Abstract
Gastric carcinoma (GC) is one of the most common causes of cancer-related death worldwide. Surgical resection is the only cure available and is dependent on the GC stage at presentation, which incorporates depth of tumor invasion, extent of lymph node and distant metastases. Accurate preoperative staging is therefore essential for optimal surgical management with consideration of preoperative and/or postoperative chemotherapy. Multidetector computed tomography (MDCT) with its ability to assess tumor depth, nodal disease and metastases is the preferred technique for staging GC. Endoscopic ultrasonography is more accurate for assessing the depth of wall invasion in early cancer, but is limited in the assessment of advanced local or stenotic cancer and detection of distant metastases. Magnetic resonance imaging (MRI), although useful for staging, is not proven to be effective. Positron emission tomography (PET) is most useful for detecting and characterizing distant metastases. Both MDCT and PET are useful for assessment of treatment response following preoperative chemotherapy and for detection of recurrence after surgical resection. This review article discusses the usefulness of imaging modalities for detecting, staging and assessing treatment response for GC and the potential role of newer applications including CT volumetry, virtual gastroscopy and perfusion CT in the management of GC.
Keywords: Gastric carcinoma, computed tomography, endoscopic ultrasonography, magnetic resonance imaging, positron emission tomography
Introduction
Gastric carcinoma (GC) is the fourth most common cancer worldwide and the second most common cause of cancer-related death[1]. Surgical excision of the GC remains the only cure available and is dependent on the stage of the disease at presentation. The extent of stomach wall invasion by the tumor, spread to the lymph nodes and the presence of distal organ metastases determines the stage of the tumor. Detection of GC in the early stages makes survival highly favorable. However, due to the non-specific symptoms of GC, patients often present at inoperable stages with locally advanced or metastatic disease.
Accurate preoperative staging of GC is essential for planning the optimal treatment method, which includes minimally invasive procedures such as endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), laparoscopic partial gastrectomy, and more radical complete gastrectomy and lymph node dissection. At more advanced stages, adjuvant and neoadjuvant chemotherapy alone or in combination with radiotherapy have been shown to improve survival[2]. Multidetector computed tomography (MDCT) is the most commonly used technique for the staging of GC as it provides higher resolution scans with thin collimation that allows excellent multiplanar reconstructions. Magnetic resonance imaging (MRI), despite its better soft tissue contrast and direct multiplanar imaging capability, is less preferred than MDCT due to prolonged scanning time and higher cost. Endoscopic ultrasonography (EUS) is regarded as a highly accurate technique for staging depth of invasion by early GC, but similar to CT regarding T2 or T3 lesions, and is limited in the case of large stenotic tumors. EUS can also be used for the detection of perigastric lymph nodes but is not a reliable technique for the detection of distant metastases. Positron emission tomography (PET) has a role for detection of distant metastases. MDCT and PET are both useful for assessment of recurrence after surgical resection and response to chemotherapy and radiation therapy. This review discusses the role of CT, MRI, EUS and PET in the diagnosis and staging of GC and assessment of treatment response and the role of newer techniques including CT volumetry, virtual gastroscopy, and perfusion CT.
Epidemiology
GC is the fourth most common cancer in the world, with an estimated 989,600 new cases reported in 2008, making up to 8% of all cancers and causing 738,000 deaths, accounting for 10% of all cancer-related deaths[2]. The GC rates increase progressively with age, with most patients aged between 50 and 70 years at presentation. The highest incidence rates are in Asia (particularly Korea, China and Japan) and many parts of South America.
In most developed countries, the rates of GC have declined dramatically over the past 50 years and similar decreasing trends have recently been noted in areas with historically high GC rates such as Korea. Decreases in incidence have been attributed in part to reduction in chronic Helicobacter pylori infection[3–5], and increased screening in Japan[6] and Korea where the disease burden is highest. Predisposing conditions that can increase the risk of developing GC include dietary factors, Helicobacter pylori gastritis, atrophic gastritis, pernicious anemia, adenomatous gastric polyps and hereditary factors[2].
Morphology, histologic types and staging
Adenocarcinomas account for 95% of all GCs[7]. Most GCs are polypoid or ulcerated[8,9]. Based on the level of invasion, GCs are divided into early gastric cancer (EGC) and advanced gastric cancer (AGC). EGC or the superficial form is limited to the mucosa and submucosa, regardless of the presence or absence of lymph node metastases and can appear as a small circumscribed, sometimes ulcerated thickening of the gastric wall[10]. AGC involves the muscularis propria or beyond and can be polypoid, ulcerating, ulcerating infiltrating and diffusely infiltrating (linitis plastica). Histologically, GC is usually classified into intestinal or diffuse histologic forms[11]. The intestinal type is presumed to arise from intestinalized gastric mucosa and they are usually nodular, polypoid, or fungating. The diffuse type is grossly ill defined and may have the appearance of a plaque or linitis plastica. The occurrence of GCs in the stomach is relatively evenly distributed with 30% occurring in the antrum, 30% in the body and 40% in the fundus and cardia[12–15].
GC is an aggressive carcinoma with 5-year survival rates ranging from 3% in the case of stage IV tumors to 85–90% in the case of stage I tumors[16–18]. The most commonly used staging system for GC was developed by the American Joint Committee on Cancer (AJCC)[18]. The tumors arising at the esophagogastric junction and in the proximal 5 cm of the stomach are classified as esophageal carcinomas. The T stages of GC are defined as T1a (tumor invades the lamina propria or muscularis mucosae), T1b (tumor invades the submucosa), T2 tumor invades the muscularis propria), T3 (tumor penetrates subserosal connective tissue), T4a (tumor invades the serosa), and T4b (tumor invades adjacent structures). The N stage categories are N1 (1–2 positive lymph nodes), N2 (3–6 positive lymph nodes), and N3 (7 or more positive lymph nodes). Positive peritoneal cytology is classified as metastatic (M1) stage. The Japanese Gastric Cancer Society classification further subdivides T1-stage GC into three grades: M, limited to the mucosa; SM1, minimal invasion into the submucosal layer; and SM2, massive invasion into the submucosal layer[19]. Morphologically, the EGC are further classified into type I (polypoid lesions protruding more than 5 mm within the gastric lumen), type IIa (elevated lesion protruding less than 5 mm), type IIb (flat lesion), type IIc (depression lesion that is concave and does not reach the muscularis mucosa), and type III (excavated lesion that is ulcerated and reaches beyond the muscularis mucosa but not the muscularis propria). The AGC are classified into type I (polypoid tumors, sharply demarcated from the surrounding mucosa, usually attached on a wide base), type II (ulcerated carcinomas with sharply demarcated and raised margins), type III (ulcerated carcinomas without definite limits, infiltrating the surrounding wall, type IV (diffusely infiltrating carcinomas in which ulceration is usually not a marked feature), and type V (carcinomas that cannot be classified into any of the above types)[19,20].
The various staging schemes are used to provide a guide for management and prognosis, which is discussed in subsequent sections. The most useful issue is differentiation between early and advanced GC, which allows for potential endoscopic resection versus more invasive surgical resection and/or neoadjuvant chemotherapy, respectively.
Detection of GC
Upper gastrointestinal endoscopy is the preferred technique for detection of GC which is also useful in obtaining histological confirmation of suspicious gastric lesions. However, up to 6.7% of GCs may be missed when an endoscopy shows no initial cancer findings[21]. Imaging techniques are useful for staging the already detected GC but can also occasionally detect unsuspected cancers. Routine clinical ultrasonography can detect liver metastases; however, its use for detection and overall staging of GC is limited as the gastric wall cannot be evaluated adequately except in dedicated research studies. Several studies have reported accuracy up to 90% or more with MDCT for detection of GC with the use of multiplanar reconstruction (MPR) and virtual gastroscopy (VG) techniques[22–27]. However, MDCT detection of EGC is moderate with accuracies ranging from 44% to 70%[23,25]. MRI and PET are not routinely used for detection of GC.
Staging of GC
EUS
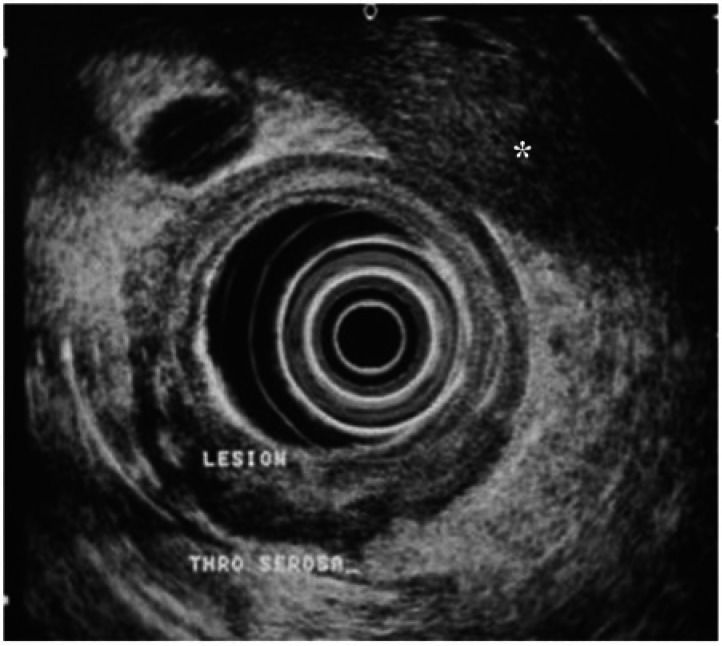
EUS is a combined technique of high-frequency ultrasound (5–12 Hz) and endoscopy that allows evaluation of the digestive tract wall and immediate adjacent structures. Although high-frequency ultrasound gives excellent resolution and delineation of the gastric wall, the depth of penetration is limited. The technique requires good apposition of the probe to the gastric wall and this is achieved by filling the stomach with deaerated water or by placing a balloon filled with deaerated water on the tip of the endoscope. EUS demonstrates normal gastric wall as a five-layered structure[28] and up to 9 distinct layers when using high-resolution probes[29]. The layers do not always correspond to the histologic layers; additional layers are caused by echoes produced by the interfaces between the histologic layers. GCs are identified on EUS (Fig. 1) as areas of focal thickening, irregularity or disruption of the layers[30].
Figure 1.
EUS of an ulcerative lesion in the antrum. The tumor (lesion) is seen to penetrate through the serosa with a hypoechoic lymph node (*) also apparent. The lesion was confirmed as a T4, N1 stage GC on histopathology after total gastrectomy (image courtesy of Professor Lawrence Ho, NUS, Singapore).
EUS has been in use since the 1980s and is reported to have very high T staging accuracy. It is regarded as the modality of choice for local staging. The accuracy of EUS for overall T staging varies between 65% and 92.1%. The sensitivity and specificity for assessing serosal involvement varies between 77.8% and 100% and between 67.9% and 100%, respectively[31]. The pooled sensitivities for T stages are reported to be between 88.1% for T1 cancers, 82.73% for T2, 89.7% for T3, and 99.2% for T4 cancers[32]. Another recent meta-analysis has shown that EUS can distinguish T1–2 tumors from T2–4 tumors with a sensitivity of 0.86 (95% confidence interval [CI] 0.81–0.90), and specificity of 0.91 (0.89–0.93)[33]. However, EUS is dependent on several factors such as operator experience and the methodology used. Furthermore, the presence of ulceration, large size, location of the tumor and diffuse type histology are important factors that influence the staging accuracy of EUS[34–36]. EUS cannot be performed adequately when the full extent of tumor cannot be assessed due to high-grade strictures[37,38]. The possibility of over staging and under staging of tumors can occur due to discrepancy resulting from additional echoes produced by the interfaces between the different histologic layers[30,39,40]. EUS is an invasive technique often requiring sedation and has procedure- and sedation-related complications. Furthermore, EUS has limited depth of penetration and therefore limited usefulness in the overall assessment of distant metastases.
The overall accuracy of EUS in N staging may be as low as 30%[41] but generally ranges from 66% to 90%[42,43]. A simple size criterion for detection of involved lymph nodes is not sensitive. EUS shows low sensitivity for detection of N2–3 stages, which is technically difficult to observe[43]. With the use of EUS-guided fine-needle aspiration (EUS-FNA) for nodal staging, the accuracy has improved and the sensitivity, specificity and positive predictive value of EUS-FNA are reported to be 92%, 98% and 97%, respectively[44]. EUS is not designed to detect distant metastases and therefore CT, MRI, and PET are used.
Despite the limitations, endoscopy and EUS continue to play a major role in the management of adenocarcinomas. Several enhancement techniques have been developed to improve the detection of abnormal mucosal lesions during endoscopy and include chromoendoscopy, narrow band imaging, and confocal laser endomicroscopy. Recently developed treatment techniques such as EMR and ESD are suitable alternatives to surgery in the management of EGC.
In addition, EMR and ESD are established alternatives to surgery in the management of EGC, defined as tumors limited to the mucosa (T1a) or submucosa (T1b). Current indications for EMR are based on the following Japanese Gastric Cancer Society criteria (please see earlier section on morphology, histologic types and staging): well-differentiated macroscopic elevated mucosal lesions <2 cm in size, or depressed type <1 cm without ulceration. Extended criteria have been proposed for ESD: Well-differentiated mucosal lesions of any size without ulceration, mucosal lesions <3 cm if ulcerated, and differentiated lesions with minute (<500 μm) submucosal invasion (SM1) and no evidence of lymphovascular invasion on EUS or MDCT[45,46].
MDCT
MDCT is considered the best modality for the staging of GC with its ability for non-invasive assessment of local extension of tumor, nodal disease and metastases. Rapid acquisition times allows for multiphasic imaging with a single injection of intravenous contrast medium and high-resolution three-dimensional image reconstructions. Previously, studies performed with single-row detector CT were prone to motion artifacts due to the long breath holds required and generally had low resolution. With the advent of MDCT and improved resolution and the possibility of MPRs, the accuracy of evaluation of invasion of the gastric wall has significantly improved from 69–84% for single detector CT[47] to 80–89%[48,49] with MDCT.
MDCT technique
Patients are scanned after fasting for 4–6 h. Gastric distension is required to differentiate gastric tumors from the collapsed normal gastric mucosa (Fig. 2). Good distension can be achieved with endoluminal contrast agents, which help to distinguish the gastric lumen and walls from adjacent structures and allow accurate evaluation of gastric wall thickness. Negative endoluminal contrast agents such as water or gas produced by effervescent granules are preferred. Positive contrast agents can obscure small enhancing tumors or may produce pseudolesions due to inadequate mixing and are therefore best avoided. About 500–750 ml of water is ingested by the patient 10–15 min before the scan and an additional 250 ml is administered just before the scan. Gas distension of the stomach can be performed with effervescent granules, which produce carbon dioxide and distend the stomach. We routinely use gas distension of the stomach with 1–2 sachets of effervescent granules (EZ Gas II Effervescent Granule Packets, EZ EM, New York) administered with a small amount (5–10 ml) of water. It is well tolerated by the patients and easier to administer, even in those with nausea. Distension can be further enhanced by inducing gastric wall hypotonia with either scopolamine-N-butyl bromide (Buscopan, Boehringer International, Ingelheim, Germany) or glucagon administered intravenously or as an intramuscular injection. The scans are usually obtained in the portal venous phase performed at 60–70 s from the start of intravenous injection of 100–150 ml of iodinated contrast at 3–4 ml/s. Additional arterial phase scans may also be obtained at 30–35 s from the start of intravenous injection, which may be useful to differentiate enhancing tumors from normal gastric mucosa. Scans are usually obtained with the patient in a supine position. The scan parameters usually used are 120 kVp, 250 mAs; collimation 64 × 0.625 mm, 3-mm helical thickness, and 1-mm reconstruction thickness. Additional scans may be obtained when necessary in lateral decubitus or oblique positions to adequately distend different stomach segments depending on the location of the tumor and the use of water or gas as the distension agent[26,50]. A recent study has shown that T staging of GC is not significantly affected by the use of either gas or water as the distending agent[51].
Figure 2.
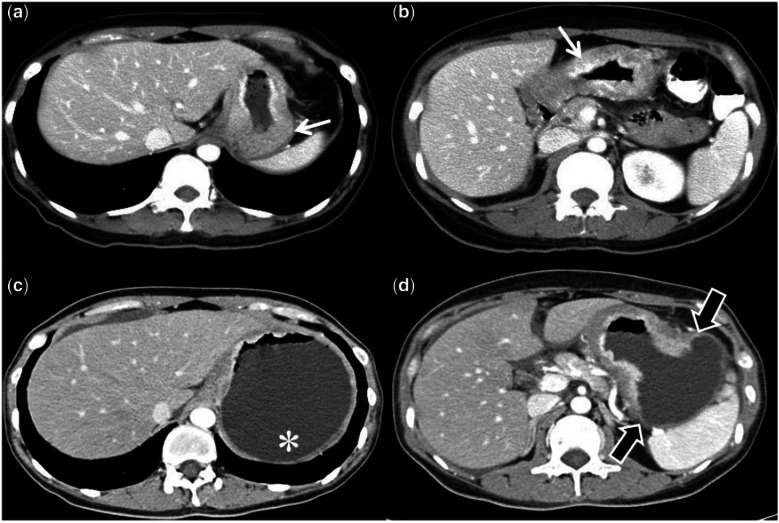
Example illustrating the importance of distension of the stomach for determining the extent of GC. A 58-year-old man with abdominal pain underwent routine CT (a, b) that showed some thickening (white arrow) of the stomach. An endoscopic biopsy confirmed an adenocarcinoma. Staging CT (c, d) shows the actual extent of the tumor (block black arrows) and the gastric fundus (*) is free of the tumor, which was not apparent in the initial CT due to collapsed normal gastric mucosa in an undistended stomach.
MDCT staging of GC
Normal gastric walls may show multilayered patterns with single, two or three layers on dynamic CT independently of hypotonia[52,53]. The innermost enhancing layer corresponds histologically to the gastric mucosa. The intermediate hypoattenuating layer of 2–3 mm represents the submucosa and the outer slightly hyperattenuating layer of variable thickness corresponds to the muscular propria and the serosa layer. However, this multilayered pattern may not be visualized in all cases and not in all parts of the stomach. The gastric wall may be seen as a single layer especially when hypotonia is induced. The characteristic CT finding in GC is disruption of the multilayered pattern of the gastric wall. GCs can cause variable thickening of the gastric wall and associated enhancement, which can also be variable. T1 tumors can show non-transmural marked enhancement with focal wall thickening or marked enhancement only without wall thickening in a single-layer pattern, or thickening and marked enhancement without abrupt obliteration of the middle and outer layers in a multilayered pattern. T2 lesions can show transmural enhancement with focal wall thickening in a single-layer pattern, or both abnormal enhancement and abrupt obliteration of the middle layer in a three-layered pattern, or of the outer layer in a two-layered pattern, and with a smooth outer border of the thickened gastric wall. Based on the multilayered gastric wall appearance, several authors have proposed a CT T staging of GC[23,27,42,49,51–55]. Table 1 shows the pathologic T stages and MDCT T staging criteria modified to the latest TNM staging[18,51]. Focal thickening greater than 5 mm in a well-distended stomach is considered to indicate a neoplastic lesion[49,54].
Table 1.
Pathologic T stages and MDCT criteria for T stages of GC (compiled from Moschetta et al.[26] and Kim et al.[49])
| Pathologic T stage | MDCT criteria |
|---|---|
| pT1: tumor invades the lamina propria, muscularis mucosae or submucosa | T1: strong enhancement with focal thickening in the inner and/or middle layer, but the outer layer shows no enhancement; enhancement of the stomach wall only but the wall is not thickened; wall thickening with intense enhancement of the inner layer and the presence of a hypodense stripe/layer |
| pT2: tumor invasion into the muscularis propria | T2–3: the entire stomach wall thickness is thickened to variable extent but there is a regular surface of outer layer of gastric wall; normal appearance of perigastric fat |
| pT3: tumor invades the subserosa | |
| pT4a: tumor perforates the serosa | T4a: the entire stomach wall is thickened with homogeneous or inhomogeneous enhancement; irregular surface of the outer layer of the gastric wall; presence of micronodules or dense stranding in the perigastric fat |
| pT4b: tumor invades adjacent structures | There is extension of the tumor into adjacent contiguous organs in addition to wall thickening |
The diagnostic accuracy of MDCT for overall T staging varies from 77% to 89%. The use of MPRs improves the accuracy of T staging as they can demonstrate tumor and perigastric fat in profile allowing better assessment of the extent of tumor invasion (Fig. 3)[23,49]. In one study, the overall accuracy of T stage prediction in 106 cases increased from 77% for axial images alone to 84% with MPR[49]. In an another study, Chen et al.[23] combined VG and MPRs, which increased the overall accuracy of T staging to 89% from 73% for axial images alone. A newer CT reconstruction method called vessel probe in MPR mode has been shown to improve the diagnostic accuracy of T staging of GC[26]. Vessel probe, initially developed for examination of small vessels can display images in orthogonal multiplanar, oblique and curved reconstructions as well as three-dimensional and curved reformat views. The vessel probe algorithm permits a more accurate view of gastric wall stratification compared with MPRs as it reduces partial volume artifacts. Another technique called transient transparent projection (TTP) was initially developed as a replacement for double-contrast barium enema images[16] and provides a topographic view of gastric lesions (Fig. 4) that may be complimentary for diagnosis.
Figure 3.
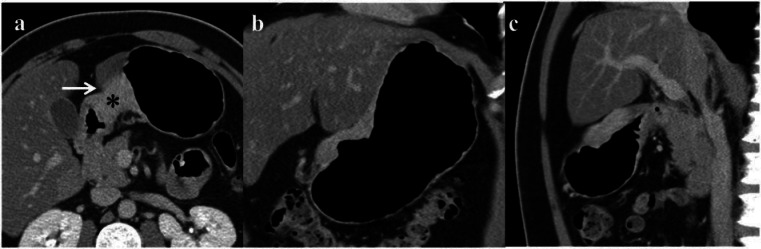
Use of multiplanar reformation for determination of invasion into adjacent organs. In this example, the GC (*) is not separable from the left lobe of the liver (arrow) on the axial image (a). On the coronal (b) and sagittal (c) reconstructions, it is clear that there is no direct invasion by the tumor but apposition of the stomach wall to the left lobe of the liver. The tumor was limited to the stomach wall at histopathology.
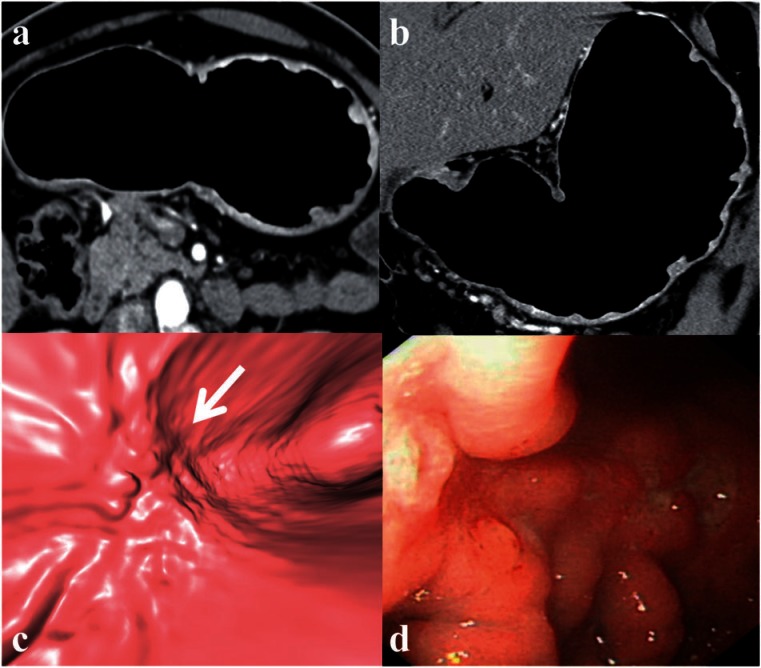
Figure 4.
Large pyloric GC. TTP (a) simulating a double-contrast barium enema image. Coronal reformat image (b) showing the view position for VG (c) which demonstrates the tumor similar to the endoscopic view (d).
The overall accuracy, sensitivity and specificity of N staging with MDCT are 79% (69–92%), 84.6% (78–92%) and 73.9% (62–85.7%) respectively[23,24,49,55–58]. The variable accuracies are due to changes in the nodal staging systems over the years and non-uniform evaluation of resected lymph nodes. Nodal staging using additional MPRs did not show significant improvement in a study by Kim et al.[49] with accuracies of 62% and 64% for axial images and MPRs, respectively. In another study by Chen et al.[23], the N stage did not significantly improve using MPR images and VG with an accuracy of only 78%. The MPRs are useful in T staging of advanced GCs[22,59] but have inherent limitations for nodal staging because of the high frequency of microscopic invasion of normal-sized lymph nodes and the poor differentiation between reactive and metastatic nodal enlargement. There is increasing evidence that examination of an insufficient number of lymph nodes may have a detrimental effect on the overall survival of patients with GC who receive curative treatment[60].
An initial study on MDCT staging using transaxial images only reported an accuracy of 72% for M stage assessment[61]. In another study, the detection of metastases did not improve with MPR compared with axial images only with an accuracy of 86% for both[49].
Serosal invasion and peritoneal metastases are of prognostic importance. The sensitivity and specificity for assessing serosal involvement with MDCT varies from 77.8% to 100% and 80% to 97%, respectively[31]. MDCT has also shown promise in the preoperative diagnosis of peritoneal carcinomatosis and can assess for the presence of ascites (Fig. 5), peritoneal nodules, mesenteric thickening and fat stranding, which are suspicious for peritoneal spread of disease. Factors predictive of peritoneal metastases include greater tumor size, more advanced T stage, and the presence of ascites[62–64].
Figure 5.
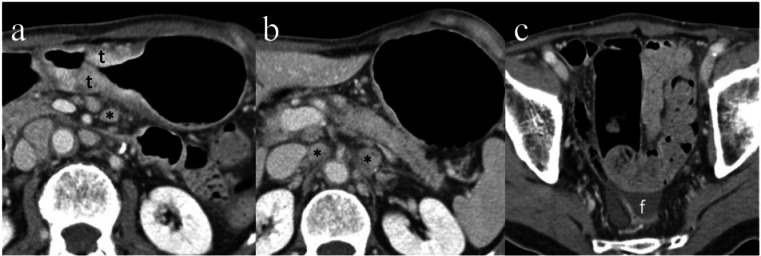
Selected axial images (a–c) from a CT study using a GC protocol. A large circumferential mass at the incisura (t) with enlarged perigastric and para-aortic lymph nodes (*) and free fluid noted in the pelvis. Peritoneal metastatic disease and metastatic para-aortic lymphadenopathy were confirmed during laparotomy and on histopathology.
MDCT has a low sensitivity of 43% for detection of small (<5 mm) peritoneal nodules compared with 89% for detection of nodules >5 mm. The overall sensitivity and specificity of MDCT for the detection of peritoneal nodules were 75% and 92%, respectively. Presence of ascites at staging CT has a sensitivity and specificity of 40% and 97% for the presence of tumor cells on cytology and 51% and 97% for the presence of peritoneal metastases, respectively[64]. Therefore the presence of free fluid in the abdomen or pelvis should alert for the possible presence of peritoneal carcinomatosis.
Despite the reported high accuracy of MDCT for the detection of serosal invasion and peritoneal metastases, these results should be regarded with caution as these are well-controlled clinical studies and may not be applicable to all populations. In view of the prognostic importance of serosal invasion, many centers still practice preoperative laparoscopy to assess for serosal invasion and peritoneal metastases, even in the absence of ascites before laparotomy for gastric resection.
VG
VG is a three-dimensional endoluminal perspective image that simulates endoluminal views at endoscopy (Figs. 4 and 6). VG images can be obtained when CT is performed with air as the distending agent and VG is reconstructed using commercially available workstations similar to virtual colonography. VG has been shown to improve the detection of GC[49,58] and enable non-invasive endoluminal evaluation of the extent and morphology of GC. VG is particularly useful in the detection of EGC (Fig. 5)[65]. Several studies support the use of VG for the detection and characterization of early gastric tumors. In a study by Kim et al.[66] VG had significantly better performance than axial images for the detection of EGC with sensitivity and specificity of 91.9% and 74% and 62.9% and 82.9%, respectively. VG also had better accuracy (0.89, P < 0.038) for detection of EGC compared with accuracies of 0.78 and 0.81 for axial and MPR images, respectively. Although VG may be useful, it requires an additional 10–20 min for processing of images, technical expertise and an experienced radiologist for interpretation.
Figure 6.
Use of VG for early detection of GC. Transverse axial (a) and coronal reformat (b) images do not demonstrate any focal thickening apart from thick mucosal folds. The VG image (c) corresponds well with the polypoidal ulceration with fold truncation seen along the lesser curve (arrow) on endoscopy (d). This was confirmed as a T1b tumor on histopathology after a partial gastrectomy.
Staging of GC: EUS versus MDCT
Until recently, EUS was the most accurate and the preferred technique for local staging of GC and CT was preferred for the detection of metastases. However, the role of MDCT in GC is increasing as the technology improves and various reconstruction methods to improve staging are being developed[67]. Several studies comparing the two techniques have shown that the accuracy of CT is increasing and approaching that of EUS for T staging[24,50,55,68]. One study showed that EUS and 4-slice MDCT had comparable accuracies of 87.5% and 83.3%, respectively[58]. The inter-observer agreement for MDCT has been reported to be almost perfect[55]. Some authors even suggest that MDCT might replace EUS for preoperative staging[42]. Ahn et al.[69] evaluated the accuracy of MDCT with MPRs compared with EUS for the preoperative staging of 434 patients with GC. The accuracies for T staging and negative predictive values for nodal disease for MDCT/EUS were 92.2%/94.1%, and 90.1%/92.6%, respectively. In another study, Hwang et al.[43] compared EUS and MDCT with MPRs for preoperative T and N staging in 277 patients with GC. The overall accuracies for T and N staging with EUS (75% and 66%) and MDCT (77% and 63%) were not significantly different. Furukawa et al.[70] compared MDCT with VG and EUS for the detection of GC and accuracy of T stage prediction in 176 patients. The prediction of T stage was similar for MDCT with VG and EUS with accuracies of 82.2% and 83.7%, respectively (P = 0.850). Both EUS and MDCT have similar accuracy for assessing serosal involvement[31]. MDCT is a better technique for distant metastases and peritoneal metastases, but EUS has also been shown to be useful for detection of ascites (Fig. 7) especially when no free fluid is visible on CT. In one study, the sensitivity and specificity of EUS for the detection of ascites were 60.7% and 99.4%, respectively, and this was significantly related to the presence of peritoneal seeding (P < 0.001)[71]. EUS is valuable for differentiating T1a and T1b disease allowing for selection of endoscopic mucosal and submucosal resection, respectively. MDCT is not as accurate for EGC but is equal to if not superior to EUS in differentiating T2, T3 or T4 tumors. EUS has low sensitivity for nodal staging and additional EUS biopsy is invasive and impractical for routine use. MDCT is commonly used for nodal staging and, if lymphadenopathy is suspected, patients can undergo neoadjuvant chemotherapy before evaluating for possible resection[43].
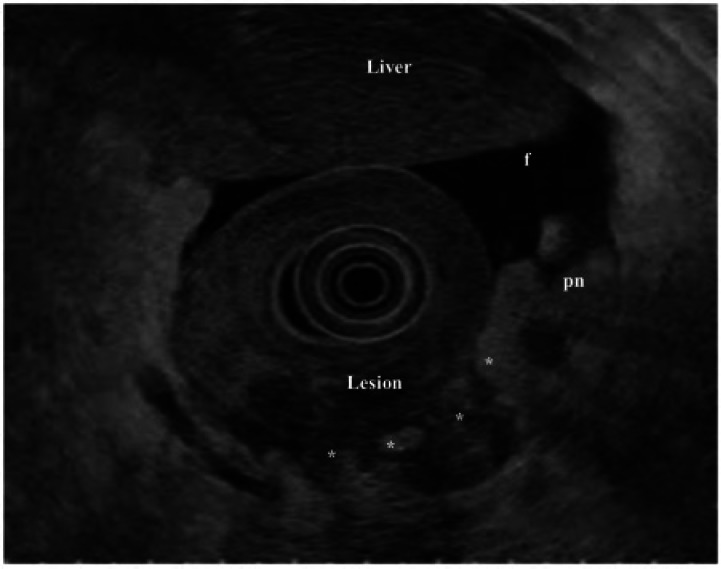
Figure 7.
EUS for peritoneal invasion. Thickening in the antrum of the stomach (lesion), which is seen to penetrate the serosa at multiple points (*). In addition, there is free fluid (f) and peritoneal nodules (pn) consistent with peritoneal carcinomatosis confirmed at diagnostic laparoscopy (image courtesy of Professor Lawrence Ho, NUS, Singapore).
MRI
MRI provides better soft tissue contrast than CT, but due to its long acquisition time and susceptibility to motion artifacts, there has been limited use of MRI for gastrointestinal tract imaging with the exception of the small bowel. Recent advances in MRI including shorter breath-hold sequences and the use of intravenous contrast agents have made it feasible for imaging abdominal organs[72]. MRI is promising for T staging of GC as individual layers may be better differentiated compared with CT. MRI is performed with gastric distension using water or effervescent granules. Two recent studies have shown favorable results for gastric tumor staging with contrast-enhanced multisequence MRI compared with the latest 64-row MDCT scanners using MPRs. Anzidei et al.[73] studied 40 patients with MRI and CT with gastric distension protocols. MDCT and MRI had accuracies of 37.5%/50% for T1 stage and 81.2%/88.7% for T2 stage, respectively, with no significant difference in accuracy for evaluation of T3/T4 tumors. This study showed that MRI may be superior for identifying early stages of gastric cancer. Another study by Maccioni et al.[74] compared MRI and MDCT for T and N staging of GC in 25 patients. The detection rates of gastric tumors were similar for MRI and MDCT (92%), although reviewers in the study were allowed to compare directly with endoscopic images. For MRI and MDCT, accuracies of 60%/48% for T stage and 68%/72% for N stage were noted, respectively. Although these studies show that MRI was comparable with MDCT for T and N staging in GC or possibly superior to MDCT for T staging (Figs. 8 and 9), the small number of patients studied does not provide strong evidence for the use of MRI over CT and EUS. Currently, the use of MRI for staging of GC is limited to special circumstances when patients are allergic to iodinated contrast media, there is concern about radiation exposure with CT or invasiveness of EUS, or as a problem-solving tool when both CT and EUS are inconclusive.
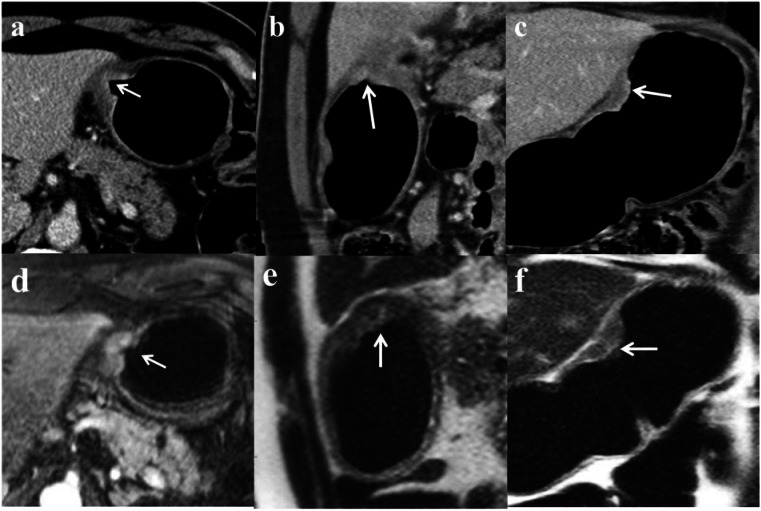
Figure 8.
Multiplanar images from a CT (a, axial; b, sagittal; and c, coronal) and MRI (d, axial T1-weighted+contrast; e, sagittal; and f, coronal T1-weighted images) of a GC. There is an ulcerated mass along the lesser curve with superficial enhancement involving <50% of the gastric wall. No surrounding fat stranding or lymph nodes were detected. The involved gastric wall is in close approximation to the left lobe of the liver with a fat plane best demonstrated on the sagittal T1-weighted MRI sequence (e). A T2N0 stage gastric tumor with no evidence of peritoneal disease or liver invasion was confirmed after a subtotal gastrectomy.
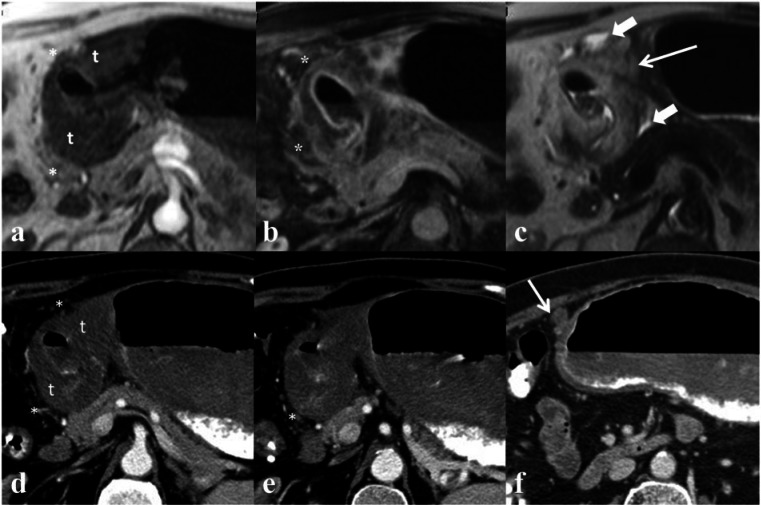
Figure 9.
Sequential axial images from an MRI (a, T1-weighted arterial phase; b, T1-weighted portal venous phase; c, T2-weighted) and CT (d–f) gastric protocol. There is a large circumferential mass in the antrum (t) with heterogeneous enhancement and surrounding fat stranding (*). Adjacent lymphadenopathy (thin arrow) and a small amount of peritoneal fluid (arrowheads) detected on the T2-weighted MRI sequence (c) suggesting peritoneal disease. Metastatic peritoneal disease was confirmed on diagnostic laparotomy and histopathology.
PET
PET with 2-[18F]fluoro-2-deoxy-d-glucose (FDG) combined with CT has been recognized as a useful diagnostic technique in clinical oncology and several studies have assessed the accuracy for nodal and metastatic staging in GC[75]. PET is of limited value in EGC but may be useful in detecting synchronous or multiple primary cancers. PET has low sensitivity for the primary tumor (Fig. 10) and lymph node metastases, therefore is limited in the preoperative work-up and best used as part of a comprehensive work-up. The major advantage of FDG-PET/CT is in the detection of distant metastases to the liver, lungs, and skeleton[76]. In contrast, FDG-PET/CT has limited accuracy in the detection of peritoneal disease[77]. Several studies have described patterns of FDG uptake specific for peritoneal metastases including diffuse uptake spreading uniformly throughout the abdomen and focal peritoneal uptake representing deposits. However, small peritoneal nodules may be missed due to the low resolution of FDG-PET/CT, and MDCT remains the most widely used technique for the detection of peritoneal metastases[78].
Figure 10.
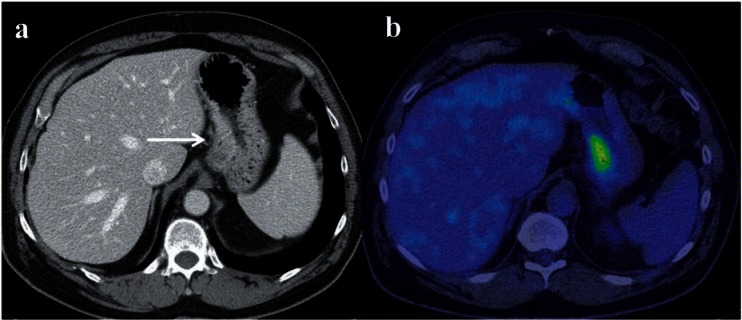
Axial contrast-enhanced CT (a) and FDG-PET/CT (b) in a case of T2 stage GC along the lesser curve (arrow) that shows uptake.
Studies comparing FDG-PET/CT and MDCT have demonstrated that FDG-PET/CT has lower or comparable sensitivity and accuracy for the detection of primary gastric tumors and nodal metastases[79,80].
Few studies have shown that uptake of tracer in the primary tumor and nodes is an independent and significant prognostic factor for predicting cancer recurrence or non-curable operations[56]. A quantitative mean standard uptake value (SUV) of greater than 5 and positive nodal FDG uptake predicted non-curative operations with a sensitivity of 35.2%, specificity of 91.0% and an accuracy of 76.7%[56]. In light of these findings, FDG-PET/CT may provide effective information to physicians to identify patients for whom resection would be non-curative and then consider preoperative treatment such as neoadjuvant chemotherapy.
Posttreatment assessment of GC
GC can be downstaged preoperatively with neoadjuvant chemotherapy to allow for curative surgical resection with improved progression-free and overall survival[2]. Assessment of response is currently performed with MDCT and/or FDG-PET/CT.
On MDCT, the Response Evaluation Criteria in Solid Tumors (RECIST) using the sum of the longest index lesion diameters (uni-dimensional) is widely considered the method of choice in the assessment of tumor response to treatment. However, this measurement is subject to high variability and most studies have focused on solid organ tumors with limited focus on response in GC. For most gastrointestinal tract tumors, the primary tumor has been considered unmeasurable according to RECIST with response assessment focusing instead on uni-dimensional measurement of involved lymph nodes. However, this method is limited by both the high incidence of normal-sized lymph nodes involved in metastatic disease and the presence of enlarged lymph nodes secondary to reactive hyperplasia[81]. An initial study by Ng et al.[82] of 21 patients with non-metastatic gastric cancer showed that CT is not accurate in identifying locoregional disease spread after neoadjuvant chemotherapy with a sensitivity and specificity of 57% and 43%, respectively. Recently, techniques including CT tumor volumetry (TV) (Fig. 11) and FDG-PET/CT have been shown to be useful for response assessment.
Figure 11.
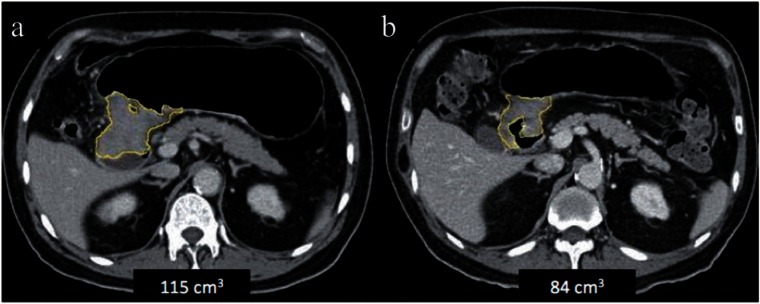
Axial CT images before (a) and after (b) neoadjuvant chemotherapy for pyloric GC. There was a 27% reduction in the tumor volume with no significant change in the gross appearance of the tumor. A partial gastrectomy was performed and complete necrosis was seen on histopathology.
TV has been demonstrated to be particularly useful for assessment of treatment response. A 15% reduction in tumor volume evaluated with MDCT has been shown to correlate with histologic response to neoadjuvant chemotherapy with 100% sensitivity and 53% specificity (P > 0.05)[83]. Wieder et al.[84] compared FDG-PET/CT and size of the tumors on MDCT before and after neoadjuvant chemotherapy. The change in FDG-PET/CT uptake was more than double the decrease in tumor size for those with a histologic response (P < 0.01). In another study, Lee et al.[81] prospectively compared CT TV with FDG-PET/CT in 33 patients with AGC. A cut-off of 35.6% reduction in tumor volume had a sensitivity of 100% and a specificity of 58.8% for response to neoadjuvant chemotherapy, whereas reduction in tumor diameter and SUV rate were not significantly correlated with response.
Larger studies demonstrating the use of TV and FDG-PET/CT are required to establish their clinical usefulness in assessing treatment response.
Recurrent GC
After complete surgical resection of GC, long-term survival is poor with a 35% 5-year survival rate and 80% dying secondary to locoregional recurrence. The optimal method for assessing early GC recurrence is unknown but various methods have been used including serum tumor markers, endoscopy, FDG-PET/CT and CT[85].
CT is the most widely used imaging method due to its availability, although postsurgical changes and altered morphology may limit its specificity. FDG-PET/CT is limited due to relatively poor uptake in most GCs. Endoscopy is invasive but is the gold standard technique for intraluminal recurrence. Tumor markers may allow for selection of more invasive or expensive techniques.
A study by Tan et al.[86] on 102 patients who underwent gastrectomy, compared intensive follow-up involving the use of routine physical examination, serum tumor markers, and the use of contrast-enhanced CT scans more than once per year versus clinical suspicion of relapse. Those with intensive follow-up had earlier detection (11.5 vs. 19.2 months, P = 0.02) but no improvement in survival. Other authors have performed similar studies looking at intensive versus regular follow-up with earlier detection of recurrence apparent but no improvement in survival[87].
Studies comparing FDG-PET/CT and MDCT have been inconclusive in determining the most useful non-invasive technique[80,88]. Bilici et al.[89] demonstrated that sensitivity, specificity, accuracy, positive and negative predictive values of FDG-PET/CT were significantly superior to those of MDCT in the detection of recurrent GC. The FDG-PET/CT results changed the treatment in nearly half of patients with subsequent therapeutic procedures in 50% and the avoidance of invasive procedures in the other 50%. However, the study focused on FDG-PET/CT performed for suspected distant metastases at diagnostic MDCT. FDG-PET/CT and MDCT could therefore be used in combination to increase the accuracy of detection of recurrent tumor.
Future directions
TV
More recently, TV has been assessed as an adjunct for staging of GC and to assess response to neoadjuvant chemotherapy. Kikuchi et al.[90] evaluated the tumor volume of resected GC specimens in 171 cases and correlated this with survival. When compared with other parameters such as the depth of tumor invasion, tumor volume was identified as a significant prognostic factor (P < 0.001; relative risk 10.351). Another study by the same group[91] assessed the significance of TV using EUS in 100 cases. This showed that increasing tumor volume was an independent risk factor for lymph node metastasis (P = 0.0121).
Currently, we have looked at the feasibility of CT TV for the assessment of T, N and M stage in about 150 patients with predominantly AGC (unpublished data). Our preliminary retrospective study has shown that CT TV is feasible for staging of the tumors and had high accuracies for the prediction of T, M and final stages (0.8) and moderate accuracy for N stage (0.7–0.8) of GC. In this study, a volume of 20 mm3 or less could predict T1-stage tumors with >90% accuracy (Fig. 12).
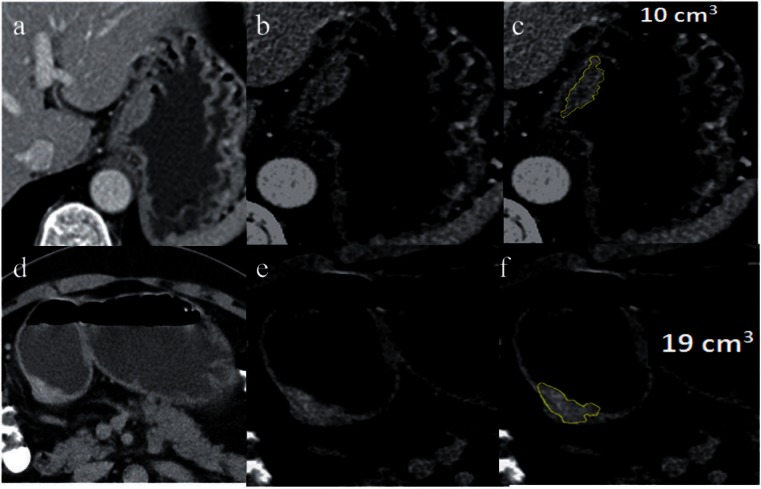
Figure 12.
CT TV for staging. Axial CT sections (a, d), magnified focal view of tumor (b, e) and volumetry overlay (c, f, yellow line showing the tumor outline) of a T2 stage tumor in the gastric cardia (top row, a–c) and a T3 stage tumor in the antrum (bottom row, d–f). These tumors appear to be similar in size on the axial images. A hypodense peripheral layer is seen in the case of the T2 tumor compared with the T3 tumor. The volume of the T2 tumor is 10 cm3 compared with 19 cm3 for the T3 tumor.
TV is a promising tool for providing additional parameters for preoperative staging and for assessment of treatment response. TV requires additional processing time and this may be substantially reduced by developing automated volumetry. Larger studies demonstrating the use of volumetry are required to establish its clinical usefulness.
Perfusion CT
Perfusion CT (P-CT) allows measurement of physiologic parameters associated with tumor perfusion and is an established marker of angiogenesis[92,93]. The main hemodynamic parameter assessed is the tumor blood flow. Preliminary studies with P-CT of GC have shown that blood volume was significantly increased in GC compared with that of normal stomach mucosa[94] and there was no difference between GC with and without lymph node metastases. Another study by Yao et al.[95] showed that a decreased blood flow value may reflect a progressive state of GC. Tumor perfusion decreased as the stage and malignant character of the GC advanced, suggesting that P-CT can assess the malignancy grade of GC non-invasively. P-CT-derived blood volume correlated significantly with microvessel density of the tumor[96], which may be valuable information during preoperative assessment with potential for targeted therapies. Future studies validating the usefulness of P-CT for individualized treatment of GC are eagerly awaited.
Conclusion
Accurate preoperative staging of GC is essential for planning optimal surgical management. MDCT is currently the preferred technique for staging of GC. EUS is the most accurate technique for staging of EGC and assessment of endoscopic treatment options. Accuracy of MDCT for staging and detection of EGC can be improved through the use of MPRs and VG, respectively. MRI is currently not recommended for staging of GC. FDG-PET/CT is most useful for detection of distant metastases and recurrent postoperative GC. Both MDCT and FDG-PET/CT are useful modalities for staging and treatment response assessment. Overall, EUS, MDCT and PET may be best utilized as complementary tools for comprehensive work-up in the management of GC.
Recent advances such as CT TV may provide additional information for preoperative staging of GC and for assessment of treatment response. Perfusion CT may be useful for characterizing the biological behavior of GC to enable targeted therapies.
Conflict of interest
The authors have no conflicts of interest to declare.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. . PMid:21296855. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. . PMid:16822992. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Bu XL, Wang QY, Hu PJ, Chen MH. Decreasing seroprevalence of Helicobacter pylori infection during 1993–2003 in Guangzhou, southern China. Helicobacter. 2007;12:164–169. doi: 10.1111/j.1523-5378.2007.00487.x. . PMid:17309754. [DOI] [PubMed] [Google Scholar]
- 4.Kawakami E, Machado RS, Ogata SK, Langner M. Decrease in prevalence of Helicobacter pylori infection during a 10-year period in Brazilian children. Arq Gastroenterol. 2008;45:147–151. doi: 10.1590/s0004-28032008000200011. PMid:18622470. [DOI] [PubMed] [Google Scholar]
- 5.Tkachenko MA, Zhannat NZ, Erman LV, et al. Dramatic changes in the prevalence of Helicobacter pylori infection during childhood: a 10-year follow-up study in Russia. J Pediatr Gastroenterol Nutr. 2007;45:428–432. doi: 10.1097/MPG.0b013e318064589f. . PMid:18030208. [DOI] [PubMed] [Google Scholar]
- 6.Lee KJ, Inoue M, Otani T, et al. Gastric cancer screening and subsequent risk of gastric cancer: a large-scale population-based cohort study, with a 13-year follow-up in Japan. Int J Cancer. 2006;118:2315–2321. doi: 10.1002/ijc.21664. . PMid:16331632. [DOI] [PubMed] [Google Scholar]
- 7.Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1–27. doi: 10.1093/oxfordjournals.epirev.a036288. PMid:3533579. [DOI] [PubMed] [Google Scholar]
- 8.Olearchyk AS. Gastric carcinoma. A critical review of 243 cases. Am J Gastroenterol. 1978;70:25–45. PMid:358826. [PubMed] [Google Scholar]
- 9.Aird I, Bentall HH, Roberts JA. A relationship between cancer of stomach and the ABO blood groups. Br Med J. 1953;1:799–801. doi: 10.1136/bmj.1.4814.799. . PMid:13032504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angelelli G, Ianora AA, Scardapane A, Pedote P, Memeo M, Rotondo A. Role of computerized tomography in the staging of gastrointestinal neoplasms. Semin Surg Oncol. 2001;20:109–121. doi: 10.1002/ssu.1024. . PMid:11398204. [DOI] [PubMed] [Google Scholar]
- 11.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. PMid:14320675. [DOI] [PubMed] [Google Scholar]
- 12.Cady B, Ramsden DA, Stein A, Haggitt RC. Gastric cancer. Contemporary aspects. Am J Surg. 1977;133:423–429. doi: 10.1016/0002-9610(77)90126-X. . PMid:848677. [DOI] [PubMed] [Google Scholar]
- 13.McBride CM, Boddie AW. Adenocarcinoma of the stomach: are we making any progress? South Med J. 1987;80:283–286. doi: 10.1097/00007611-198703000-00002. . PMid:3824008. [DOI] [PubMed] [Google Scholar]
- 14.Cady B, Rossi RL, Silverman ML, Piccione W, Heck TA. Gastric adenocarcinoma. A disease in transition. Arch Surg. 1989;124:303–308. doi: 10.1001/archsurg.1989.01410030049009. . PMid:2465751. [DOI] [PubMed] [Google Scholar]
- 15.Antonioli DA, Goldman H. Changes in the location and type of gastric adenocarcinoma. Cancer. 1982;50:775–781. doi: 10.1002/1097-0142(19820815)50:4<775::AID-CNCR2820500425>3.0.CO;2-W. . PMid:7093911. [DOI] [PubMed] [Google Scholar]
- 16.Kim JH, Eun HW, Goo DE, Shim CS, Auh YH. Imaging of various gastric lesions with 2D MPR and CT gastrography performed with multidetector CT. Radiographics. 2006;26:1101–1116. doi: 10.1148/rg.264055089. discussion 1117–1118. [DOI] [PubMed] [Google Scholar]
- 17.Ba-Ssalamah A, Prokop M, Uffmann M, Pokieser P, Teleky B, Lechner G. Dedicated multidetector CT of the stomach: spectrum of diseases. Radiographics. 2003;23:625–644. doi: 10.1148/rg.233025127. . PMid:12740465. [DOI] [PubMed] [Google Scholar]
- 18.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. . PMid:20180029. [DOI] [PubMed] [Google Scholar]
- 19.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. 2nd English edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. PMid:11957040. [DOI] [PubMed] [Google Scholar]
- 20.Borrmann R. Geshwulste des magens und duodenums. In: Henke F, Lubarsch O, editors. Handbuch der speziellen pathologischen anatomieund histologis. Berlin: Springer-Verlag; 1926. [Google Scholar]
- 21.Raftopoulos SC, Segarajasingam DS, Burke V, Ee HC, Yusoff IF. A cohort study of missed and new cancers after esophagogastroduodenoscopy. Am J Gastroenterol. 2010;105:1292–1297. doi: 10.1038/ajg.2009.736. . PMid:20068557. [DOI] [PubMed] [Google Scholar]
- 22.Kim YH, Lee KH, Park SH, et al. Staging of T3 and T4 gastric carcinoma with multidetector CT: added value of multiplanar reformations for prediction of adjacent organ invasion. Radiology. 2009;250:767–775. doi: 10.1148/radiol.2502071872. . PMid:19095785. [DOI] [PubMed] [Google Scholar]
- 23.Chen CY, Hsu JS, Wu DC, et al. Gastric cancer: preoperative local staging with 3D multi-detector row CT–correlation with surgical and histopathologic results. Radiology. 2007;242:472–482. doi: 10.1148/radiol.2422051557. . PMid:17255419. [DOI] [PubMed] [Google Scholar]
- 24.Yang DM, Kim HC, Jin W, et al. 64 multidetector-row computed tomography for preoperative evaluation of gastric cancer: histological correlation. J Comput Assist Tomogr. 2007;31:98–103. doi: 10.1097/01.rct.0000234072.16209.ab. . PMid:17259840. [DOI] [PubMed] [Google Scholar]
- 25.Yan C, Zhu ZG, Yan M, et al. Value of multidetector-row computed tomography in the preoperative T and N staging of gastric carcinoma: a large-scale Chinese study. J Surg Oncol. 2009;100:205–214. doi: 10.1002/jso.21316. . PMid:19530124. [DOI] [PubMed] [Google Scholar]
- 26.Moschetta M, Stabile Ianora AA, Anglani A, Marzullo A, Scardapane A, Angelelli G. Preoperative T staging of gastric carcinoma obtained by MDCT vessel probe reconstructions and correlations with histological findings. Eur Radiol. 2010;20:138–145. doi: 10.1007/s00330-009-1482-7. . PMid:19504100. [DOI] [PubMed] [Google Scholar]
- 27.Kim AY, Kim HJ, Ha HK. Gastric cancer by multidetector row CT: preoperative staging. Abdom Imaging. 2005;30:465–472. doi: 10.1007/s00261-004-0273-5. . PMid:15785907. [DOI] [PubMed] [Google Scholar]
- 28.Kuntz C, Herfarth C. Imaging diagnosis for staging of gastric cancer. Semin Surg Oncol. 1999;17:96–102. doi: 10.1002/(SICI)1098-2388(199909)17:2<96::AID-SSU3>3.0.CO;2-4. . PMid:10449680. [DOI] [PubMed] [Google Scholar]
- 29.Bergman JJ, Fockens P. Endoscopic ultrasonography in patients with gastro-esophageal cancer. Eur J Ultrasound. 1999;10:127–138. doi: 10.1016/S0929-8266(99)00055-5. . PMid:10586017. [DOI] [PubMed] [Google Scholar]
- 30.Botet JF, Lightdale CJ, Zauber AG, et al. Preoperative staging of gastric cancer: comparison of endoscopic US and dynamic CT. Radiology. 1991;181:426–432. doi: 10.1148/radiology.181.2.1924784. PMid:1924784. [DOI] [PubMed] [Google Scholar]
- 31.Kwee RM, Kwee TC. Imaging in local staging of gastric cancer: a systematic review. J Clin Oncol. 2007;25:2107–2116. doi: 10.1200/JCO.2006.09.5224. . PMid:17513817. [DOI] [PubMed] [Google Scholar]
- 32.Puli SR, Batapati Krishna Reddy J, Bechtold ML, Antillon MR, Ibdah JA. How good is endoscopic ultrasound for TNM staging of gastric cancers? A meta-analysis and systematic review. World J Gastroenterol. 2008;14:4011–4019. doi: 10.3748/wjg.14.4011. . PMid:18609685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mocellin S, Marchet A, Nitti D. EUS for the staging of gastric cancer: a meta-analysis. Gastrointest Endosc. 2011;73:1122–1134. doi: 10.1016/j.gie.2011.01.030. . PMid:21444080. [DOI] [PubMed] [Google Scholar]
- 34.Kim JH, Song KS, Youn YH, et al. Clinicopathologic factors influence accurate endosonographic assessment for early gastric cancer. Gastrointest Endosc. 2007;66:901–908. doi: 10.1016/j.gie.2007.06.012. . PMid:17963876. [DOI] [PubMed] [Google Scholar]
- 35.Hizawa K, Iwai K, Esaki M, Matsumoto T, Suekane H, Iida M. Is endoscopic ultrasonography indispensable in assessing the appropriateness of endoscopic resection for gastric cancer? Endoscopy. 2002;34:973–978. doi: 10.1055/s-2002-35851. . PMid:12471541. [DOI] [PubMed] [Google Scholar]
- 36.Akashi K, Yanai H, Nishikawa J, et al. Ulcerous change decreases the accuracy of endoscopic ultrasonography diagnosis for the invasive depth of early gastric cancer. Int J Gastrointest Cancer. 2006;37:133–138. doi: 10.1007/s12029-007-9004-9. PMid:18080789. [DOI] [PubMed] [Google Scholar]
- 37.Chen CH, Yang CC, Yeh YH. Preoperative staging of gastric cancer by endoscopic ultrasound: the prognostic usefulness of ascites detected by endoscopic ultrasound. J Clin Gastroenterol. 2002;35:321–327. doi: 10.1097/00004836-200210000-00008. . PMid:12352295. [DOI] [PubMed] [Google Scholar]
- 38.Willis S, Truong S, Gribnitz S, Fass J, Schumpelick V. Endoscopic ultrasonography in the preoperative staging of gastric cancer: accuracy and impact on surgical therapy. Surg Endosc. 2000;14:951–954. doi: 10.1007/s004640010040. . PMid:11080410. [DOI] [PubMed] [Google Scholar]
- 39.Saito N, Takeshita K, Habu H, Endo M. The use of endoscopic ultrasound in determining the depth of cancer invasion in patients with gastric cancer. Surg Endosc. 1991;5:14–19. doi: 10.1007/BF00591380. . PMid:1871669. [DOI] [PubMed] [Google Scholar]
- 40.Ichikawa T, Saito K, Yoshioka N, et al. Detection and characterization of focal liver lesions: a Japanese phase III, multicenter comparison between gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced computed tomography predominantly in patients with hepatocellular carcinoma and chronic liver disease. Invest Radiol. 2010;45:133–141. doi: 10.1097/RLI.0b013e3181caea5b. . PMid:20098330. [DOI] [PubMed] [Google Scholar]
- 41.Polkowski M, Palucki J, Wronska E, Szawlowski A, Nasierowska-Guttmejer A, Butruk E. Endosonography versus helical computed tomography for locoregional staging of gastric cancer. Endoscopy. 2004;36:617–623. doi: 10.1055/s-2004-814522. . PMid:15243885. [DOI] [PubMed] [Google Scholar]
- 42.Habermann CR, Weiss F, Riecken R, et al. Preoperative staging of gastric adenocarcinoma: comparison of helical CT and endoscopic US. Radiology. 2004;230:465–471. doi: 10.1148/radiol.2302020828. . PMid:14752188. [DOI] [PubMed] [Google Scholar]
- 43.Hwang SW, Lee DH, Lee SH, et al. Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J Gastroenterol Hepatol. 2010;25:512–518. doi: 10.1111/j.1440-1746.2009.06106.x. [DOI] [PubMed] [Google Scholar]
- 44.Anand D, Barroeta JE, Gupta PK, Kochman M, Baloch ZW. Endoscopic ultrasound guided fine needle aspiration of non-pancreatic lesions: an institutional experience. J Clin Pathol. 2007;60:1254–1262. doi: 10.1136/jcp.2006.045955. . PMid:17220205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asano M. Endoscopic submucosal dissection and surgical treatment for gastrointestinal cancer. World J Gastrointest Endosc. 2012;4:438–447. doi: 10.4253/wjge.v4.i10.438. . PMid:23189214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1–11. doi: 10.1007/s10120-006-0408-1. . PMid:17334711. [DOI] [PubMed] [Google Scholar]
- 47.Cho JS, Kim JK, Rho SM, Lee HY, Jeong HY, Lee CS. Preoperative assessment of gastric carcinoma: value of two-phase dynamic CT with mechanical iv. injection of contrast material. AJR Am J Roentgenol. 1994;163:69–75. doi: 10.2214/ajr.163.1.8010251. . PMid:8010251. [DOI] [PubMed] [Google Scholar]
- 48.Kim HS, Han HY, Choi JA, et al. Preoperative evaluation of gastric cancer: value of spiral CT during gastric arteriography (CTGA) Abdom Imaging. 2001;26:123–130. doi: 10.1007/s002610000167. . PMid:11178686. [DOI] [PubMed] [Google Scholar]
- 49.Kim HJ, Kim AY, Oh ST, et al. Gastric cancer staging at multi-detector row CT gastrography: comparison of transverse and volumetric CT scanning. Radiology. 2005;236:879–885. doi: 10.1148/radiol.2363041101. . PMid:16020558. [DOI] [PubMed] [Google Scholar]
- 50.Chen CY, Hsu JS, Wu DC, et al. Gastric cancer: preoperative local staging with 3D multi-detector row CT–correlation with surgical and histopathologic results. Radiology. 2007;242:472–482. doi: 10.1148/radiol.2422051557. . PMid:17255419. [DOI] [PubMed] [Google Scholar]
- 51.Kumano S, Okada M, Shimono T, et al. T-staging of gastric cancer of air-filling multidetector-row CT: comparison with hydro-multidetector-row CT. Eur J Radiol. 2012;81:2953–2960. doi: 10.1016/j.ejrad.2011.12.039. . PMid:22304982. [DOI] [PubMed] [Google Scholar]
- 52.Minami M, Kawauchi N, Itai Y, Niki T, Sasaki Y. Gastric tumors: radiologic-pathologic correlation and accuracy of T staging with dynamic CT. Radiology. 1992;185:173–178. doi: 10.1148/radiology.185.1.1523303. PMid:1523303. [DOI] [PubMed] [Google Scholar]
- 53.D'Elia F, Zingarelli A, Palli D, Grani M. Hydro-dynamic CT preoperative staging of gastric cancer: correlation with pathological findings. A prospective study of 107 cases. Eur Radiol. 2000;10:1877–1885. doi: 10.1007/s003300000537. . PMid:11305564. [DOI] [PubMed] [Google Scholar]
- 54.Fukuya T, Honda H, Kaneko K, et al. Efficacy of helical CT in T-staging of gastric cancer. J Comput Assist Tomogr. 1997;21:73–81. doi: 10.1097/00004728-199701000-00014. . PMid:9022773. [DOI] [PubMed] [Google Scholar]
- 55.Kumano S, Murakami T, Kim T, et al. T staging of gastric cancer: role of multi-detector row CT. Radiology. 2005;237:961–966. doi: 10.1148/radiol.2373041380. . PMid:16251394. [DOI] [PubMed] [Google Scholar]
- 56.Hur H, Kim SH, Kim W, Song KY, Park CH, Jeon HM. The efficacy of preoperative PET/CT for prediction of curability in surgery for locally advanced gastric carcinoma. World J Surg Oncol. 2010;8:86. doi: 10.1186/1477-7819-8-86. . PMid:20932345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen BB, Liang PC, Liu KL, et al. Preoperative diagnosis of gastric tumors by three-dimensional multidetector row ct and double contrast barium meal study: correlation with surgical and histologic results. J Formos Med Assoc. 2007;106:943–952. doi: 10.1016/S0929-6646(08)60065-0. . PMid:18063516. [DOI] [PubMed] [Google Scholar]
- 58.Bhandari S, Shim CS, Kim JH, et al. Usefulness of three-dimensional, multidetector row CT (virtual gastroscopy and multiplanar reconstruction) in the evaluation of gastric cancer: a comparison with conventional endoscopy, EUS, and histopathology. Gastrointest Endosc. 2004;59:619–626. doi: 10.1016/S0016-5107(04)00169-5. . PMid:15114303. [DOI] [PubMed] [Google Scholar]
- 59.Kim YN, Choi D, Kim SH, et al. Gastric cancer staging at isotropic MDCT including coronal and sagittal MPR images: endoscopically diagnosed early vs. advanced gastric cancer. Abdom Imaging. 2009;34:26–34. doi: 10.1007/s00261-008-9380-z. . PMid:18311495. [DOI] [PubMed] [Google Scholar]
- 60.Son T, Hyung WJ, Lee JH, et al. Clinical implication of an insufficient number of examined lymph nodes after curative resection for gastric cancer. Cancer. 2012;118:4687–4693. doi: 10.1002/cncr.27426. . PMid:22415925. [DOI] [PubMed] [Google Scholar]
- 61.Wakelin SJ, Deans C, Crofts TJ, Allan PL, Plevris JN, Paterson-Brown S. A comparison of computerised tomography, laparoscopic ultrasound and endoscopic ultrasound in the preoperative staging of oesophago-gastric carcinoma. Eur J Radiol. 2002;41:161–167. doi: 10.1016/S0720-048X(01)00418-1. . PMid:11809546. [DOI] [PubMed] [Google Scholar]
- 62.Kim JW, Shin SS, Heo SH, et al. Diagnostic performance of 64-section CT using CT gastrography in preoperative T staging of gastric cancer according to 7th edition of AJCC cancer staging manual. Eur Radiol. 2012;22:654–662. doi: 10.1007/s00330-011-2283-3. . PMid:21965037. [DOI] [PubMed] [Google Scholar]
- 63.Marin D, Catalano C, Baski M, et al. 64-Section multi-detector row CT in the preoperative diagnosis of peritoneal carcinomatosis: correlation with histopathological findings. Abdom Imaging. 2010;35:694–700. doi: 10.1007/s00261-008-9464-9. . PMid:19455272. [DOI] [PubMed] [Google Scholar]
- 64.Yajima K, Kanda T, Ohashi M, et al. Clinical and diagnostic significance of preoperative computed tomography findings of ascites in patients with advanced gastric cancer. Am J Surg. 2006;192:185–190. doi: 10.1016/j.amjsurg.2006.05.007. . PMid:16860627. [DOI] [PubMed] [Google Scholar]
- 65.Shen Y, Kang HK, Jeong YY, et al. Evaluation of early gastric cancer at multidetector CT with multiplanar reformation and virtual endoscopy. Radiographics. 2011;31:189–199. doi: 10.1148/rg.311105502. . PMid:21257941. [DOI] [PubMed] [Google Scholar]
- 66.Kim JH, Eun HW, Hong SS, Kim YJ, Han JK, Choi BI. Gastric cancer detection using MDCT compared with 2D axial CT: diagnostic accuracy of three different reconstruction techniques. Abdom Imaging. 2012;37:541–548. doi: 10.1007/s00261-011-9823-9. . PMid:22080389. [DOI] [PubMed] [Google Scholar]
- 67.Blackshaw GR, Stephens MR, Lewis WG, et al. Progressive CT system technology and experience improve the perceived preoperative stage of gastric cancer. Gastric Cancer. 2005;8:29–34. doi: 10.1007/s10120-004-0311-6. . PMid:15747171. [DOI] [PubMed] [Google Scholar]
- 68.Shimizu K, Ito K, Matsunaga N, Shimizu A, Kawakami Y. Diagnosis of gastric cancer with MDCT using the water-filling method and multiplanar reconstruction: CT-histologic correlation. AJR Am J Roentgenol. 2005;185:1152–1158. doi: 10.2214/AJR.04.0651. . PMid:16247125. [DOI] [PubMed] [Google Scholar]
- 69.Ahn HS, Lee HJ, Yoo MW, et al. Diagnostic accuracy of T and N stages with endoscopy, stomach protocol CT, and endoscopic ultrasonography in early gastric cancer. J Surg Oncol. 2009;99:20–27. doi: 10.1002/jso.21170. . PMid:18937292. [DOI] [PubMed] [Google Scholar]
- 70.Furukawa K, Miyahara R, Itoh A, et al. Diagnosis of the invasion depth of gastric cancer using MDCT with virtual gastroscopy: comparison with staging with endoscopic ultrasound. AJR Am J Roentgenol. 2011;197:867–875. doi: 10.2214/AJR.10.5872. . PMid:21940574. [DOI] [PubMed] [Google Scholar]
- 71.Chu KM, Kwok KF, Law S, Wong KH. A prospective evaluation of catheter probe EUS for the detection of ascites in patients with gastric carcinoma. Gastrointest Endosc. 2004;59:471–474. doi: 10.1016/S0016-5107(03)02873-6. . PMid:15044880. [DOI] [PubMed] [Google Scholar]
- 72.Keogan MT, Edelman RR. Technologic advances in abdominal MR imaging. Radiology. 2001;220:310–320. doi: 10.1148/radiology.220.2.r01au22310. PMid:11477231. [DOI] [PubMed] [Google Scholar]
- 73.Anzidei M, Napoli A, Zaccagna F, et al. Diagnostic performance of 64-MDCT and 1.5-T MRI with high-resolution sequences in the T staging of gastric cancer: a comparative analysis with histopathology. Radiol Med. 2009;114:1065–1079. doi: 10.1007/s11547-009-0455-x. . PMid:19774440. [DOI] [PubMed] [Google Scholar]
- 74.Maccioni F, Marcelli G, Al Ansari N, et al. Preoperative T and N staging of gastric cancer: magnetic resonance imaging (MRI) versus multi detector computed tomography (MDCT) Clin Ter. 2010;161:e57–62. PMid:20499021. [PubMed] [Google Scholar]
- 75.Lim JS, Yun MJ, Kim MJ, et al. CT and PET in stomach cancer: preoperative staging and monitoring of response to therapy. Radiographics. 2006;26:143–156. doi: 10.1148/rg.261055078. . PMid:16418249. [DOI] [PubMed] [Google Scholar]
- 76.Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology. 2002;224:748–756. doi: 10.1148/radiol.2243011362. . PMid:12202709. [DOI] [PubMed] [Google Scholar]
- 77.Wang Z, Chen JQ. Imaging in assessing hepatic and peritoneal metastases of gastric cancer: a systematic review. BMC Gastroenterol. 2011;11:19. doi: 10.1186/1471-230X-11-19. . PMid:21385469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smyth EC, Shah MA. Role of 18F 2-fluoro-2-deoxyglucose positron emission tomography in upper gastrointestinal malignancies. World J Gastroenterol. 2011;17:5059–5074. doi: 10.3748/wjg.v17.i46.5059. . PMid:22171140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim EY, Lee WJ, Choi D, et al. The value of PET/CT for preoperative staging of advanced gastric cancer: comparison with contrast-enhanced CT. Eur J Radiol. 2011;79:183–188. doi: 10.1016/j.ejrad.2010.02.005. . PMid:20226612. [DOI] [PubMed] [Google Scholar]
- 80.Ha TK, Choi YY, Song SY, Kwon SJ. F18-fluorodeoxyglucose-positron emission tomography and computed tomography is not accurate in preoperative staging of gastric cancer. J Korean Surg Soc. 2011;81:104–110. doi: 10.4174/jkss.2011.81.2.104. . PMid:22066108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee SM, Kim SH, Lee JM, et al. Usefulness of CT volumetry for primary gastric lesions in predicting pathologic response to neoadjuvant chemotherapy in advanced gastric cancer. Abdom Imaging. 2009;34:430–440. doi: 10.1007/s00261-008-9420-8. . PMid:18546037. [DOI] [PubMed] [Google Scholar]
- 82.Ng CS, Husband JE, MacVicar AD, Ross P, Cunningham DC. Correlation of CT with histopathological findings in patients with gastric and gastro-oesophageal carcinomas following neoadjuvant chemotherapy. Clin Radiol. 1998;53:422–427. doi: 10.1016/S0009-9260(98)80270-5. . PMid:9651057. [DOI] [PubMed] [Google Scholar]
- 83.Beer AJ, Wieder HA, Lordick F, et al. Adenocarcinomas of esophagogastric junction: multi-detector row CT to evaluate early response to neoadjuvant chemotherapy. Radiology. 2006;239:472–480. doi: 10.1148/radiol.2391050043. . PMid:16543584. [DOI] [PubMed] [Google Scholar]
- 84.Wieder HA, Beer AJ, Lordick F, et al. Comparison of changes in tumor metabolic activity and tumor size during chemotherapy of adenocarcinomas of the esophagogastric junction. J Nucl Med. 2005;46:2029–2034. PMid:16330567. [PubMed] [Google Scholar]
- 85.Kim DW, Park SA, Kim CG. Detecting the recurrence of gastric cancer after curative resection: comparison of FDG PET/CT and contrast-enhanced abdominal CT. J Korean Med Sci. 2011;26:875–880. doi: 10.3346/jkms.2011.26.7.875. . PMid:21738339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tan IT, So BY. Value of intensive follow-up of patients after curative surgery for gastric carcinoma. J Surg Oncol. 2007;96:503–506. doi: 10.1002/jso.20823. . PMid:17680634. [DOI] [PubMed] [Google Scholar]
- 87.Böhner H, Zimmer T, Hopfenmüller W, Berger G, Buhr HJ. Detection and prognosis of recurrent gastric cancer–is routine follow-up after gastrectomy worthwhile? Hepatogastroenterology. 2000;47:1489–1494. [PubMed] [Google Scholar]
- 88.Sim SH, Kim YJ, Oh DY, et al. The role of PET/CT in detection of gastric cancer recurrence. BMC Cancer. 2009;9:73. doi: 10.1186/1471-2407-9-73. . PMid:19250554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bilici A, Ustaalioglu BB, Seker M, et al. The role of 18F-FDG PET/CT in the assessment of suspected recurrent gastric cancer after initial surgical resection: can the results of FDG PET/CT influence patients' treatment decision making? Eur J Nucl Med Mol Imaging. 2011;38:64–73. doi: 10.1007/s00259-010-1611-1. . PMid:20838995. [DOI] [PubMed] [Google Scholar]
- 90.Kikuchi S, Hiki Y, Shimao H, Sakakibara Y, Kakita A. Tumor volume: a novel prognostic factor in patients who undergo curative resection for gastric cancer. Langenbecks Arch Surg. 2000;385:225–228. doi: 10.1007/s004230050269. . PMid:10857495. [DOI] [PubMed] [Google Scholar]
- 91.Kikuchi S, Sakuramoto S, Kobayashi N, et al. A new staging system based on tumor volume in gastric cancer. Anticancer Res. 2001;21:2933–2936. PMid:11712789. [PubMed] [Google Scholar]
- 92.Cuenod CA, Fournier L, Balvay D, Guinebretière JM. Tumor angiogenesis: pathophysiology and implications for contrast-enhanced MRI and CT assessment. Abdom Imaging. 2006;31:188–193. doi: 10.1007/s00261-005-0386-5. . PMid:16447089. [DOI] [PubMed] [Google Scholar]
- 93.Lee TY, Purdie TG, Stewart E. CT imaging of angiogenesis. Q J Nucl Med. 2003;47:171–187. PMid:12897709. [PubMed] [Google Scholar]
- 94.Yao J, Yang ZG, Chen TW, Li Y, Yang L. Perfusion changes in gastric adenocarcinoma: evaluation with 64-section MDCT. Abdom Imaging. 2010;35:195–202. doi: 10.1007/s00261-009-9503-1. . PMid:19259725. [DOI] [PubMed] [Google Scholar]
- 95.Satoh A, Shuto K, Okazumi S, et al. Role of perfusion CT in assessing tumor blood flow and malignancy level of gastric cancer. Dig Surg. 2010;27:253–260. doi: 10.1159/000288703. . PMid:20668380. [DOI] [PubMed] [Google Scholar]
- 96.Yao J, Yang ZG, Chen HJ, Chen TW, Huang J. Gastric adenocarcinoma: can perfusion CT help to noninvasively evaluate tumor angiogenesis? Abdom Imaging. 2011;36:15–21. doi: 10.1007/s00261-010-9609-5. . PMid:20336293. [DOI] [PubMed] [Google Scholar]