Abstract
Sarcomas of the prostate are rare tumours. Their clinicopathologic features are well described, however, the imaging features of these tumours have rarely been documented. The purpose of this article is to illustrate the imaging findings of prostate sarcomas, with an emphasis on their appearance on magnetic resonance imaging and to identify features that may help to differentiate them from the commoner prostate adenocarcinomas.
Keywords: Prostate, sarcomas, magnetic resonance imaging, computed tomography, positron emission tomography
Introduction
Sarcomas of the prostate are rare tumours, accounting for 0.1–0.2% of all primary prostatic neoplasms[1,2]. Rhabdomyosarcomas are the most common form, accounting for 42% of prostate sarcomas and occur primarily in children and adolescents[3,4]. Leiomyosarcomas represent 25% of all prostate sarcomas and are more likely to occur in older men[2,5]. Other subtypes are rarer. Prostate sarcomas are predominantly mesenchymal in origin but, less commonly, they may also arise from stromal components of the prostate gland.
The imaging features of these tumours have rarely been documented in the literature and have appeared mainly as case reports. Imaging, however, has an important role in the management of patients with prostate sarcomas. A lack of awareness of the imaging appearances may hinder the diagnosis and care of these patients. To our knowledge, this is the largest reported series of the magnetic resonance (MR) imaging appearances of prostate sarcomas.
In this review, the imaging appearances of prostate sarcomas are described, based on a retrospective review of the imaging findings in 13 patients with prostate sarcoma at our institution, which is a tertiary referral centre for cancer care. For each tumour, the pattern of growth and metastatic spread and the MR imaging, computed tomography (CT) and fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT appearances were assessed when available. The histologic classification of these 13 cases of prostate sarcoma was 5 rhabdomyosarcomas (of which 3 were of the embryonal subtype and 2 were alveolar subtypes), 4 leiomyosarcomas, 1 stromal sarcoma, 1 malignant peripheral nerve sheath tumour (MPNST), 1 spindle cell sarcoma and 1 postradiation-induced sarcoma not otherwise specified.
Clinical findings
Prostate sarcomas are typically rapidly growing and aggressive tumours. They present with local symptoms of their effect on adjacent structures, urinary dysfunction being the commonest symptom at presentation. Other symptoms include tenesmus, pain and haematuria from invasion of the bladder floor and urethra. Clinically, no reliable tumour markers for prostate sarcoma have been identified[6]. The serum prostate-specific antigen (PSA) value is usually normal and this is not surprising considering the non-epithelial origin of these tumours[5,7]. This feature is a helpful aid in distinguishing prostate sarcoma from carcinoma. Also, rhabdomyosarcomas, the commonest form of prostate sarcoma, occur primarily in children and young adults, compared with prostate adenocarcinomas, which occur in older men.
The clinical findings from our study are summarized in Table 1.
Table 1.
Histology, age, serum PSA level and presenting symptoms for each case of prostate sarcoma
| Tumour type | Age | PSA (ng/ml) | Presenting symptoms |
|---|---|---|---|
| Embryonal rhabdomyosarcoma | 18 | 2.5 | Urinary dysfunction |
| Embryonal rhabdomyosarcoma | 15 | – | Urinary dysfunction, haematuria |
| Embryonal rhabdomyosarcoma | 16 | – | Urinary dysfunction |
| Alveolar rhabdomyosarcoma | 15 | – | Urinary dysfunction, flank pain |
| Alveolar rhabdomyosarcoma | 17 | – | Urinary dysfunction |
| Leiomyosarcoma | 54 | 0.3 | Urinary dysfunction, tenesmus |
| Leiomyosarcoma | 62 | 0.2 | Urinary dysfunction, perineal pain |
| Leiomyosarcoma | 62 | 0.3 | Urinary dysfunction, perineal pain, tenesmus |
| Leiomyosarcoma | 63 | 0.6 | Urinary dysfunction |
| Stromal sarcoma | 46 | – | Urinary dysfunction |
| MPNST | 33 | – | Testicular pain |
| Spindle cell sarcoma | 42 | – | Urinary dysfunction |
| Postradiation-induced sarcoma | 81 | 0.3 | Asymptomatic, diagnosed on biopsy |
Growth pattern
Prostate sarcomas are usually large at presentation; the mean size in this study was 7.9 cm. Because of their large size at the time of diagnosis, distinguishing on imaging between sarcomas arising from the prostate and those arising from the bladder base may be difficult. The tumour shape varies from round/lobulated and well-defined masses to irregular and ill-defined lesions. Tumour shape alone is not a reliable predictor of the histologic subtype of prostate sarcomas, with well-defined and irregular masses seen in both embryonal rhabdomyosarcomas and leiomyosarcomas. However, both cases of alveolar rhabdomyosarcoma in this study appeared as irregular ill-defined masses (Fig. 1). This may reflect the more aggressive nature of alveolar rhabdomyosarcomas and it is important to identify these rare cases of alveolar rhabdomyosarcoma involving the prostate because this histologic subtype is unfavourable and necessitates more aggressive chemotherapy[1]. Also, all of the rarer prostate sarcomas (MPNST, stromal, spindle cell and postradiation-induced sarcomas) appeared as round/lobulated well-defined masses.
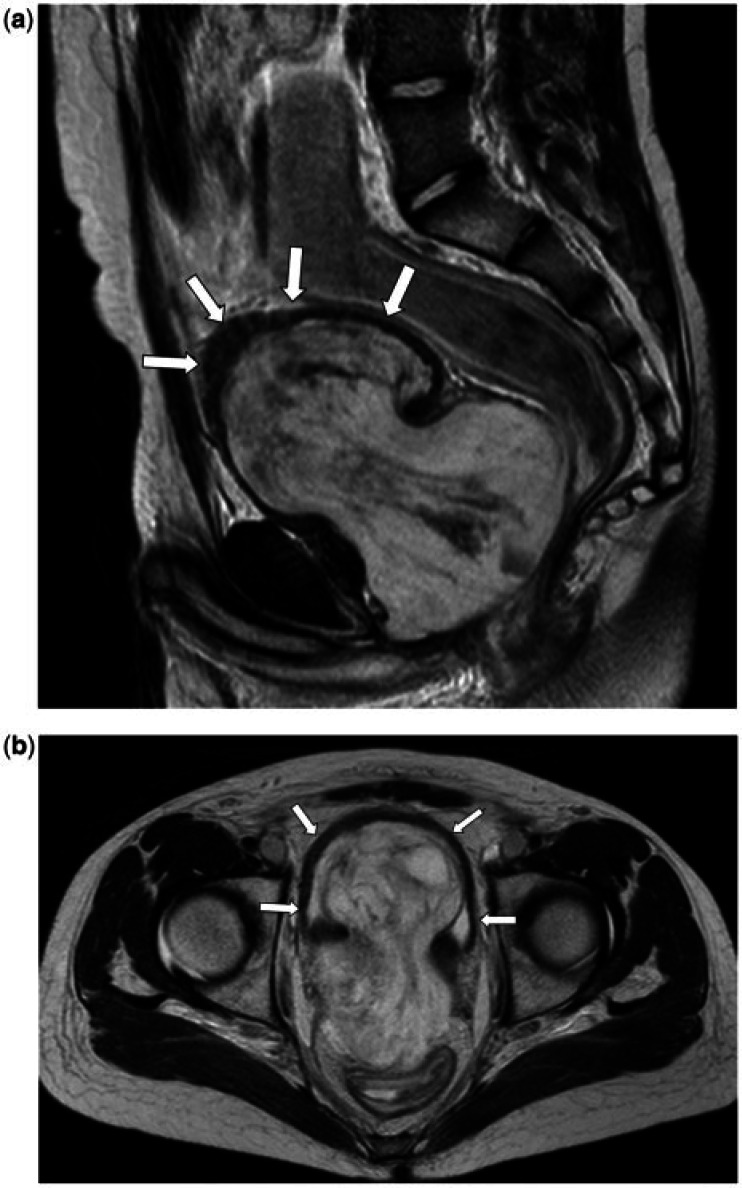
Figure 1.
A 17-year-old male (a, b) and 15-year old male (c) both with a prostatic alveolar rhabdomyosarcoma. (a) Sagittal T2-weighted and (b) coronal T2-weighted MR images show the prostate tumour as an irregular, ill-defined soft tissue mass, which is predominantly of intermediate T2 signal intensity. It extends beyond the prostate, breaching the urogenital diaphragm, extending into the penile bulb (black arrow) and invades the anterior wall of the rectum (white arrow). (c) Axial T2-weighted MR images. The tumour demonstrates the aggressive infiltrative features of alveolar rhabdomyosaromas, invading the seminal vesicles, vas deferens (black open arrow), bladder and extending up to the mesorectal fascia posteriorly. There are enlarged left pelvic side wall nodes (white asterisk). Ascites is also noted (black asterisk).
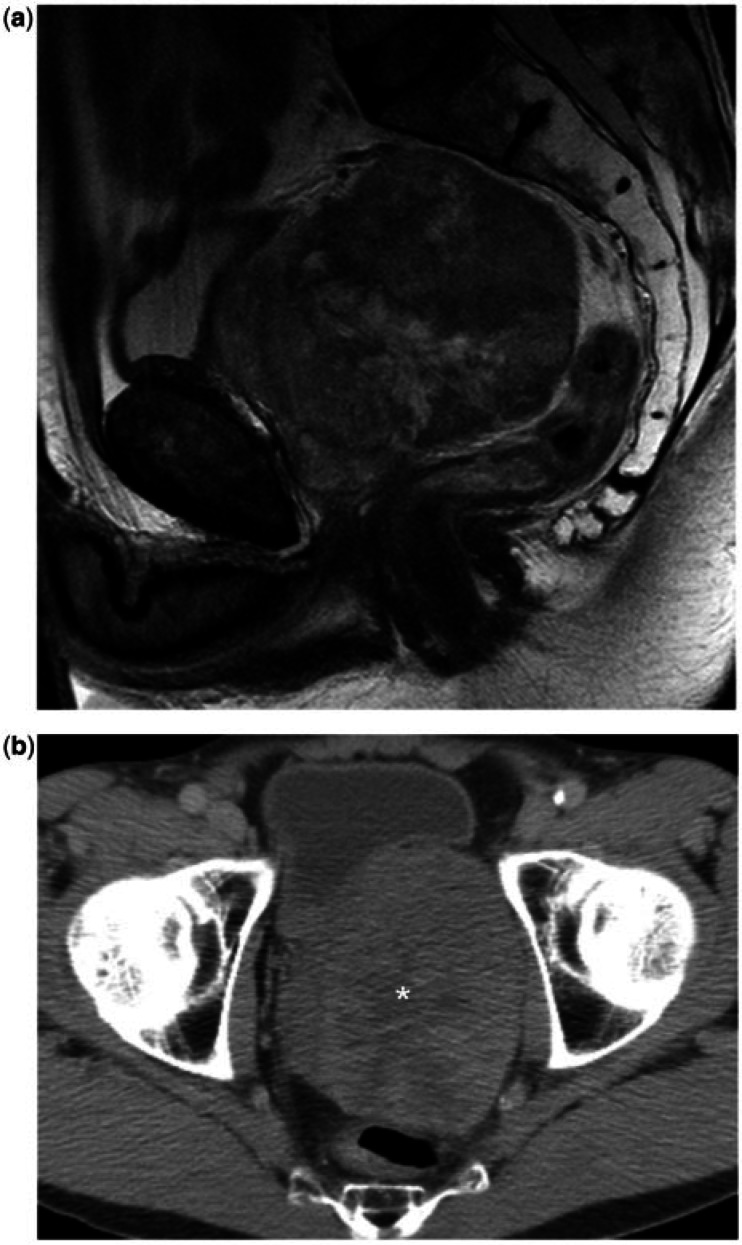
Most of the prostate sarcomas in our study occupied most of or the entire prostate, with loss of the normal zonal anatomy as seen on MR imaging. However, in 5 of the 13 cases, MR imaging showed that the tumours were likely to arise from the central gland, with compression of a recognizable peripheral zone. This imaging feature could be helpful in differentiating sarcomas from prostate adenocarcinoma, which more often arises from the peripheral zone. Extracapsular extension of tumour was also seen in all cases of prostate sarcomas; this feature reportedly occurs in only about 35% of prostate adenocarcinomas[8]. As a result, tumour invasion of adjacent structures is common, occurring in 10 of the 13 cases in this study, with the seminal vesicles being the most commonly involved structure (n = 8), followed by bladder (n = 6), rectum (n = 2) and ureter (n = 1). Despite their locally aggressive features, prostate sarcomas can also exert a significant mass effect on adjacent structures such as the bladder and rectum (Fig. 2). The presence of displacement of adjacent organs, as opposed to solely direct invasion, is not a feature associated with prostate adenocarcinoma, which extends by infiltrating its adjacent tissues.
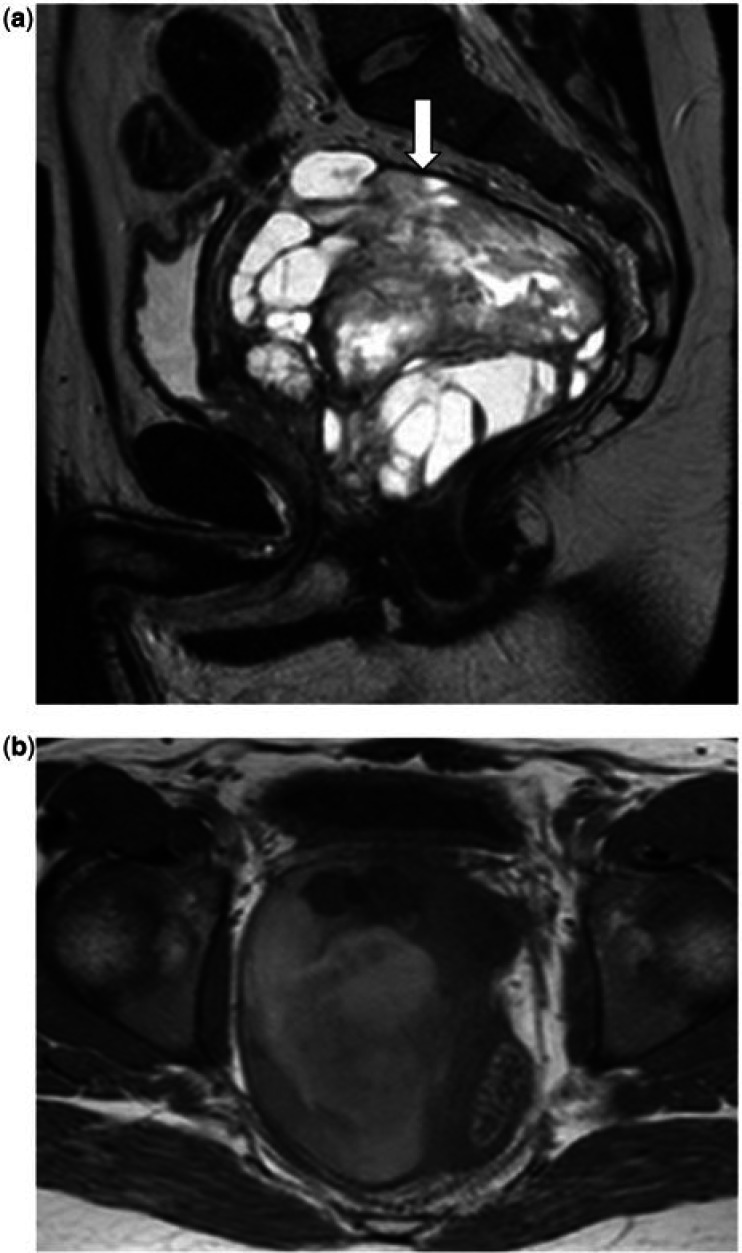
Figure 2.
A 15-year-old male with a prostatic embryonal rhabdomyosarcoma. (a) Sagittal T2-weighted and (b) axial T2-weighted images show the tumour as a well-defined lobulated mass that replaces and extends beyond the prostate, exerting a mass effect anteriorly on the bladder (arrows) and on the lower rectum posteriorly. The mass consists of a T2 hyperintense stroma containing sheets of intermediate-low signal tissue.
The growth pattern for each prostate sarcoma from our study is summarized in Table 2.
Table 2.
Growth pattern for each case of prostate sarcoma
| Histology | Embryonal rhabdomyosarcoma (n = 3) | Alveolar rhabdomyosarcoma (n = 2) | Leiomyosarcoma (n = 4) | Stromal sarcoma (n = 1) | MPNST (n = 1) | Spindle cell sarcoma (n = 1) | Postradiation-induced sarcoma (n = 1) | All cases (n = 13) |
|---|---|---|---|---|---|---|---|---|
| Mean size (cm) | 7.7 | 7.4 | 7.5 | 11.7 | 10.8 | 10.0 | 3.5 | 7.9 |
| Shape and outline | A = 1, B = 2 | A = 0, B = 2 | A = 3, B = 1 | A = 1, B = 0 | A = 1, B = 0 | A = 1, B = 0 | A = 1, B = 0 | A = 8, B = 5 |
| Pseudocapsule | 1 | 0 | 2 | 1 | 1 | 0 | 1 | 6 |
| Zonal preservation | 1 | 0 | 2 | 0 | 1 | 0 | 1 | 5 |
| Extension beyond prostate | 3 | 2 | 4 | 1 | 1 | 1 | 1 | 13 |
| Breach of urogenital diaphragm | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 3 |
| Bladder invasion | 3 | 1 | 1 | 0 | 0 | 1 | 0 | 6 |
| Seminal vesicle invasion | 1 | 2 | 3 | 0 | 0 | 1 | 1 | 8 |
| Rectal invasion | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 2 |
| Ureteric Invasion | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Hydronephrosis | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 4 |
| Lymph node involvement |
|
|
|
1. 0 | 1. 0 | 1. Pelvic | 1. 0 |
|
| Metastases |
|
|
|
1. 0 | 1. Lung, bone, liver | 1. Lung | 1. Liver |
|
A, rounded or lobulated and well-circumscribed tumour; B, irregular and ill-defined tumour.
MR characteristics
Due to its high soft tissue contrast resolution, MR imaging is the modality of choice for assessing the primary site of disease. It helps to determine the site of origin of the tumour, its local extent, tissue characteristics, presence of local adenopathy and aids in planning surgical resection.
The MR signal characteristics of prostate sarcomas documented in this study are similar to those seen in other studies[9,10]. Most prostate sarcomas appear as homogeneous low signal masses on the T1-weighted images. In the 1 case of MPNST, the lesion was heterogeneous on the T1-weighted sequences, containing areas of high, intermediate, and low signal in keeping with blood breakdown products (Fig. 3). On the T2-weighted sequences, prostate sarcomas invariably appear as heterogeneous masses with areas of intermediate and high T2 signal. Necrosis and cystic change in these tumours is common, because of their high malignancy and rapid growth[5,9]. Homogeneous T2 low signal was only seen in 1 case of leiomyosarcoma in this study. This is likely to be due to the fact that this was the smallest lesion in the study, measuring only 2.9 cm.
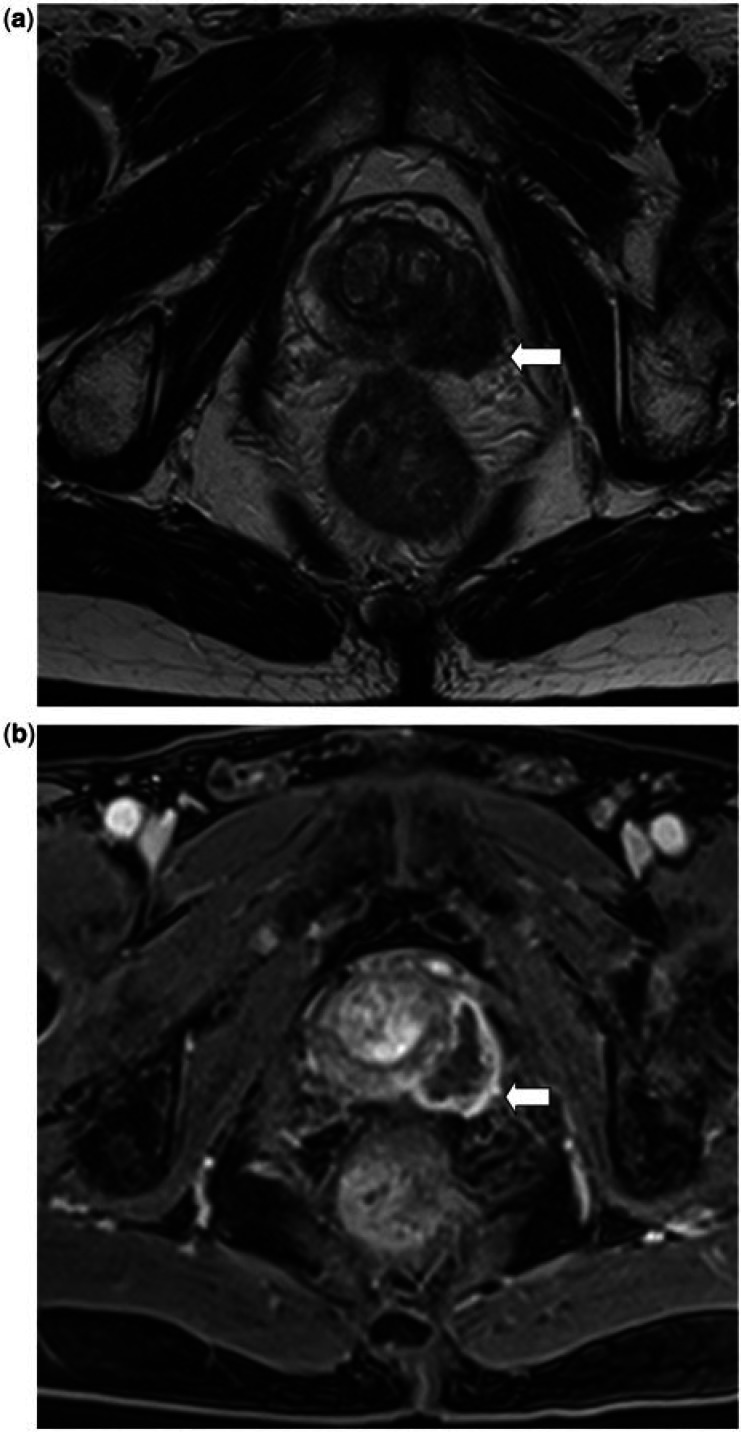
Figure 3.
A 33-year-old man with a MPNST of the prostate. (a) Sagittal T2-weighted MR image shows the tumour as a very heterogeneous mass, with areas of high, intermediate and low T2 signal seen within it, and areas of necrosis/cystic change. The mass is well defined with a T2 low signal intensity pseudocapsule seen along its superior and posterior border (white arrow). (b) Axial T1-weighted MR image shows that the mass is heterogeneous; the tumour is of relatively low T1 signal but contains areas of T1 high signal intensity in keeping with haemorrhage.
Prostate sarcomas that present as well-defined masses may demonstrate a well-defined T2 low signal compressible pseudocapsule, which may be complete or incomplete (Fig. 3). This imaging feature of prostate sarcomas has also been previously described in the literature and is best seen at the border with the pelvic fat on the superior and posterior aspects of the mass[9,11,12]. The pseudocapsule results from the interface formed between tumour that has extended beyond the prostate and the adjacent compressed periprostatic soft tissues.
Contrast-enhanced MR imaging was only performed on 1 patient with a leiomyosarcoma in this study; the tumour showed avid peripheral enhancement (Fig. 4). The lack of central enhancement within this lesion can be explained by the histologic findings, which showed that the central component of the lesion consisted of myxoid and hyalinized material. Other studies showed that prostate sarcomas demonstrate heterogeneous enhancement, reflecting the heterogeneous pathologic appearance of these lesions, which may be solid or of mixed pattern, with areas of cystic change and intratumoral necrosis[9,13].
Figure 4.
A 63-year-old man with a prostatic leiomyosarcoma. (a) Axial T2-weighted MR image shows a slightly ill-defined lesion arising from the peripheral zone of the prostate, which is predominantly of homogeneous low T2 signal intensity and shows extension into the transition zone and extracapsular extension. (b) Axial T1-weighted fat-suppressed postcontrast MR image demonstrates avid peripheral enhancement of the tumour with central necrosis.
To our knowledge the diffusion-weighted (DW)-MR imaging appearance of prostate sarcomas has not been previously described. The DW-MR appearances of these lesions may vary, reflecting their varied pathologic makeup (Fig. 5). In 3 of the 4 cases which underwent DW-MR imaging, the tumours demonstrated impeded diffusion, and the signal intensity of the lesions at a b value of 1000 sc/mm2 was high, with a low apparent diffusion coefficient (ADC) (mean 0.70 × 10–3 mm2/s), in keeping with their cellular composition. In one case of leiomyosarcoma, however, the tumour displayed a low ADC value (0.51 × 10–3 mm2/s), but the b1000 signal intensity of the lesion was also low. Histologic analysis of this lesion demonstrated that it predominantly consisted of a myxoid and hyalinized matrix. The low b1000 signal intensity was probably a consequence of the T2 blackout effect as this lesion was also of very low signal intensity on the T2-weighted images[14].
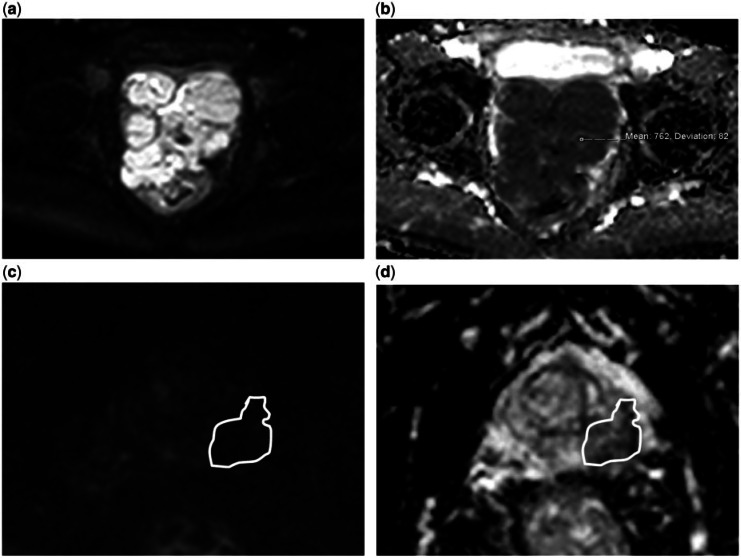
Figure 5.
A 54-year-old man (a, b) and a 62-year-old man (c, d), both with a prostatic leiomyosarcoma. The images show the different diffusion appearances between the 2 cases. (a) DW image with a b value of 1000 s/mm2 and (b) ADC map shows that the tumour demonstrates impeded diffusion and has a mean ADC value of 0.76 × 10–3 mm2/s. (c) DW image with a b value of 1000 s/mm2 and (d) ADC map shows that this tumour does not demonstrate impeded diffusion and has a mean ADC value of 0.51 × 10–3 mm2/s but is also of low signal on the b1000 image.
MR spectroscopy features of prostate sarcomas have been described in a small series[10]. Prostate sarcomas have a marked increase in the ratio of choline/citrate, helping to differentiate them from benign hyperplasia but not from prostate adenocarcinoma, which demonstrates similar features.
The MR signal characteristics of the prostate sarcomas are summarized in Table 3.
Table 3.
Imaging characteristics for each case of prostate sarcoma
| Histology | Embryonal rhabdomyosarcoma (n = 3) | Alveolar rhabdomyosarcoma (n = 2) | Leiomyosarcoma (n = 4) | Stromal sarcoma (n = 1) | MPNST (n = 1) | Spindle cell sarcoma (n = 1) | Postradiation-induced sarcoma (n = 1) | All cases (n = 13) |
|---|---|---|---|---|---|---|---|---|
| T1 homogeneity | ||||||||
| Homogeneous | 3 | 2 | 4 | 1 | 0 | 1 | 1 | 12 |
| Heterogeneous | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| T1 signal |
|
|
|
1. Low | 1. High, intermediate and low | 1. Low | 1. Low |
|
| T2 homogeneity | ||||||||
| Homogeneous | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Mildly heterogeneous | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 4 |
| Very heterogeneous | 2 | 0 | 3 | 1 | 1 | 1 | 0 | 8 |
| T2 signal |
|
|
|
1. High, Intermediate and Low | 1. High and Intermediate | 1. High and Intermediate | 1. Intermediate and Low |
|
| Necrosis or cystic change | 2 | 1 | 3 | 1 | 1 | 1 | 0 | 9 |
| Haemorrhage | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| CT appearances |
|
|
|
1. A | 1. No CT | 1. No CT | 1. B |
|
A, heterogeneous soft tissue mass with areas of low attenuation in keeping with necrosis/cystic change; B, heterogeneous soft tissue mass; C, homogeneous soft tissue mass.
CT characteristics
The CT characteristics of prostate sarcomas are non-specific, with most lesions appearing as large pelvic masses of heterogeneous attenuation with areas of necrosis/cystic change and heterogeneous enhancement (Fig. 6). Calcification is not a feature, nor is the presence of macroscopic fat. These CT features are also supported by other studies in the literature[9,15]. Due to the poorer soft tissue contrast resolution of CT compared with MR, the precise origin of the tumour is often difficult to determine on CT; it is difficult to differentiate between tumours arising from the prostate and those arising from the bladder base. However, CT is useful for assessing the extent of local adenopathy and is the imaging modality of choice for detection of distant metastases.
Figure 6.
A 54-year-old man with a prostatic leiomyosarcoma. (A) Sagittal T2-weighted MR image shows the tumour as a large, well-defined, rounded mass arising from the superior aspect of the prostate, with heterogeneous T2 signal intensity. (b) Axial CT image shows the lesion as a heterogeneous mass, containing areas of necrosis/cystic change (asterisk).
The CT appearances of prostate sarcomas are summarized in Table 3.
FDG-PET/CT characteristics
One patient with an embryonal rhabdomyosarcoma underwent pretreatment FDG-PET/CT, which demonstrated heterogeneous increased metabolic activity within the tumour, with a maximum standardized uptake value (SUVmax) of 9.5. The findings are in keeping with other studies that have shown that rhabdomyosarcomas are PET positive and that FDG-PET/CT is a useful adjunct in staging rhabdomyosarcomas[16,17]. These studies show that the accuracy of TNM staging and in particular M staging of FDG-PET/CT is higher than that of conventional cross-sectional imaging. Although none of the patients with leiomyosarcoma in this study underwent FDG-PET/CT imaging, other studies have shown that leiomyosarcomas are also FDG avid and that the SUVmax from FDG-PET is a likely predictor of tumour behaviour[18].
Metastases
The first site of distant spread in prostate sarcomas is often to the regional lymph nodes, seen in 46% of our cases. As expected, spread occurs first to the pelvic nodes, followed by spread to the retroperitoneal nodes and then less commonly to the mediastinal nodes. Studies have shown that nodal involvement is common in prostatic rhabdomyosarcomas[19,20], but is uncommon in prostatic leiomyosarcomas, reportedly occurring in only 10% of cases[21,22]. This is supported by our findings; all patients with rhabdomyosarcoma had nodal involvement and none of our patients with leiomyosarcoma had nodal spread. This is another feature that could be used to help differentiate between these 2 histologic subtypes on imaging.
Distant metastases were common in our study group, occurring in 69% of cases, 89% of which were present at the time of diagnosis. Metastases were seen in 4 of the 5 patients with rhabdomyosarcoma and in 2 of the 4 patients with leiomyosarcoma. The principal sites of distant organ spread in rhabdomyosarcoma were the lungs followed by bones, and in leiomyosarcoma distant spread was commonest to the lungs and liver. The same patterns of metastatic spread have been documented in other studies, however, at lower incidences. These have been recorded in 10–20% of patients with rhabdomyosarcomas, with metastatic disease most likely found in the lungs or bones[9,11]. In a review of 54 published cases, Vandoros et al.[23] showed that 24% of patients with leiomyosarcoma had metastases at diagnosis (lung 18%, liver 12% and bone 6%). Bone metastases are frequently osteolytic, as seen in 3 of the 4 cases with bone metastases in our study and are generalized throughout the skeleton. This feature can help differentiate prostate sarcomas from prostate adenocarcinomas, in which metastases are usually osteoblastic and largely confined to the pelvis and vertebrae[24]. In our 1 case of prostate stromal sarcoma, no metastases were seen. As prostate stromal sarcoma is a rare tumour, there are limited data about its prognosis but a few case reports of low-grade stromal sarcomas showed that these tumours normally do not metastasize, but early local invasion and distant metastases are seen in the high-grade counterpart[25].
The patterns of metastatic spread for the different subtypes of prostate sarcoma are summarized in Table 2.
Differentiating between the subtypes of prostate sarcoma
Although most prostate sarcomas appear as heterogeneous masses, there are certain MR imaging features that may aid in differentiating the histologic subtypes. Embryonal rhabdomyosarcomas may be well defined and protrude into the bladder lumen and tend to consist of a T2 hyperintense stroma containing sheets of intermediate to low T2 signal intensity tissue (Fig. 2).These findings are consistent with their gross pathologic appearances; embryonal rhabdomyosarcomas are typically firm, fleshy, lobulated masses, which may have a deceptively benign appearance and contain sheets of both malignant round and spindle cells in a variably myxoid matrix. As previously mentioned, alveolar rhabdomyosarcomas are less well defined and have a more infiltrative pattern due to their more aggressive nature (Fig. 1). MPNSTs and leiomyosarcomas appear more heterogeneous compared with embryonal rhabdomyosarcomas (Figs. 3 and 6). Pathologic assessment of both these tumours has shown that they have a tendency for necrosis, haemorrhage and cystic degeneration[26]. As previously mentioned, knowledge of the different patterns of nodal and distant organ spread between rhabdomyosarcomas and leiomyosarcomas may aid in the diagnosis. Lymph node involvement is common in rhabdomyosarcomas but is not commonly seen with leiomyosarcomas. Although lung is the primary site for distant spread in both rhabdomyosarcomas and leiomyosarcomas, bone metastases are more common in rhabdomyosarcomas and liver metastases are more common in leiomyosarcomas. Rhabdomyosarcomas are also more common and occur in a younger age group compared with leiomyosarcomas.
Differentiating prostate sarcomas from prostate adenocarcinomas
Differentiating between prostate sarcoma and prostate adenocarcinoma is essential because of the differences in their management and prognoses. Patients with prostate sarcoma should be treated in specialist centres with combined modality treatment involving chemotherapy, radiotherapy and surgery, whereas management of patients with prostate adenocarcinoma varies between active surveillance, hormone therapy, chemotherapy, brachytherapy and surgery depending on the grade and stage of disease and can be carried out in most hospitals. The diagnosis should be made before surgery by biopsy and although rare, there is a small risk of seeding along the biopsy tract with tumour cells when performing biopsies of soft tissue sarcomas; however, this is not a concern when biopsying prostate adenocarcinoma[27]. Once the diagnosis of prostate sarcoma has been made, staging should include CT imaging of the chest and liver to look for metastatic spread to these sites.
Several features may aid in differentiating prostate sarcomas from prostate adenocarcinomas, allowing early detection and thus earlier treatment. As previously mentioned, prostate adenocarcinoma is a disease of the elderly, being rare before the age of 50 years, with most cases identified in patients who are asymptomatic but have an increased level of serum PSA. Although some prostate sarcomas, such as leiomyosarcomas, also occur in the elderly, rhabdomyosarcomas, which is the commonest histologic subtype, predominantly occur in children and young adults. Serum PSA levels are not normally increased and patients present with local symptoms such as urinary dysfunction, pain and haematuria. Prostate sarcomas typically present as solitary large pelvic masses, which on MR appear heterogeneous with intermediate and high signal intensity areas seen on the T2-weighted images homogeneous with low signal intensity on T1-weighted imaging. The masses commonly demonstrate areas of necrosis or cystic change and a pseudocapsule may be present. Prostate adenocarcinomas are commonly multifocal, predominantly arising within the peripheral zone of the prostate as ill-defined areas of homogeneously low T1 and T2 signal intensity. Prostate sarcomas invariably extend beyond the prostate and thus local invasion of adjacent structures is common, whereas most prostate adenocarcinomas are organ confined at the time of diagnosis. Although prostate sarcomas can be ill-defined and infiltrative, the presence of a well-defined mass that extends beyond the prostate and displaces rather than invades adjacent structures is very suggestive of prostate sarcoma. This is not a feature associated with prostate adenocarcinoma, which extends by infiltrating its adjacent tissues. The pattern of metastatic spread may also aid in differentiating prostate sarcoma from adenocarcinoma; lung is the commonest site of distant organ spread in prostate sarcoma whereas bone is the commonest site for prostate adenocarcinoma metastases. Osteoblastic metastases occur in prostate adenocarcinoma, whereas bone metastases in sarcomas tend to be osteolytic. Also, liver metastases are rarely seen with prostate adenocarcinoma but are not uncommon with prostate leiomyosarcoma.
Conclusion
Although the imaging appearances of prostate sarcomas vary, they commonly appear as large heterogeneous masses extending beyond the prostate. There are certain imaging features that may help to differentiate between the different histologic subtypes but, more importantly, there are imaging features that can help differentiate prostate sarcoma from adenocarcinoma, thus helping to avoid delays in diagnosis and allowing the appropriate staging investigations to be requested promptly.
Conflict of interest
The authors have no conflicts of interest to declare.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Eble JN, Sauter G, Epstein JI, Sesterhann IA. World Health Organization classification of tumours. Pathology and genetics of tumours of the urinary system and male genital organs. Lyon: IARC Press; 2004. pp. p. 1–354. [Google Scholar]
- 2.Scmidt JD, Welch MJ. Sarcoma of the prostate. Cancer. 1976;37:1908–1912. doi: 10.1002/1097-0142(197604)37:4<1908::AID-CNCR2820370441>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Tetu B, Srigley JR, Bostwick DG. Soft tissue tumours. In: Bostwick DG, editor. Pathology of the prostate. New York: Churchill Livingstone; 1990. p. p. 117. [Google Scholar]
- 4.Maurer HM. The Intergroup Rhabdomyosarcoma Study - 1. A final report. Cancer. 1988;61:209–220. doi: 10.1002/1097-0142(19880115)61:2<209::AID-CNCR2820610202>3.0.CO;2-L. . PMid:3275486. [DOI] [PubMed] [Google Scholar]
- 5.Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76:1422–1427. doi: 10.1002/1097-0142(19951015)76:8<1422::AID-CNCR2820760819>3.0.CO;2-L. . PMid:8620418. [DOI] [PubMed] [Google Scholar]
- 6.Malogolowkin MH, Ortega JA. Rhabdomyosarcoma of childhood. Pediatr Ann. 1988;17:251. doi: 10.3928/0090-4481-19880401-06. PMid:3290813. [DOI] [PubMed] [Google Scholar]
- 7.Sexton WJ, Lance RE, Reyes AO, Pisters PWT, Tu S-M, Pister LL. Adult prostate sarcoma: the M.D. Anderson cancer centre experience. J Urol. 2001;166:52–525. doi: 10.1016/s0022-5347(05)65974-5. [DOI] [PubMed] [Google Scholar]
- 8.Sohayda C, Kupelian PA, Levin HS, Klein EA. Extent of extracapsular extension in localized prostate cancer. Urology. 2000;55:382–386. doi: 10.1016/S0090-4295(99)00458-6. . PMid:10699615. [DOI] [PubMed] [Google Scholar]
- 9.Agrons GA, Wagner BJ, Lonergan GJ, Dickey GE, Kaufman MS. From the archives of the AFIP. Genitourinary rhabdomyosarcoma in children: radiologic-pathologic correlation. Radiographics. 1997;17:919–937. doi: 10.1148/radiographics.17.4.9225391. PMid:9225391. [DOI] [PubMed] [Google Scholar]
- 10.Ren F, Lu J, Wang J, Ye J, Shao C, Wang M. Adult prostate carcinoma: radiological-clinical correlation. Clin Radiol. 2009;64:171–177. doi: 10.1016/j.crad.2008.07.013. . PMid:19103347. [DOI] [PubMed] [Google Scholar]
- 11.Varghese SL, Grossfeld GD. The prostate gland: malignancies and other adenocarcinomas. Radiol Clin North Am. 2000;38:179–202. doi: 10.1016/S0033-8389(05)70155-X. . PMid:10664672. [DOI] [PubMed] [Google Scholar]
- 12.Bartolozzi C, Selli C, Olmastroni M, Menchi I, Di Candio G. Rhabdomyosarcoma of the prostate: MR findings. AJR. 1988;150:1333–1334. doi: 10.2214/ajr.150.6.1333. . PMid:3259375. [DOI] [PubMed] [Google Scholar]
- 13.Chang J, Lee H, Lee S, et al. Unusual tumours involving the prostate: radiological-pathological findings. Br J Radiol. 2008;81:907–915. doi: 10.1259/bjr/68294775. . PMid:18662962. [DOI] [PubMed] [Google Scholar]
- 14.Thomassin-Naggara I, Daraï E, Cuenod CA, et al. Contribution of diffusion-weighted MR imaging for predicting benignity of complex adnexal masses. Eur Radiol. 2009;19:1544–1552. doi: 10.1007/s00330-009-1299-4. . PMid:19214523. [DOI] [PubMed] [Google Scholar]
- 15.Cohen M. Tumours involving multiple tissues or organs. Imaging of children with cancer. St Louis, MO: Mosby Year Book; 1992. pp. p. 308–337. [Google Scholar]
- 16.Klem ML, Grewal RK, Wexler LH, Schöder H, Meyers PA, Wolden SL. PET for staging in rhabdomyosarcoma: an evaluation of PET as an adjunct to current staging tools. J Pediatr Hematol Oncol. 2007;29:9–14. doi: 10.1097/MPH.0b013e3180307693. . PMid:17230060. [DOI] [PubMed] [Google Scholar]
- 17.Tateishi U, Hosono A, Makimoto A, et al. Comparative study of FDG PET/CT and conventional imaging in the staging of rhabdomyosarcoma. Ann Nucl Med. 2009;23:155–161. doi: 10.1007/s12149-008-0219-z. . PMid:19225939. [DOI] [PubMed] [Google Scholar]
- 18.Punt SE, Eary JF, O’Sullivan J, Conrad EU. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: imaging characteristics. Nucl Med Commun. 2009;30:546–549. doi: 10.1097/MNM.0b013e32832bcaec. . PMid:19440162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence W, Jr, Hays DM. Surgical lesions from the Intergroup Rhabdomyosarcoma Study. Natl Cancer Inst Monogr. 1981;56:159–163. PMid:7029287. [PubMed] [Google Scholar]
- 20.Gaiger AM, Soule EH, Newton WA., Jr Pathology of rhabdomyosarcoma: Experience of the Intergroup Rhabdomyosarcoma Study, 1972–1978. Natl Cancer Inst Monogr. 1981;56:19–27. PMid:7029289. [PubMed] [Google Scholar]
- 21.Lawrence W, Jr, Donegan WL, Natarajan N, Mettlin C, Beart R, Winchester D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg. 1987;205:349. doi: 10.1097/00000658-198704000-00003. . PMid:3566372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torosian MH, Friedrich C, Godbold J, Hajdu SI, Brennan MF. Soft tissue sarcoma: initial characteristics and prognostic factors in patients with and without metastatic disease. Semin Surg Oncol. 1988;4:13. doi: 10.1002/ssu.2980040105. . PMid:3353619. [DOI] [PubMed] [Google Scholar]
- 23.Vandoros GP, Manolidis T, Karamouzis MV, et al. Leiomyosarcoma of the prostate: case report and review of 54 previously published cases. Sarcoma. 2008;458709:1–5. doi: 10.1155/2008/458709. doi:10.1155/2008/458709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waring PM. Prostatic embryonal rhabdomyosarcoma in adults. A clinicopathological review. Cancer. 1992;69:755–762. doi: 10.1002/1097-0142(19920201)69:3<755::AID-CNCR2820690324>3.0.CO;2-Y. . PMid:1730126. [DOI] [PubMed] [Google Scholar]
- 25.Tavora F, Furtado P, Cheng L. Prostatic stromal sarcoma. 2010. Available at: http://emedicine.medscape.com/article/1743611-overview. Accessed 19 August 2012.
- 26.Rosai J, Ackerman LV. Soft tissues. In: Rosai J, editor. Surgical pathology. St Louis, MO: Mosby; 2004. p. p. 2298. [Google Scholar]
- 27.Schwartz HC, Spengler DM. Needle tract recurrences after closed biopsy for sarcoma: three cases and review of the literature. Ann Surg Oncol. 1997;4:228–236. doi: 10.1007/BF02306615. . PMid:9142384. [DOI] [PubMed] [Google Scholar]