google Scholar]
- 96.Zhu W, Xu W, Jiang R, et al. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol. 2006;80:267–274. doi: 10.1016/j.yexmp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 97.Suzuki K, Sun R, Origuchi M. Mesenchymal stromal cells promote tumor growth through the enhancement of neovascularization. Mol Med. 2011;17:579–587. doi: 10.2119/molmed.2010.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang XP, Sun Z, Miyagi Y, et al. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122:2419–2429. doi: 10.1161/CIRCULATIONAHA.110.955971. [DOI] [PubMed] [Google Scholar]
- 99.Eliopoulos N, Stagg J, Lejeune L, et al. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106:4057–4065. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- 100.Sun Y, Chen L, Hou XG, et al. Differentiation of bone marrow-derived mesenchymal stem cells from diabetic patients into insulin producing cells in vitro. Chin Med J. 2007;120:771–776. [PubMed] [Google Scholar]
- 101.Phadnis SM, Ghaskadbi SM, Hardikar AA, et al. Mesenchymal stem cells derived from bone marrow of diabetic patients portrait unique markers influenced by the diabetic microenvironment. Rev Diabet Stud. 2009;6:260–270. doi: 10.1900/RDS.2009.6.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Madhira SL, Challa SS, Chalasani M, et al. Promise(s) of mesenchymal stem cells as an in vitro model system to depict pre-diabetic/diabetic milieu in WNIN/GR-Ob mutant rats. PLoS One. 2012;7:e48061. doi: 10.1371/journal.pone.0048061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shin L, Peterson DA. Impaired therapeutic capacity of autologous stem cells in a model of type 2 diabetes. Stem Cells Translational Medicine. 2012;1:125–135. doi: 10.5966/sctm.2012-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kume S, Kato S, Yamagishi S, et al. Advanced glycation end-products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J Bone Miner Res. 2005;20:1647–1658. doi: 10.1359/JBMR.050514. [DOI] [PubMed] [Google Scholar]
- 105.Mizuno H, Tobita M, Uysal AC. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30:804–810. doi: 10.1002/stem.1076. [DOI] [PubMed] [Google Scholar]
- 106.Gir P, Oni G, Brown SA, et al. Human adipose stem cells: Current clinical applications. Plast Reconstr Surg. 2012;129:1277–1290. doi: 10.1097/PRS.0b013e31824ecae6. [DOI] [PubMed] [Google Scholar]
- 107.Bassi EJ, Moraes-Vieira PM, Moreira-Sá CS, et al. Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes. 2012;61:2534–2545. doi: 10.2337/db11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fan CG, Zhang QJ, Zhou JR. Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev. 2011;7:195–207. doi: 10.1007/s12015-010-9168-8. [DOI] [PubMed] [Google Scholar]
- 109.Zhao Y, Jiang Z, Zhao T, et al. Reversal of type 1 diabetes via islet β cell regeneration following immune modulation by cord blood-derived multipotent stem cells. BMC Med. 2012;10:3. doi: 10.1186/1741-7015-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao Y, Mazzone T. Human cord blood stem cells and the journey to a cure for type 1 diabetes. Autoimmun Rev. 2010;10:103–107. doi: 10.1016/j.autrev.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 111.Chandra V, Swetha G, Muthyala S, et al. Islet-like cell aggregates generated from human adipose tissue derived stem cells ameliorate experimental diabetes in mice. PLoS One. 2011;6:e20615. doi: 10.1371/journal.pone.0020615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Okura H, Komoda H, Fumimoto Y, et al. Transdifferentiation of human adipose tissue-derived stromal cells into insulin-producing clusters. J Artif Organs. 2009;12:123–130. doi: 10.1007/s10047-009-0455-6. [DOI] [PubMed] [Google Scholar]
- 113.Ohmura Y, Tanemura M, Kawaguchi N, et al. Combined transplantation of pancreatic islets and adipose tissue-derived stem cells enhances the survival and insulin function of islet grafts in diabetic mice. Transplantation. 2010;90:1366–1373. doi: 10.1097/TP.0b013e3181ffba31. [DOI] [PubMed] [Google Scholar]
- 114.Cavallari G, Olivi E, Bianchi F, et al. Mesenchymal stem cells and islet co-transplantation in diabetic rats: Improved islet graft revascularization and function by human adipose tissue-derived stem cells preconditioned with natural molecules. Cell Transplant. 2012;21:2771–2781. doi: 10.3727/096368912X637046. [DOI] [PubMed] [Google Scholar]
- 115.Vanikar AV, Dave SD, Thakkar UG, et al. Cotransplantation of adipose tissue-derived insulin-secreting mesenchymal stem cells and hematopoietic stem cells: A novel therapy for insulin-dependent diabetes mellitus. Stem Cells Int. 2010;2010:582382. doi: 10.4061/2010/582382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mayani H. Umbilical cord blood: Lessons learned and lingering challenges after more than 20 years of basic and clinical research. Arch Med Res. 2011;42:645–651. doi: 10.1016/j.arcmed.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 117.Prabakar KR, Domínguez-Bendala J, Molano RD, et al. Generation of glucose-responsive, insulin-producing cells from human umbilical cord blood-derived mesenchymal stem cells. Cell Transplant. 2012;21:1321–1339. doi: 10.3727/096368911X612530. [DOI] [PubMed] [Google Scholar]
- 118.Phuc PV, Nhung TH, Loan DT, et al. Differentiating of banked human umbilical cord blood-derived mesenchymal stem cells into insulin-secreting cells. In Vitro Cell Dev Biol Anim. 2011;47:54–63. doi: 10.1007/s11626-010-9356-5. [DOI] [PubMed] [Google Scholar]
- 119.Forraz N, McGuckin CP. The umbilical cord: A rich and ethical stem cell source to advance regenerative medicine. Cell Prolif. 2011;44:60–69. doi: 10.1111/j.1365-2184.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hu J, Yu X, Wang Z, et al. Long term effects of the implantation of Wharton's jelly-derived mesenchymal stem cells from the umbilical cord for newly onset type 1 diabetes mellitus. Endocr J. 2012 doi: 10.1507/endocrj.ej12-0343. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]