Abstract
Objective
Different HIV-1 antigen specificities appear in sequence after HIV-1 transmission and the immunoglobulin G (IgG) subclass responses to HIV antigens are distinct from each other. The initial predominant IgG subclass response to HIV-1 infection consists of IgG1 and IgG3 antibodies with a noted decline in some IgG3 antibodies during acute HIV-1 infection. Thus, we postulate that multiple antigen-specific IgG3 responses may serve as surrogates for the relative time since HIV-1 acquisition.
Design
We determined the magnitude, peak, and half-life of HIV-1 antigen-specific IgG1 and IgG3 antibodies in 41 HIV-1-infected individuals followed longitudinally from acute infection during the first appearance of HIV-1-specific antibodies through approximately 6 months after infection.
Methods
We used quantitative HIV-1-binding antibody multiplex assays and exponential decay models to estimate concentrations of IgG1 and IgG3 antibodies to eight different HIV-1 proteins including gp140 Env, gp120 Env, gp41 Env, p66 reverse transcriptase, p31 Integrase, Tat, Nef, and p55 Gag proteins during acute/recent HIV-1 infection.
Results
Among HIV-1-specific IgG3 responses, anti-gp41 IgG3 antibodies were the first to appear. We found that anti-gp41 Env IgG3 and anti-p66 reverse transcriptase IgG3 antibodies, in addition to anti-Gag IgG3 antibodies, each consistently and measurably declined after acute infection, in contrast to the persistent antigen-specific IgG1 responses.
Conclusion
The detailed measurements of the decline in multiple HIV-specific IgG3 responses simultaneous with persistent IgG1 responses during acute and recent HIV-1 infection could serve as markers for detection of incident HIV infection.
Keywords: HIV-1 acute infection, HIV-1 incidence, immunoglobulin G subclass
Introduction
Recent studies of the earliest events following HIV-1 transmission by the transmitted/founder virus demonstrate early destruction of B-cell generative microenvironments [1], and that the initial antibody response ineffectively controls virus replication [2]. Detailed understanding of the antibody specificities and subclasses elicited after HIV-1 transmission can inform vaccine designs that aim to elicit functional antibodies more readily and more robustly than natural HIV-1 infection. Furthermore, these specific antibody responses may be used as a surrogate of the time since HIV-1 transmission. The latter idea is important because antibody responses, potentially combined with other markers, can be used to define incident HIV infection [3].
Current incidence tests are based on the evolution of the HIV-specific antibody response during the first several months after transmission, wherein assays measuring the quantity or avidity of HIV-specific antibodies can discriminate recent from chronic infection. One commonly used strategy, the BED IgG-Capture Enzyme Immunoassay (BED EIA), measures the proportion of HIV-1 gp41-specific binding antibodies to total immunoglobulin G (IgG) from subtypes B, E, and D by a capture ELISA [4]. Other strategies, such as the Abbott AxSYM HIV 1/2/gO, use a third-generation EIA that exploits the avidity maturation of HIV-specific antibody response to determine time from transmission [5]. Although these assays have been extensively used to determine incidence, they tend to result in a large number of misclassified recent infections and may, therefore, overestimate incidence, especially in non-clade B populations [6–8]. In addition, the ability of these assays to accurately distinguish recent infection in individuals on antiretroviral therapy (ART) and with low CD4 cell counts is difficult [9,10]. Additional measurements are needed that could be used in a multivariate approach to increase specificity in the setting of non-clade B infections and ART use.
Anti-Env HIV-1 plasma antibodies are predominantly IgG1 subclass, whereas anti-Env IgG3 is the second most predominant IgG subclass [11]. Antibody effector functions (e.g., complement fixation, Fc receptor binding) are determined by antigen specificity and antibody isotype and subclass, and anti-Env IgG1 and IgG3 are predominantly responsible for antibody effector functions (reviewed in [12]). Differential regulation of anti-Env and anti-Gag antibodies has been previously described [13] and anti-Gag IgG3 antibodies were found more frequently in early infection [14–17]. Moreover, using western blot analyses, Gag-specific IgG3 was shown to decline 1–4 months after infection [18], concordant with a decrease in anti-HIV IgG3 during disease progression [15,17]. Although anti-Gag antibodies do not have known direct antiviral activity, they may be indicative of an active T-helper-cell response [13]. Furthermore, shorter duration of antigen-specific IgG subclasses may reflect inherent differences in antibody subclass durability (i.e., IgG3) or that certain specificities are from predominantly short-lived memory B cells.
We determined the kinetics of HIV-specific IgG subclass antibody responses in an HIV-1 acute cohort from the USA, South Africa, and Malawi. Generally, there was an overall decline in HIV-specific IgG3 responses in all individuals, whereas HIV-specific IgG1 responses tended to rise and remained elevated throughout the study period. We further explored the applicability of HIV-specific IgG3 antibody responses to determining incidence by applying an exponential decay model to determine the peak IgG3 antibody concentrations as well as the half-life of these antibodies during acute HIV-1 infection (AHI). In addition, we determined the effect of ART use, viral load, and individual location on the peak and half-life of each HIV-specific IgG3 response. We propose that these measurements could be part of a multivariate algorithm that could allow for an estimate of the relative timing from HIV-1 transmission.
Methods
Participants
Plasma samples were collected over time from 41 participants enrolled during AHI, each with between five and 15 visits. As shown in Table 1, 26 of the 41 participants (63.4%) were from the USA and, of those, 11 (42.3%) began ART within 4 weeks of enrollment. An additional two participants from the USA began ART at 12 and 16 weeks after enrollment. No participants from South Africa/Malawi were on ARTwhile plasma samples were being collected. Fifteen (36.5%) of the participants were whites and five (12.2%) were female. Participants’ ages ranged from 17 to 60 years with an average age of 28.4 years. HIV-1 clade typing was done on a subset of participants at enrollment and virus clade corresponded to location (clade B, North America; clade C, Africa) for each participant. CD4 cell counts were done on most participants, but data was not obtained for all study visits (Supplemental Digital Content 1, http://links.lww.com/QAD/A161). Viral load levels were also measured for most visits for each participant (Supplemental Digital Content 2, http://links.lww.com/QAD/A161) and viral load set point was determined by averaging all viral load measurements within a defined set point window [19]. All participants were assigned to Fiebig stages 1 through 6 upon enrollment into the Center for HIV-AIDS Vaccine Immunology (CHAVI) 001 prospective study (Table 2) [20–22]. Upon enrollment, four participants were in Fiebig stages I and II (viral RNA-positive only in stage I and viral RNA-positive and p24 antigen-positive in stage II, antibody EIA nonreactive and occurring approximately 10–24.3 days from transmission); four participants were in Fiebig stage III (viral RNA, p24 antigen, EIA-positive, western blot-negative and occurring approximately 20.3–27.5 days from transmission); 11 participants were in Fiebig stage IV (viral RNA, p24 antigen, EIA-positive, western blot indeterminate and approximately 23.5–33.1 days from transmission), and the rest of the participants (22) were in Fiebig stages V and VI (viral RNA, p24 antigen, ELISA-positive, western blot-positive, p31 negative to positive in stage VI, with stage Voccurring approximately 29.1–102.6 days from transmission).
Table 1.
Clinical data for CHAVI 001 participants (n=41).
| Blacks/whites/unknown | 25/15/1 |
| Mean age (range) | 28.4 (17–60) |
| Male/female | 36/5 |
| USA/Malawi/South Africa | 26/11/4 |
| On ART/off ARTa | 13/28 |
ART, antiretroviral treatment.
Thirteen participants began ART at some point prior to the last sample collection, with 11 participants starting ART within 4 weeks of enrollment. Some participants had interrupted ART during the study.
Table 2.
Fiebig staging data for CHAVI 001 participants.
| Fiebig stage | Approximate timeframe after transmission (days)a |
Number of participants |
|---|---|---|
| I and II | 10–24.3 | 4 |
| III | 20.3–27.5 | 4 |
| IV | 23.5–33.1 | 11 |
| V and VI | Stage V=29.1–102.6; stage VI=98.6 – open ended |
22 |
Derived from [22], allowing 10–14 days for the eclipse phase.
This CHAVI Acute and Chronic Cohorts study was reviewed and approved by the Institutional Review Boards of Duke University Medical Center. All participants provided written informed consent for study participation.
HIV-1 multiplex-binding antibody assays
Customized multiplex HIV-1-binding assays were performed as previously described [2] to determine IgG1 and IgG3 responses specific for recombinant HIV-1 p55 Gag (Protein Sciences, Meriden, Connecticut, USA), recombinant HIV-1 gp41 MN (Immunodiagnostics, Woburn, Massachusetts, USA), a previously described artificial multi-clade group M consensus gp120 Env protein (Con6 gp120) (Drs Liao and Haynes, Duke University)[23], HIV-1 p66 reverse transcriptase (RT) (Protein Sciences), HIV-1 recombinant Nef (Genway, San Diego, California, USA), recombinant HIV-1 Tat (Advanced Bioscience, Kensington, Maryland, USA), and recombinant HIV-1 p31 Integrase (Genway) [2]. HIV-specific antibody subclasses were detected with mouse antihuman IgG1 (BD Pharmingen, San Diego, California, USA) and mouse antihuman IgG3 (Calbiochem, Gibbstown, New Jersey, USA) on a Bio-Plex instrument (BioRad, Hercules, California, USA) and microgram per milliliter equivalents were calculated using a 4PL curve analysis with IgG subclass standards. Mouse antihuman IgG1 and IgG3 detection antibodies were tested for cross-reactivity to IgG1, IgG2, IgG3, and IgG4 and found to be highly specific for subclass detection. HIVIG (Quality Biological, Gaithersburg, Maryland, USA) and a constant HIV-positive serum titration were utilized as positive controls and negative controls were included in every assay. All assays were run under good clinical laboratory practice compliant conditions, including tracking of positive controls by Levy–Jennings charts. Positivity criteria (mean fluorescence intensity plus 3 standard deviations) for antibody–antigen pairs were predetermined using a set of plasmas from 30-seronegative participants. Food and Drug Administration compliant software, Bio-Plex Manager 5.0 (BioRad), was utilized for the analysis of specimens.
Statistical analysis
To obtain estimates of peak antigen values and half-life, exponential decay models were created for each antigen,
in which y is the antigen value, xt is the number of days after observed peak response for the antigen of interest, β0 is the estimated peak value (level at day 0), and β1 is the exponential rate of decay such that ln(2)/β1 is the estimated half-life. The models only include values at or postpeak, assume an asymptote of zero, and a random effect for β0, and were fit using PROC NLMIXED in SAS 9.2. Approximate 95% confidence intervals (CIs) for the half-life estimates were generated based on a Student’s t-test distribution using the delta method to estimate the variance of the half-life estimates. Specifically,
in which μ equals the estimate of β1 from the model. To explore the impact of ART, viral load, and participant location status on antigen peak and half-life estimates, additional models were created for each of these status variables that included subgroup-specific estimates of peak value and half-life. Specifically,
in which subgroups are defined as use of ART (yes vs. no), viral load (≤5000 IU/ml vs. >5000 IU/ml), and participant location (USA vs. South Africa/Malawi), and ART and viral status are time-varying covariates such that values may change for a participant over time. Because ART was only used in participants from the USA, the ART models included only the USA participants. Likewise, the participant location models included only observations prior to ART initiation. To compare the timing of peak between antigens, adjusted mean estimates for each antigen at each visit were obtained from a longitudinal model that adjusts for Fiebig staging at study enrollment in order to account for the differences in the amount of time after infection upon enrollment.
The models include all observed antigen values, treat each visit as a categorical variable, and were fit using PROCMIXED in SAS 9.2.
Results
To evaluate the magnitude and kinetics of IgG subclass responses to AHI, we measured HIV-specific IgG1 and IgG3 antibody responses in 41 individuals in the CHAVI 001 cohort. Table 3 shows the seroprevalence of HIV-specific IgG1 and IgG3 antibodies in these individuals. HIV-1 p55 Gag-specific and gp41Env-specific IgG1 was detected in all participants, and IgG1 specific for p66 reverse transcriptase, p31 Integrase, and gp120 Env was detected in at least 85% of participants. All 41 participants had detectable p55-specific and gp41-specific IgG3, and IgG3 specific for p66 reverse transcriptase, p31 Integrase, and gp120 Env was detected in most (>80%) but not all of the participants. HIV-1 Nef-specific and Tat-specific IgG3 were detected in only 16 (39%) and 20 participants (49%), respectively. These data show that IgG1 and IgG3 antibodies directed toward p55 Gag, gp41 Env, gp120 Env, and p66 reverse transcriptase are detectable in most participants during AHI.
Table 3.
Seroprevalence of HIV-specific IgG1 and IgG3 antibodies during acute HIV-1 infection.
| Positive number (%) |
||
|---|---|---|
| IgG1 (n=41) | IgG3 (n=41) | |
| p55 Gag | 41 (100) | 41 (100.0) |
| gp41 Env | 41 (100) | 41 (100.0) |
| p66RT | 40 (97.6) | 39 (95.1) |
| p31 Integrase | 37 (90.2) | 33 (80.5) |
| gp120 Env | 35 (85.4) | 35 (85.4) |
| Tat | 9 (22.0) | 20 (48.8) |
| Nef | 19a (50.0) | 16 (39.0) |
n=38.
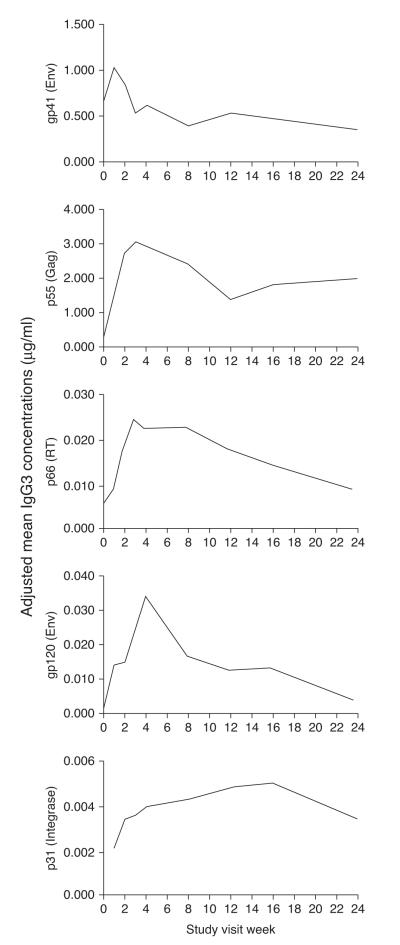
We previously showed that HIV gp41-specific antibodies are the first to arise during AHI and they are followed by p55 Gag-specific, p66 reverse transcriptase-specific, gp120 Env-specific, and p31 Integrase-specific antibodies [2,12]. To assess the postinfection timeframe of the peak of the IgG3 and IgG1 antibody response, the visit at which the estimated mean peak occurred was obtained from the longitudinal models that adjust for Fiebig staging (as participants were enrolled at different Fiebig stages). The adjusted mean titers for HIV-1 gp41 Env-specific IgG3 peaked approximately 1 week after enrollment, followed by p55 Gag-specific and p66 reverse transcriptase-specific IgG3 at 3 weeks after enrollment, gp120 Env-specific IgG3 at 4 weeks after enrollment, and p31 Integrase-specific IgG3 at 16 weeks after enrollment (Fig. 1) [22]. HIV-specific IgG1 antibodies did not show an overall discernable peak (Fig. 2b). These results show that, in contrast to HIV-specific IgG1, HIV-specific IgG3 antibodies decline over time and the timing of the peak of individual HIV-specific IgG3 antibody responses is a reflection of their sequential elicitation during AHI.
Fig. 1.
HIV-specific IgG3 antibody responses peak sequentially during acute HIV-1 infection. Mean concentrations of HIV-specific IgG3 are shown in a longitudinal model in which responses were aligned to study enrollment and adjusted for time after HIV-1 acquisition according to the classification by Fiebig et al. [22].
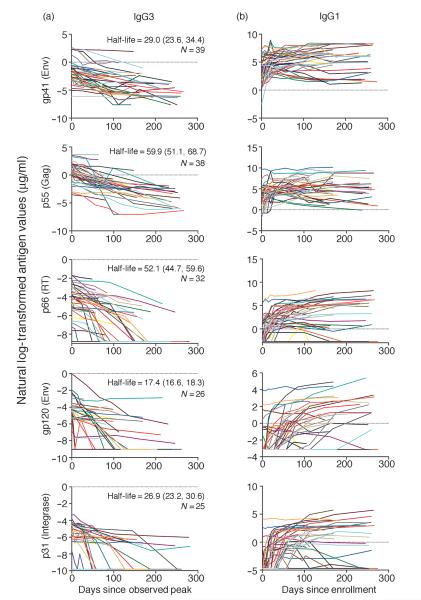
Fig. 2.
Levels of HIV-1-specific IgG1 remain stable, whereas HIV-1-specific IgG3 declines over time during acute HIV-1 infection. Declining HIV-1-specific IgG3 antibody responses were aligned according to the peak of each response, modeled over time, and response half-life was estimated using the exponential decay model. Half-life in days (95% confidence interval) and N (number of participants with at least one positive time point after peak) are depicted in each panel (a). HIV-1-specific IgG1 antibody levels did not decline and, therefore, were not fit to the exponential decay model. For this reason, IgG1 antibody responses were aligned to enrollment into the study (b).
Levels of HIV-specific IgG1 and IgG3 antibodies were also examined through 43 weeks after enrollment and models were attempted to estimate the half-life [ln(2)/β2] of each antibody response. Figure 2 shows the kinetics of HIV-specific IgG1 and IgG3 antibody responses during AHI. HIV-specific IgG3 showed an overall decline, whereas HIV-specific IgG1 antibody levels remained more stable and, thus, would not fit the exponential decay model. Therefore, half-life of the HIV-specific IgG1 antibody responses for all responding participants could not be estimated and as such; these responses are aligned by enrollment instead of peak response, as shown for HIV-specific IgG3 (Fig. 2). In Supplemental Digital Content 3, http://links.lww.com/QAD/A161, HIV-specific IgG3 antibody responses are aligned to enrollment and, therefore, the rise and decline of these responses can be observed for individual participants. Using the exponential decay model, half-life was estimated for p55 Gag-specific, gp41 Env-specific, gp120 Env-specific, p31 Integrase-specific, and p66 reverse transcriptase-specific IgG3 antibody responses, with gp120 Env-specific IgG3 having the shortest half-life estimate of 17.4 (95% CI 16.6, 18.3) days and p55 Gag-specific IgG3 having the longest half-life estimate of 59.9 (95% CI 51.1, 68.7) days (Fig. 2 and Table 4). Because Nef and Tat IgG3 antibody responses were detected in less than half of all participants and would, therefore, not be very useful to determine incidence, the results of the exponential decay model are not shown. These results demonstrate that HIV-specific IgG3 antibodies tend to decline during AHI in a defined manner, whereas HIV-specific IgG1 antibodies tend to remain stable or elevate over time.
Table 4.
.Effects of antiretroviral use, viral load, and virus clade on IgG3 antibody half-life.
| Half-life (days) |
|||||
|---|---|---|---|---|---|
| Antibody Response | Model | Estimate type | Estimate | 95% CI | P value |
| IgG3 p55 | Exponential decay model | Overall | 59.91 | 51.05–68.74 | 0.0092 |
| Viral load only model | Low | 49.09 | 36.19–61.99 | ||
| High | 77.24 | 60.85–93.63 | |||
| ART use only modela | No | 22.10 | 17.57–26.65 | <0.0001 | |
| Yes | 47.40 | 44.37–50.44 | |||
| Location only modelc | USA | 13.59 | 0.70–26.49 | 0.0921 | |
| Africab | 71.71 | 56.42–87.01 | |||
| IgG3 p66 | Exponential decay model | Overall | 52.14 | 44.67–59.61 | 0.0119 |
| Viral load only model | Low | 42.41 | 33.39–51.43 | ||
| High | 62.7 | 49.96–75.44 | |||
| ART use only modela | No | 50.11 | 44.09–56.12 | <0.0001 | |
| Yes | 20.54 | 18.04–23.05 | |||
| Location only modelc | USA | 51.07 | 40.20–61.93 | 0.0146 | |
| Africa | 74.66 | 60.23–89.10 | |||
| IgG3 p31 | Exponential decay model | Overall | 26.86 | 23.18–30.55 | |
| Viral load only model | Low | 25.04 | 19.84–30.23 | 0.2394 | |
| High | 29.47 | 23.67–35.27 | |||
| ART use only modela | No | 33.12 | 23.33–42.92 | 0.0046 | |
| Yes | 20.44 | 16.84–24.05 | |||
| Location only modelc | USA | 33.08 | 22.9–43.26 | 0.0039 | |
| Africa | 40.57 | 28.50–52.64 | |||
| IgG3 gp41 | Exponential decay model | Overall | 28.99 | 23.56–34.42 | |
| Location only modelc | USA | 70.16 | 42.16–98.15 | 0.0001 | |
| Africa | 24.96 | 19.13–30.78 | |||
| IgG3 gp120 | Exponential decay model | Overall | 17.43 | 16.58–18.28 | 0.0143 |
| Viral load only model | Low | 11.65 | 7.23–16.06 | ||
| High | 17.57 | 16.83–18.30 | |||
| ART use only modela | No | 17.49 | 17.15–17.83 | <0.0001 | |
| Yes | 11.33 | 10.41–12.26 | |||
| Location only modelc | USA | 17.50 | 17.05–17.94 | <0.0001 | |
| Africa | 529.95 | 183.6–876.3 | |||
ART, antiretroviral therapy; CI, confidence interval.
USA participants only.
Malawi/South Africa, predominantly clade C.
Samples prior to start of ART only.
The exponential decay model was also used to obtain estimates for specific antibody concentrations (95% CI) at the observed peak for p55 Gag-specific IgG3 and gp41-specific IgG3, which were 4.67 (95% CI 1.76, 7.57) and 1.49 μg/ml (95% CI 0.37, 2.62), respectively (Table 5). The peak titers for the other IgG3 specificities were 0.1 μg/ml or less. The estimated fold decrease (95% CI) from the observed peak response to day 150 postpeak was 5.7 (95% CI 4.22, 7.12) for p55 Gag-specific IgG3 and 36.11 (95% CI 11.87, 60.35) for gp41 Env-specific IgG3. Estimated antibody concentrations declined to 0.82 μg/ml for p55 Gag-specific IgG3 and 0.04 μg/ml for gp41 Env-specific IgG3 by 150 days from the peak response. Together with the half-life values obtained from the exponential decay model, these data show a measurable decline in HIV-specific IgG3 antibodies during AHI.
Table 5.
IgG3 concentration estimates from the exponential decay model.
| Concentration at peak (μg/ml) |
Concentration at half-life (μg/ml) |
Concentration at 150 days postpeak (μg/ml) |
Fold decrease at 150 days postpeak |
|||||
|---|---|---|---|---|---|---|---|---|
| Specificity | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI |
| p55 Gag | 4.67 | 1.76–7.57 | 2.33 | 0.88–3.78 | 0.82 | 0.28–1.37 | 5.67 | 4.22–7.12 |
| gp41 Env | 1.49 | 0.37–2.62 | 0.75 | 0.18–1.31 | 0.04 | 0.00–0.08 | 36.11 | 11.87–60.35 |
| p66 RT | 0.03 | 0.02–0.05 | 0.0161 | 0.01–0.02 | 0.0044 | 0.0022–0.0065 | 7.35 5. | 25–9.44 |
| gp120 Env | 0.05 | 0.00–0.10 | 0.03 | 0.0022,0.05 | 0.0001 | 0.00–0.0003 | 389.80 | 276.24–503.36 |
| p31 | 0.01 | 0.01–0.01 | 0.0048 | 0.0027–0.0069 | 0.0002 | 0.0001–0.0003 | 47.97 | 22.5–73.43 |
CI, confidence interval; Est, estimate.
Antibody responses were also modeled to account for the effects of ART use, viral load (≤5000 vs. >5000 IU/ml), location (USA, predominantly clade B or South Africa/Malawi, predominantly clade C) on the half-life (Table 4), and peak concentration (Supplemental Digital Content 4, http://links.lww.com/QAD/A161) of HIV-specific IgG3 responses. To separate any possible effects of ART from location on antibody half-life, only participants from the USA were evaluated for the effects of ART on IgG3 responses (because all but one of the participants on ART were in the USA). ART significantly lengthened the half-life estimate of p55 Gag-specific IgG3 responses: participants off ART had p55 Gag-specific IgG3 responses with a half-life estimate of 22.10 (95% CI 17.57, 26.65) days and those on ART had a longer half-life estimate of 47.40 (95% CI 44.37, 50.04) days. Because of the variation in the kinetics of gp41 Env-specific IgG3 responses, the effects of ART on the half-life of this response could not be ascertained. In contrast to its effects on the half-life of p55 Gag-specific IgG3, ART significantly shortened the half-life estimate of p66 reverse transcriptase-specific IgG3 responses by over two-fold: participants on ART had a half-life estimate of 20.54 (95% CI 18.04, 23.05) days and those off ART had a half-life estimate of 50.11 (95% CI 44.09, 56.12) days (Table 4). ART also significantly shortened the half-lives of p31 Integrase-specific and gp120 Env-specific IgG3 by 1.6-fold and 1.5-fold, respectively. ART use significantly increased the peak concentration of gp120 Env-specific IgG3 responses, but did not affect the peak concentrations of any of the other IgG3 responses (Supplemental Digital Content 4, http://links.lww.com/QAD/A161). Also, because HIV-specific IgG1 antibodies did not decline during AHI and the exponential decay model could not be applied, the influence of ARTon the kinetics of these responses was not determined. In summary, ART use tends to prolong the IgG3 antibody response to p55 Gag, but shortens the IgG3 antibody response to p66 reverse transcriptase, p31 Integrase, and gp120 Env.
The effects of viral load and location on IgG3 antibody half-life and peak concentration were also evaluated (Table 4). All 41 participants were analyzed for the effects of viral load on the IgG3 response. Participants with a viral load of more than 5000 IU/ml had a significantly longer IgG3 antibody half-life for responses to p55 Gag, p66 reverse transcriptase, and gp120 Env. Viral load levels did not have an effect on the half-life of p31 Integrase-specific IgG3 and a final model could not be obtained for the effects of viral load on gp41 Env-specific IgG3 half-life. Viral load levels did not significantly affect the peak concentration of any of the HIV-specific IgG3 antibody responses (Supplemental Digital Content 4, http://links.lww.com/QAD/A161).
For the evaluation of the effects of participant location on IgG3 antibody half-life and peak concentration, only observations prior to ART initiation were analyzed. This eliminates any possible influence of ART use that may interfere with the effects of location on IgG3 kinetics. We observed that participants in South Africa/Malawi had significantly longer antibody half-lives for p66 reverse transcriptase, p31 Integrase, and gp120 Env IgG3 compared with participants in the USA (Table 4). Participants in South Africa/Malawi also had a longer antibody half-life for p55 Gag-specific IgG3, but the difference between antibody half-lives in participants in the USA vs. South Africa/Malawi was not significant. However, participants in South Africa/Malawi had a shorter gp41 Env-specific antibody half-life compared with participants in the USA. There were no location-specific effects on the peak of IgG3 antibody responses (Supplemental Digital Content 4, http://links.lww.com/QAD/A161). Together, these results show that participants with viral load levels above 5000 IU/ml had longer IgG3 antibody half-lives for p55 Gag-specific, p66 reverse transcriptase-specific, and gp120 Env-specific responses. In addition, participants in Africa had p66 reverse transcriptase-specific, p31 Integrase-specific, and gp120 Env-specific IgG3 responses that were of significantly longer duration compared with participants in the USA.
Discussion
Here, we have examined the HIV-specific IgG1 and IgG3 responses to AHI in a defined manner and propose that these measurements can be used in an algorithm to determine incident HIV-1 infection. Our focus was maintained on IgG1 and IgG3 subclasses, as HIV-specific IgG2 antibodies are not detected in most individuals and the elicitation of HIV-specific IgG4 may be delayed in comparison (reviewed in [12]). Here, we have found that, although IgG1 and IgG3 responses are elicited to gp41 Env, p55 Gag, p66 reverse transcriptase, gp120 Env, and p31 Integrase in a majority of participants, IgG3 responses to these antigens typically decline. In contrast, HIV-1 IgG1 antibody levels are typically maintained over the same period. We estimated the half-life of the HIV-specific IgG3 antibody response as well as determined concentrations of these antibodies at their peak and nadir (approximately 150 days postpeak).
ART use was found to have a significant impact on the half-life of p55 IgG3, wherein the antibody response of participants on ART had a two-fold longer half-life compared with untreated participants. Although there was not enough CD4 cell count data available to make an association in this study, this finding is consistent with the idea that the Gag-specific antibody response may be more dependent on CD4 T-cell help than responses to other HIV-1 antigens [13]. In contrast to p55 Gag-specific IgG3, the half-life of p66 reverse transcriptase-specific, p31 integrase-specific, and gp120 Env-specific IgG3 was decreased in participants on ART, suggesting that virus replication may be a more important factor than CD4+ T-cell responses in maintaining particular HIV-specific antibody responses. There was not enough CD4 cell count data available during visits in which the peak and subsequent initial decline of IgG3 antibodies was observed to significantly determine the effect of CD4 cell counts on specific antibody responses. Participants in Malawi/South Africa had longer IgG3 antibody half-lives than participants in the USA for p66 reverse transcriptase-specific, p31 Integrase-specific, and gp120 Env-specific responses. A similar, though nonsignificant, trend was also observed for p55 Gag-specific IgG3 half-life. Participant location had the opposite effect on gp41 Env-specific IgG3 half-life. As only samples prior to ART initiation were evaluated in this particular analysis, these data suggest that there are location-specific effects on the duration of HIV-specific IgG3 responses. Importantly, there did not appear to be any significant clade-specific differences in the ability of the antigens we used to detect HIV-specific antibodies in our participants. All 15 participants in Malawi/South Africa (predominantly clade C) were positive for p66 reverse transcriptase-specific, p31 Integrase-specific, and gp120 Env-specific IgG3 (data not shown). This is not surprising, given that p66 reverse transcriptase and p31 Integrase are relatively conserved proteins and the gp120 Env that we used was an artificial multi-clade group M consensus gp120 Env protein (Con6 gp120) [23].
In a recent clonal analyses of the initial HIV-1 reactive antibodies, increased levels of IgA and IgG3 antigen-specific antibodies were identified in AHI when compared with vaccine-induced influenza antibodies [24]. Thus, some of the initial anti-HIV-1 antibody response may be due to stimulation of specific subsets of B cells that preferentially switch from IgM to IgG3, for example, marginal zone B cells [25]. In addition, the sequential appearance of gp41-specfic IgG3 followed by p55 Gag-specific and gp120 Env-specific IgG3 is consistent with our previous findings involving overall HIV-specific IgG responses [2]. These results are also consistent with the finding that the initial HIV-1 gp41 Env-specific response is due to stimulation of a preexisting pool of cross-reactive memory B cells that had been previously activated by non-HIV-1 antigens (Liao et al., in preparation).
Using the antibody concentrations of Gag, Env, and reverse transcriptase at peak, at half-life point and at 150 days, the relative time since transmission could be estimated for an unknown sample. Strategies have been developed for the purpose of incidence testing [4,26,27]. However, improved tests for cross-sectional incidence testing are needed, as current testing paradigms may not accurately account for variables such as ART, CD4 cell count, and chronic patient populations [6,9,28–31]. Evaluation of the timing and concentrations of p55 Gag, gp41 Env, and p66 reverse transcriptase IgG3 antibodies as reported here are some of the immune measurements that could be evaluated for the purpose of improving incidence testing in global populations.
Supplementary Material
Acknowledgements
The authors thank Drs Salim Abdool-Karim, Gift Kamanga and the participants and staff of the CHAVI 001 clinical teams. We thank Drs Stuart Shapiro and Bernard Branson for helpful discussion and Vicki Ashley, Yong Lin, Claire Beard, Michele Donathan and Doris Murray for technical assistance.
N.L.Y. contributed to study design, performed the experiments, analyzed the data, and wrote the article. J.T.L. performed the experiments. T.L.N. analyzed the data and wrote parts of the article. N.V. analyzed the data. K.S. contributed in study planning and design. K.E.S. performed the experiments and analyzed data. T.N.D. contributed to study design. B.F.H. contributed to study design and data interpretation and wrote parts of the article. M.C. contributed to study design and data interpretation and wrote parts of the article. G.D.T. designed the study, analyzed the data, and wrote the article.
This work was supported by the National Institutes of Health (NIH/NIAID/ DAIDS): CHAVI (AI067854), HIV Vaccine Trials Network HVTN (5 U01 AI46725-05), Bill and Melinda Gates Foundation (3830913), and the Duke University Center for AIDS Research Grant (P30 AI 64518).
Footnotes
Conflicts of interest US Patent Application Serial No. 61/388, 711 is a patent pending, which involves the work described herein.
References
- 1.Levesque MC, Moody MA, Hwang KK, Marshall DJ, Whitesides JF, Amos JD, et al. Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS Med. 2009;6:e1000107. doi: 10.1371/journal.pmed.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma antigp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen MS, Fidler S. HIV prevention 2010: where are we now and where are we going? Curr Opin HIV AIDS. 2010;5:265–268. doi: 10.1097/COH.0b013e32833acafa. [DOI] [PubMed] [Google Scholar]
- 4.Parekh BS, Kennedy MS, Dobbs T, Pau CP, Byers R, Green T, et al. Quantitative detection of increasing HIV type 1 antibodies after seroconversion: a simple assay for detecting recent HIV infection and estimating incidence. AIDS Res Hum Retrovir. 2002;18:295–307. doi: 10.1089/088922202753472874. [DOI] [PubMed] [Google Scholar]
- 5.Suligoi B, Galli C, Massi M, Di Sora F, Sciandra M, Pezzotti P, et al. Precision and accuracy of a procedure for detecting recent human immunodeficiency virus infections by calculating the antibody avidity index by an automated immunoassay-based method. J Clin Microbiol. 2002;40:4015–4020. doi: 10.1128/JCM.40.11.4015-4020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakarovitch C, Rouet F, Murphy G, Minga AK, Alioum A, Dabis F, et al. Do tests devised to detect recent HIV-1 infection provide reliable estimates of incidence in Africa? J Acquir Immune Defic Syndr. 2007;45:115–122. doi: 10.1097/QAI.0b013e318050d277. [DOI] [PubMed] [Google Scholar]
- 7.Pandori MW, Hackett J, Jr, Louie B, Vallari A, Dowling T, Liska S, et al. Assessment of the ability of a fourth-generation immunoassay for human immunodeficiency virus (HIV) antibody and p24 antigen to detect both acute and recent HIV infections in a high-risk setting. J Clin Microbiol. 2009;47:2639–2642. doi: 10.1128/JCM.00119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karita E, Price M, Hunter E, Chomba E, Allen S, Fei L, et al. Investigating the utility of the HIV-1 BED capture enzyme immunoassay using cross-sectional and longitudinal seroconverter specimens from Africa. AIDS. 2007;21:403–408. doi: 10.1097/QAD.0b013e32801481b7. [DOI] [PubMed] [Google Scholar]
- 9.Marinda ET, Hargrove J, Preiser W, Slabbert H, van Zyl G, Levin J, et al. Significantly diminished long-term specificity of the BED capture enzyme immunoassay among patients with HIV-1 with very low CD4 counts and those on antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;53:496–499. doi: 10.1097/qai.0b013e3181b61938. [DOI] [PubMed] [Google Scholar]
- 10.Hladik W, Olara D, Mermin J, Moore D, Were W, Alexander L, et al. Effect of CD4(+) T cell count and antiretroviral treatment on two serological HIV incidence assays. AIDS Res Hum Retroviruses. 2011 doi: 10.1089/AID.2010.0347. [Epub ahead of print] doi:10.1089/aid.2010.0347. [DOI] [PubMed] [Google Scholar]
- 11.Broliden PA, Morfeldt-Mansson L, Rosen J, Jondal M, Wahren B. Fine specificity of IgG subclass response to group antigens in HIV-1-infected patients. Clin Exp Immunol. 1989;76:216–221. [PMC free article] [PubMed] [Google Scholar]
- 12.Tomaras GD, Haynes BF. HIV-1-specific antibody responses during acute and chronic HIV-1 infection. Curr Opin HIV AIDS. 2009;4:373–379. doi: 10.1097/COH.0b013e32832f00c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Binley JM, Klasse PJ, Cao Y, Jones I, Markowitz M, Ho DD, et al. Differential regulation of the antibody responses to Gag and Env proteins of human immunodeficiency virus type 1. J Virol. 1997;71:2799–2809. doi: 10.1128/jvi.71.4.2799-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klasse J, Blomberg J. Patterns of antibodies to human immunodeficiency virus proteins in different subclasses of IgG. J Infect Dis. 1987;156:1026–1030. doi: 10.1093/infdis/156.6.1026. [DOI] [PubMed] [Google Scholar]
- 15.McDougal JS, Kennedy MS, Nicholson JK, Spira TJ, Jaffe HW, Kaplan JE, et al. Antibody response to human immunodeficiency virus in homosexual men. Relation of antibody specificity, titer, and isotype to clinical status, severity of immunodeficiency, and disease progression. J Clin Invest. 1987;80:316–324. doi: 10.1172/JCI113075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khalife J, Guy B, Capron M, Kieny MP, Ameisen JC, Montagnier L, et al. Isotypic restriction of the antibody response to human immunodeficiency virus. AIDS Res Hum Retroviruses. 1988;4:3–9. doi: 10.1089/aid.1988.4.3. [DOI] [PubMed] [Google Scholar]
- 17.Ljunggren K, Broliden PA, Morfeldt-Manson L, Jondal M, Wahren B. IgG subclass response to HIV in relation to antibody-dependent cellular cytotoxicity at different clinical stages. Clin Exp Immunol. 1988;73:343–347. [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson KM, Johnson EI, Croom HA, Richards KM, Doughty L, Cunningham PH, et al. Incidence immunoassay for distinguishing recent from established HIV-1 infection in therapy-naive populations. AIDS. 2004;18:2253–2259. doi: 10.1097/00002030-200411190-00005. [DOI] [PubMed] [Google Scholar]
- 19.Fellay J, Shianna KV, Ge D, Colombo S, Ledergerber B, Weale M, et al. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317:944–947. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiebig EW, Heldebrant CM, Smith RI, Conrad AJ, Delwart EL, Busch MP. Intermittent low-level viremia in very early primary HIV-1 infection. J Acquir Immune Defic Syndr. 2005;39:133–137. [PubMed] [Google Scholar]
- 21.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol. 2010;10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 23.Gao F, Weaver EA, Lu Z, Li Y, Liao HX, Ma B, et al. Antigenicity and immunogenicity of a synthetic human immunodeficiency virus type 1 group m consensus envelope glycoprotein. J Virol. 2005;79:1154–1163. doi: 10.1128/JVI.79.2.1154-1163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao HX, Chen X, Dixon A, Munshaw S, Moody MA, Zhang R, et al. Characterization of the Plasma Cell Repertoire in Acute HIV-1 Infection (AHI) Retrovirology. 2009;6(Suppl 3):73. [Google Scholar]
- 25.Gatto D, Ruedl C, Odermatt B, Bachmann MF. Rapid response of marginal zone B cells to viral particles. J Immunol. 2004;173:4308–4316. doi: 10.4049/jimmunol.173.7.4308. [DOI] [PubMed] [Google Scholar]
- 26.Janssen RS, Satten GA, Stramer SL, Rawal BD, O’Brien TR, Weiblen BJ, et al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA. 1998;280:42–48. doi: 10.1001/jama.280.1.42. [DOI] [PubMed] [Google Scholar]
- 27.Curtis KA, Kennedy MS, Charurat M, Nasidi A, Delaney K, Spira TJ, Owen MS. Development and characterization of a bead-based, multiplex assay for estimation of recent HIV type 1 infection. AIDS Res Hum Retroviruses. doi: 10.1089/aid.2011.0037. [Epub ahead of print] doi:10.1089/aid.2011.0037. [DOI] [PubMed] [Google Scholar]
- 28.Hallett TB, Ghys P, Barnighausen T, Yan P, Garnett GP. Errors in ‘BED’-derived estimates of HIV incidence will vary by place, time and age. PLoS One. 2009;4:e5720. doi: 10.1371/journal.pone.0005720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnighausen T, McWalter TA, Rosner Z, Newell ML, Welte A. HIV incidence estimation using the BED capture enzyme immunoassay: systematic review and sensitivity analysis. Epidemiology. 2010;21:685–697. doi: 10.1097/EDE.0b013e3181e9e978. [DOI] [PubMed] [Google Scholar]
- 30.McDougal JS, Parekh BS, Peterson ML, Branson BM, Dobbs T, Ackers M, et al. Comparison of HIV type 1 incidence observed during longitudinal follow-up with incidence estimated by cross-sectional analysis using the BED capture enzyme immunoassay. AIDS Res Hum Retroviruses. 2006;22:945–952. doi: 10.1089/aid.2006.22.945. [DOI] [PubMed] [Google Scholar]
- 31.Wang R, Lagakos SW. Augmented cross-sectional prevalence testing for estimating HIV Incidence. Biometrics. 2010;66:864–874. doi: 10.1111/j.1541-0420.2009.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.