Abstract
Low levels of human copper transporter 1 (hCtr1) mRNA are associated with a shorter progression-free survival after platinum-based therapy. Pretreatment with a copper-lowering agent such as trientine enhanced hCtr1-mediated platinum uptake. Therefore, we conducted a pilot study (NCT01178112) of carboplatin and trientine with the goal of resensitizing patients with advanced cancer to platinum chemotherapy. This case report reviews the outcomes of 5 patients with platinum-resistant high-grade epithelial ovarian cancer enrolled on the study to date. Overall, they tolerated treatment well. Severe adverse events that occurred in 2 patients were myelosuppression, notably anemia requiring transfusion. Dose-limiting toxicity was not observed within the first 28 days (cycle 1). After 2 cycles of therapy, partial remission was achieved in 1 patient (10+ months), stable disease in 3 patients (2, 3.5+, and 5 months, respectively), and 1 patient had progressive disease. These cases provide preliminary clinical evidence that the role of decreasing copper levels in reversing platinum resistance merits additional clinical investigation. Evaluation of this novel strategy is warranted in larger studies to assess the efficacy of this approach for treating platinum-resistant advanced epithelial ovarian cancer in patients with high copper levels.
Introduction
The standard first-line treatment for advanced epithelial ovarian cancer is cytoreductive surgery, followed by adjuvant therapy with a platinum-based regimen. Despite a salutary initial response, many patients relapse and eventually succumb to their disease, following development of drug resistance. Among the mechanisms that mediate platinum resistance (1, 2), elevated copper level–induced downregulation of the major copper influx transporter human copper transporter 1 (hCtr1) plays a major role (3). Recent discoveries revealed that hCtr1 regulates intracellular copper homeostasis, which, in turn, controls hCtr1 expression via a homeostatic feedback loop (4). Copper-lowering agents increased the expression of hCtr1, subsequently resensitizing tumor cells to platinum therapy (5). Here, we report preliminary evidence that a copper-lowering agent may be able to, at least partially, reverse platinum resistance in patients with platinum-resistant high-grade epithelial ovarian cancer.
Materials and Methods
To test the hypothesis that resistance to platinum therapy can be reversed through the use of a copper-lowering agent, we are conducting a pilot study (NCT01178112) at MD Anderson Cancer Center (6), in which carboplatin is combined with trientine [triethylenetetramine: N,N’-bis (2-aminoethyl)ethane-1,2-diamine; Fig. 1], a copper-lowering agent (5), to treat patients with advanced malignancies. Five patients enrolled to date with a histologically proven diagnosis of platinum-resistant high-grade epithelial ovarian cancer are reviewed (Table 1). Platinum resistance was defined as radiographic disease progression within 6 months of completion of a platinum-based regimen. After giving informed consent, patients received the study treatment [i.v. carboplatin area under the concentration curve (AUC 4; dose level 1) or AUC 6 (dose level 3) once every 4 weeks, plus oral trientine 500 mg 4 times a day (2 times with meals, and 2 times without meals) initially, with dose adjustment to maintain serum ceruloplasmin levels at 5–15 mg/dL]. Tumor responses were evaluated using Response Evaluation Criteria in Solid Tumors version 1.1 (7), and toxicity was assessed using Common Terminology Criteria for Adverse Events version 4.0 (8). Serum ceruloplasmin and copper levels were monitored periodically (weekly initially, and then less frequently as appropriate). This study was conducted in accordance with MD Anderson Institutional Review Board guidelines.
Figure 1.
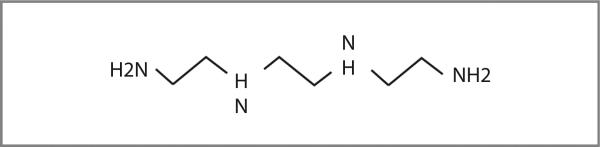
Trientine structure.
Table 1.
Tumor responses and changes in serum ceruloplasmin and copper levels
| Predose |
After 1 cycle |
After 2 cycles |
|||||
|---|---|---|---|---|---|---|---|
| Patient | Tumor response after 2 cycles | Ceruloplasmin (mg/dL) | Copper (μg/mL) | Ceruloplasmin (mg/dL) | Copper (μg/mL) | Ceruloplasmin (mg/dL) | Copper (μg/mL) |
| 1 | +14% | 39.1 | 2.01 | 32.9 (16%) | 1.45 (28%) | 24.9 (36%) | 1.11 (45%) |
| 2 | –35% | 34 | 1.71 | 23.4 (31%) | 1.00 (42%) | 15.9 (53%) | 0.56 (67%) |
| 3 | –10% | 26 | 1.02 | 20.5 (21%) | 0.67 (34%) | 16.9 (35%) | 0.44 (57%) |
| 4 | +120% | 42.2 | 1.96 | 39.8 (6%) | 1.62 (17%) | 40.6 (4%) | 1.73 (12%) |
| 5 | –2% | 31 | 1.57 | 26.8 (14%) | 1.21 (23%) | 26.1 (16%) | 1.11 (29%) |
NOTE: The values in parentheses indicate percentage reductions compared with baseline levels.
Case Reports
Patient 1
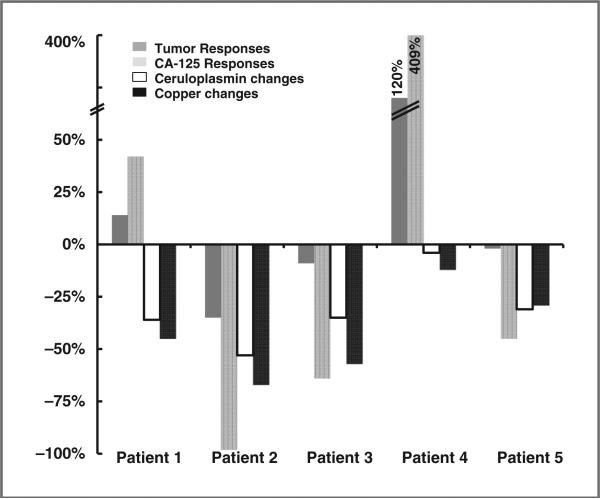
A 69-year-old white woman, whose status was postcytoreductive surgery for stage IIIC high-grade serous ovarian cancer in November 2004, received 5 lines of systemic therapy: paclitaxel plus carboplatin (becoming platinum resistant in 5 months), letrozole, topotecan, liposomal doxorubicin, and bevacizumab plus temsirolimus. In July 2010, she was enrolled at dose level 1. After 2 cycles of therapy, the patient was removed from the study for grade 3 hyperbilirubilemia caused by tumor-related intrahepatic biliary duct obstruction. The patient's tumor bulk increased by 14%, and the tumor marker CA-125 increased by 42%, whereas her serum ceruloplasmin and copper levels decreased slightly, as shown in Table 1 and Fig. 2.
Figure 2.
Changes in tumor sizes, CA-125, serum ceruloplasmin, and copper levels in 5 patients with platinum-resistant high-grade epithelial ovarian cancer who received 2 cycles of therapy with trientine and carboplatin. All patients received carboplatin at AUC 6, except for patient 1 who received carboplatin at AUC 4. Patient 5 had a BRCA1 mutation.
Patient 2
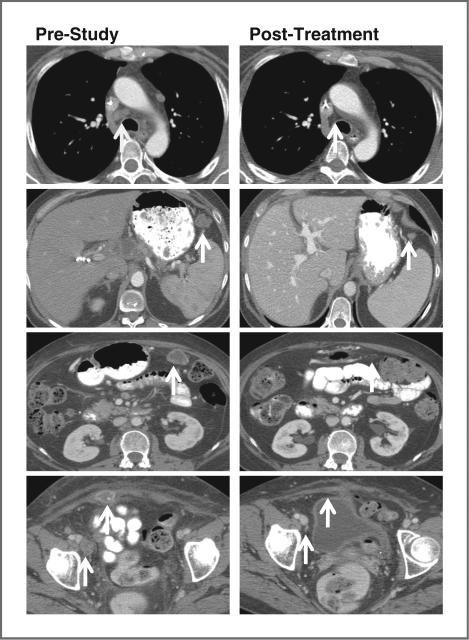
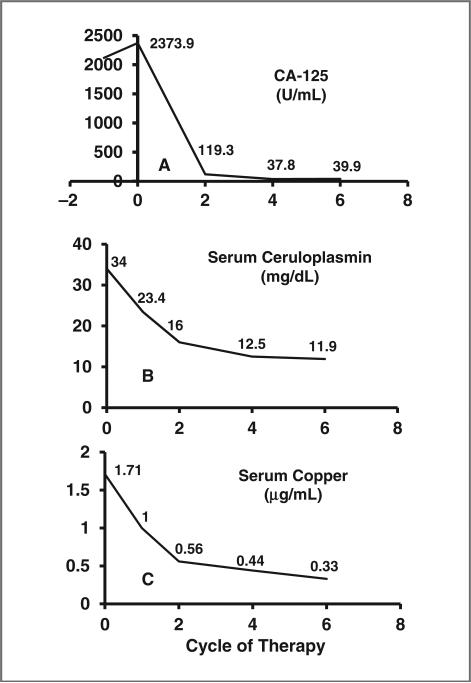
A 55-year-old white woman with a 6-year history of high-grade serous ovarian cancer initially underwent cytoreductive surgery and subsequently received 11 lines of systemic therapy: paclitaxel plus carboplatin, topotecan, liposomal doxorubicin, gemcitabine, etoposide, paclitaxel plus carboplatin (showing platinum resistance in 6 weeks), docetaxel, bevacizumab plus cyclophosphamide, paclitaxel, letrozole, and docetaxel plus sirolimus. In January 2011, the patient enrolled at dose level 3. She experienced grade 3 thrombocytopenia and anemia, and grade 2 fatigue, which improved significantly after carboplatin was decreased to AUC 5, 2 cycles later. Her overall tumor burden was reduced by 35%, 59%, and 70% after 2, 4, and 6 cycles, respectively, as shown in Fig. 3. The patient's CA-125 level decreased by 98% (Fig. 4A). After 10+ months, the patient continues on the study uneventfully. Her ceruloplasmin copper levels decreased significantly (Figs. 4B and 4C).
Figure 3.
Tumor responses in patient 2 in 2 panels of computed tomography (CT) scans of chest, abdomen, and pelvis. Tumor resolution of mediastinal lymphadenopathy and reduction of abdominal wall mass, peritoneal implants, and right inguinal lymphadenopathy are indicated by white arrows. The left panels represent CT scans prior to study enrollment, and the right panels are of corresponding sites after 6 cycles of therapy with carboplatin plus trientine, respectively.
Figure 4.
Changes in the levels of tumor marker CA-125, serum ceruloplasmin, and copper after treatment with carboplatin plus trientine in patient 2 in A, B, and C, respectively. Decreases in the CA-125 tumor marker (A) were associated with decreases in serum ceruloplasmin levels (B), and serum copper levels (C).
Patient 3
A 60-year-old African American woman, whose status was postcytoreductive surgery for stage IIIC high-grade mixed epithelial ovarian cancer in February 2005, received 11 lines of systemic therapy: carboplatin and interferon plus filgrastim, carboplatin and gemcitabine (becoming platinum resistant in 6 weeks), letrozole, liposomal doxorubicin, bevacizumab and cyclophosphamide, topotecan and bevacizumab, topotecan, paclitaxel, docetaxel, tamoxifen, a c-MET inhibitor, and palliative pelvic radiation for vaginal bleeding. In May 2011, the patient was enrolled at dose level 3. She experienced grade 4 newly diagnosed acquired sideroblastic anemia, which required transfusion. The patient achieved a 10% tumor reduction lasting 5 months, associated with reduced serum ceruloplasmin and copper levels (Fig. 2; Table 1). The tumor marker CA-125 decreased by 64%. The patient was removed from the study for vaginal bleeding.
Patient 4
A 57-year-old white woman underwent cytoreductive surgery for stage IIIC high-grade endometrioid ovarian cancer 4 years ago. She subsequently received 5 lines of systemic therapy: paclitaxel and carboplatin plus bevacizumab, liposomal doxorubicin plus carboplatin (becoming platinum resistant in 4 weeks), liposomal doxorubicin, docetaxel plus vandetanib, and an AKT inhibitor plus a mitogen-activated protein–extracellular signal-regulated kinase kinase inhibitor before she was enrolled at dose level 3 in June 2011. No grade 2 or higher treatment-related adverse events occurred. After 2 months, she was removed from the study for tumor progression by 120%. Her CA-125 increased by 409%, and her ceruloplasmin and copper levels decreased slightly.
Patient 5
A 49-year-old Asian woman with a known BRCA-1 mutation underwent cytoreductive surgery for stage IIIC high-grade serous ovarian cancer in September 2006, and subsequently received 5 lines of systemic therapy: paclitaxel plus carboplatin (becoming platinum resistant in 6 months), topotecan, liposomal doxorubicin, bevacizumab plus gemcitabine, and bevacizumab plus cyclophosphamide. In July 2010, she was enrolled at dose level 3. The patient had stable disease for 3.5+ months with moderately reduced serum ceruloplasmin and copper levels. Her tumor marker CA-125 decreased by 45%.
Discussion
Decreased platinum uptake due to excessive copper levels may serve as a critical step in the development of platinum resistance (4, 9, 10). Elevated copper levels in cancer cells and higher serum ceruloplasmin and copper levels have been observed in patients with advanced epithelial ovarian cancer (11). Low levels of hCtr1 mRNA have been associated with a shorter progression-free survival after platinum-based therapy (12).
Prolonged use of a copper-lowering agent to keep serum ceruloplasmin levels between 5 and 15 mg/dL did not cause clinical toxicity, because critical copper-dependent cellular processes were not affected (4). Treatment with copper-lowering agents alone in patients with advanced cancer produced no tumor responses (13–17). One study with tetrathiomolybdate, another copper-lowering agent, plus irinotecan, fluorouracil, and leucovorin showed a 25% response rate in patients with metastatic colorectal cancer (18). However, no clinical trial has yet been reported using a copper-lowering agent plus a platinum agent in patients with advanced cancer.
Salvage therapy in platinum-resistant patients generally produces low response rates (less than 10%), and durable responses are rare (19, 20). Clearly, a novel strategy is needed to improve treatment of platinum-resistant ovarian cancer. Here, we report 5 patients with platinum-resistant high-grade epithelial ovarian cancer who were treated with carboplatin plus trientine. Our preliminary clinical findings support the hypothesis that decreased copper levels in patients with platinum-resistant epithelial ovarian cancer may resensitize cancer cells to carboplatin therapy. The patients whose copper levels, after 1 and 2 cycles of therapy with trientine, were decreased the most had the greatest reduction in tumor bulk, as seen in patients 1, 2, and 3. In contrast, the fourth patient did not respond to carboplatin, and the concentration of copper in her body, as reflected by levels of surrogate bio-markers (serum ceruloplasmin and copper levels), did not change significantly after treatment with trientine. It is of great interest that patient 5, who had a BRCA1 mutation, achieved a minor tumor response, suggesting that even mildly increased platinum uptake leads to increased antitumor activity, because BRCA1 mutated cancer is sensitive to platinum agents (21).
The major limitation of the study is the small number of patients, which abrogates drawing definitive conclusions. Other mechanisms possibly at play include antiangiogenesis. Another limitation is that, rather than being caused by decreased copper levels, the decreases observed in serum ceruloplasmin and copper levels might be a predictive prognostic marker, because patients who achieve rapid decreases in these levels after trientine treatment are most likely to respond to chemotherapy. Another possible mechanism accounting for our observations could be that decreased serum ceruloplasmin and copper levels in response to trientine treatment reflect the efficacy of chemotherapy in responding patients due to posttherapy-improved acute phase reactions.
To the best of our knowledge, this report provides first-in-human preliminary data showing that at least partial resensitization of cancer cells to platinum therapy may be achieved through the use of a copper-lowering agent. These interesting preliminary clinical findings suggest that a larger study using a copper-lowering agent in combination with a platinum-based regimen is warranted for evaluation in treating patients with platinum-resistant epithelial ovarian cancer with high copper levels.
Acknowledgments
The authors thank Thuan Nguyen and Adrienne Howard in the Department of Investigational Cancer Therapeutics at MD Anderson Cancer Center for coordinating this clinical trial and Joann Aaron in the Department of Investigational Cancer Therapeutics at MD Anderson Cancer Center for editing this manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest
R. Kurzrock received a commercial research grant and honoraria from Merck. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Matsuo K, Lin YG, Roman LD, Sood AK. Overcoming platinum resistance in ovarian carcinoma. Expert Opin Investig Drugs. 2010;19:1339–54. doi: 10.1517/13543784.2010.515585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu S, Hu W, Iyer R, Kavanagh JJ, Coleman RL, Levenback CF, et al. Phase 1b-2a study to reverse platinum resistance through use of a hypomethylating agent, azacitidine, in patients with platinum-resistant or platinum-refractory epithelial ovarian cancer. Cancer. 2011;117:1661–9. doi: 10.1002/cncr.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuo MT, Chen HH, Song IS, Savaraj N, Ishikawa T. The roles of copper transporters in cisplatin resistance. Cancer Metastasis Rev. 2007;26:71–83. doi: 10.1007/s10555-007-9045-3. [DOI] [PubMed] [Google Scholar]
- 4.Howell SB, Safaei R, Larson CA, Sailor MJ. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol Pharmacol. 2010;77:887–94. doi: 10.1124/mol.109.063172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu J. Triethylenetetramine pharmacology and its clinical applications. Mol Cancer Ther. 2010;9:2458–67. doi: 10.1158/1535-7163.MCT-10-0523. [DOI] [PubMed] [Google Scholar]
- 6.ClinicalTrials.gov. U.S. NIH; Bethesda (MD): [2012 Mar]. Available from: http://clinicaltrials.gov/ct2/show/NCT01178112. [Google Scholar]
- 7.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute . Common Terminology Criteria for Adverse Events v.4.0 (CTCAE) U.S. NCI; Bethesda (MD): [2012 Mar 24]. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40. [Google Scholar]
- 9.Chen HH, Song IS, Hossain A, Choi MK, Yamane Y, Liang ZD, et al. Elevated glutathione levels confer cellular sensitization to cisplatin toxicity by up-regulation of copper transporter hCtr1. Mol Pharmacol. 2008;74:697–704. doi: 10.1124/mol.108.047969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang ZD, Stockton D, Savaraj N, Tien Kuo M. Mechanistic comparison of human high-affinity copper transporter 1-mediated transport between copper ion and cisplatin. Mol Pharmacol. 2009;76:843–53. doi: 10.1124/mol.109.056416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan A, Wong F, Arumanayagam M. Serum ultrafiltrable copper, total copper and caeruloplasmin concentrations in gynaecological carcinomas. Ann Clin Biochem. 1993;30:545–9. doi: 10.1177/000456329303000603. [DOI] [PubMed] [Google Scholar]
- 12.Lee YY, Choi CH, Do IG, Song SY, Lee W, Park HS, et al. Prognostic value of the copper transporters, CTR1 and CTR2, in patients with ovarian carcinoma receiving platinum-based chemotherapy. Gynecol Oncol. 2011;122:361–5. doi: 10.1016/j.ygyno.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Henry NL, Dunn R, Merjaver S, Pan Q, Pienta KJ, Brewer G, et al. Phase II trial of copper depletion with tetrathiomolybdate as an antiangiogenesis strategy in patients with hormone-refractory prostate cancer. Oncology. 2006;71:168–75. doi: 10.1159/000106066. [DOI] [PubMed] [Google Scholar]
- 14.Lowndes SA, Adams A, Timms A, Fisher N, Smythe J, Watt SM, et al. Phase I study of copper-binding agent ATN-224 in patients with advanced solid tumors. Clin Cancer Res. 2008;14:7526–34. doi: 10.1158/1078-0432.CCR-08-0315. [DOI] [PubMed] [Google Scholar]
- 15.Pass HI, Brewer GJ, Dick R, Carbone M, Merajver S. A phase II trial of tetrathiomolybdate after surgery for malignant mesothelioma: final results. Ann Thorac Surg. 2008;86:383–9. doi: 10.1016/j.athoracsur.2008.03.016. discussion 390. [DOI] [PubMed] [Google Scholar]
- 16.Redman BG, Esper P, Pan Q, Dunn RL, Hussain HK, Chenevert T, et al. Phase II trial of tetrathiomolybdate in patients with advanced kidney cancer. Clin Cancer Res. 2003;9:1666–72. [PubMed] [Google Scholar]
- 17.Brewer GJ, Dick RD, Grover DK, LeClaire V, Tseng M, Wicha M, et al. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study. Clin Cancer Res. 2000;6:1–10. [PubMed] [Google Scholar]
- 18.Gartner EM, Griffith KA, Pan Q, Brewer GJ, Henja GF, Merajver SD, et al. A pilot trial of the anti-angiogenic copper lowering agent tetrathiomolybdate in combination with irinotecan, 5-flurouracil, and leucovorin for metastatic colorectal cancer. Invest New Drugs. 2009;27:159–65. doi: 10.1007/s10637-008-9165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kavanagh J, Tresukosol D, Edwards C, Freedman R, Gonzalez de Leon C, Fishman A, et al. Carboplatin reinduction after taxane in patients with platinum-refractory epithelial ovarian cancer. J Clin Oncol. 1995;13:1584–8. doi: 10.1200/JCO.1995.13.7.1584. [DOI] [PubMed] [Google Scholar]
- 20.Leitao MM, Jr, Hummer A, Dizon DS, Aghajanian C, Hensley M, Sabbatini P, et al. Platinum retreatment of platinum-resistant ovarian cancer after nonplatinum therapy. Gynecol Oncol. 2003;91:123–9. doi: 10.1016/s0090-8258(03)00464-5. [DOI] [PubMed] [Google Scholar]
- 21.Chirnomas D, Taniguchi T, de la Vega M, Vaidya AP, Vasserman M, Hartman AR, et al. Chemosensitization to cisplatin by inhibitors of the Fanconi anemia/BRCA pathway. Mol Cancer Ther. 2006;5:952–61. doi: 10.1158/1535-7163.MCT-05-0493. [DOI] [PubMed] [Google Scholar]