Abstract
The sterol regulatory element-binding proteins (SREBPs) play an important role in regulating lipid homeostasis. Translated as inactive precursors that are localized in the endoplasmic reticulum (ER) membrane, SREBPs are activated through a proteolytic process in response to intracellular demands for lipids. The cleaved amino-terminal fragments of SREBPs then translocate into the nucleus as homodimers and stimulate the transcription of target genes by binding to the sterol response elements (SREs) in their promoters. Numerous studies using cell culture or genetically modified mouse models have demonstrated that the major target genes of SREBPs include rate-limiting enzymes in the pathways of fatty acid and cholesterol biosynthesis as well as the low-density lipoprotein (LDL) receptor. The proteolytic maturation of SREBPs has been well studied in the past. However, recent studies have also improved our understanding on the regulation of nuclear SREBPs. In the nucleus, SREBPs interact with specific transcriptional cofactors, such as CBP/p300 and the Mediator complex, resulting in stimulation or inhibition of their transcriptional activities. In addition, nuclear SREBP protein stability is dynamically regulated by phosphorylation and acetylation. Such protein-protein interactions and post-translational modifications elegantly link the extracellular signals, such as insulin, or intracellular signals, such as oxidative stress, to lipid biosynthesis by modulating the transcriptional activity of SREBPs. Under normal physiological states, lipid homeostasis is strictly maintained. However, the SREBP pathways are often dysregulated in pathophysiological conditions, such as obesity, type 2 diabetes, and fatty liver diseases. Thus, the novel regulatory mechanisms of SREBPs may provide new opportunities for fighting these metabolic diseases.
Keywords: SREBP, Transcription, Cofactor, Lipid metabolism, Mediator complex, Acetylation, Phosphorylation, Oxidative stress
Introduction
Obesity is a worldwide health problem now. The prevalence of obesity in modern society is closely correlated with the increased incidence of major human diseases, including metabolic syndrome, type 2 diabetes, nonalcoholic fatty liver disease, atherosclerotic heart disease, and certain types of cancer[1,2]. Data from the US Center for Disease Control and Prevention indicate that such epidemic has constituted the leading cause of death and a major health burden in the United States. Similar health problems are also prevalent in other developed countries, and are starting to accumulate in the rising economic powers such as China. Over-nutrition and sedentary life-styles are likely the major reasons for the increased prevalence of obesity. Although the molecular mechanisms of how obesity causes its associated diseases are still poorly defined, one common feature of these diseases is dysregulation of lipid homeostasis[3]. Numerous studies have shown that dysregulation of lipid homeostasis is closely associated with type 2 diabetes, and cardiovascular disease [1,3–5]. However, the physiologic and patho-physiologic regulation of lipid homeostasis is a highly complex process that is not fully understood at the molecular level. Among the known lipogenic regulators, the SREBP transcription factors are pivotal activators of key enzymes responsible for biosynthesis of fatty acids and cholesterol[6–9] and play an important role in the development of those metabolic diseases[10,11].
In mammals, there are three SREBP proteins, SREBP-1a, -1c and -2, encoded by two genes, srebf1 and srebf2. The target genes of SREBPs include the rate-limiting lipogenic and cholesterogenic genes, such as fatty acid synthase, HMG-CoA reductase and the LDL receptor[12,13]. Thus, SREBP activation promotes fatty acid and cholesterol biosynthesis, and cholesterol uptake. The function of SREBPs on fatty acid biosynthesis is highly conserved, although many lower organisms, such as Drosophila and C. elegans, have only one SREBP ortholog and do not synthesize cholesterol. Activation of SREBPs is tightly regulated at multiple layers, including 1) transcription; 2) precursor maturation; 3) nuclear protein stability; and 4) transcriptional activity. For example, SREBP-1c, which is the predominant form of srebf1 in hepatocytes, is activated upon food intake at the transcriptional level, primarily by insulin[14]. All SREBPs are synthesized as inactive precursors that are tethered to the ER membrane[12]. Reduction of intracellular sterols stimulates the transportation of SREBP-2 to the Golgi where it undergoes proteolytic processing. Then, the N-terminal portion of SREBP-2 translocates into the nucleus and activates transcription of target genes[15]. Similarly, the two SREBP-1 isoforms, SREBP-1a and -1c, are also processed in the Golgi to generate the nuclear forms of SREBP-1 proteins [16,17]. Unlike SREBP-2, SREBP-1c maturation is primarily activated by insulin[14]. In the nucleus, the protein stability of mature SREBPs is regulated mainly by phosphorylation and acetylation in the highly conserved domains. Furthermore, through recruiting specific transcriptional cofactors, including CBP/p300 and the Mediator complex, the transcriptional activity of SREBPs can be either stimulated or repressed.
The proteolytic process of SREBP maturation has been extensively investigated in the past. Those studies have uncovered a negative feedback mechanism of the SREBP maturation process. However, the regulation of nuclear SREBPs is less understood. Recent studies have revealed protein-protein interactions and post-translational modifications in regulating nuclear SREBPs. The new regulatory mechanisms have also improved our understanding of how extracellular and intracellular signals control the abundance and activity of SREBPs in the nucleus. Here, we summarize some of the recent advances on nuclear SREBP regulation.
SREBP Transcription Factors
SREBPs are members of the basic helix-loop-helix leucine zipper (bHLH-Zip) family of transcription factors[11]. Among the three mammalian SREBP isoforms, both SREBP-1a and -1c proteins are produced from the same gene, srebf1, by two distinct promoters and alternative splicing[18]. Their amino acid sequences differ only at the N-terminus, in which SREBP-1c contains three unique amino acids but lacks 27 others that are only present in SREBP-1a. The unique region of SREBP-1a is part of its trans-activation domain, and thus SREBP-1a displays a stronger transcriptional activity[19]. SREBP-1a and -1c also have different expression profiles: SREBP-1a is highly expressed in proliferating cells, such as cancer cells, while SREBP-1c is the predominant form in normal cells, particularly hepatocytes[20]. SREBP-2 is transcribed from srebf2 gene. The target genes of the three SREBP isoforms are distinct. SREBP-1 mainly activates genes encoding enzymes required in fatty acid and triglyceride biosynthesis, whereas SREBP-2 target genes are involved in the cholesterol pathway.
SREBPs are translated as inactive precursors and retained in the ER membrane via association with SREBP cleavage activating protein (SACP), which contains a sterol-sensing domain. In the presence of sterol, the SREBP-SCAP complex interacts with Insigs (insulin-induced protein -1 and -2) to inhibit further modifications of SREBPs. Upon decrease of intracellular sterol levels, Insigs are released from the SREBP-SCAP complex, and degraded by the proteasome system. SCAP escorts SREBPs to the Golgi apparatus with the assistance of COPII vesicles, where SREBPs undergo a two-step photolytic cleavage by site-1 protease (S1P) and site-2 protease (S2P). Upon cleavage, the amino-terminal fragments of SREBPs translocate to the nucleus as ho-modimers and bind to SREs within the promoters of target genes.
Lipids in the form of triglycerides are normally stored in adipose tissues. Accumulation of lipids in non-adipose tissues, such as pancreas, skeletal muscle, and liver, is often associated with type 2 diabetes and its complications[21]. As a key and conserved activator of fatty acid biosynthesis, SREBP-1c has been implicated in type 2 diabetes[11]. Genetically, single nucleotide polymorphisms and other sequence variations of the SREBP-1 gene are linked to type 2 diabetes in human beings[22–24]. In addition, SREBP-1c expression is correlated to insulin resistance in morbid obesity[25]. In pancreatic islets, increased expression of SREBP-1c correlated with the development of diabetes in Zucker obese fa/fa rats, a widely used model of diabetes[26]. Moreover, it has been shown that SREBP-1c levels are elevated in the livers of several animal models of insulin resistance including Zucker obese fa/fa rats[26], ob/ob mice[27], insulin receptor substrate-2 knockout mice[28], and high-fat diet-induced obese mice[29]. These observations suggest that hepatic SREBP-1c plays a role in the development of insulin resistance.
SREBP transcription factors are also involved in other diseases. In particular, increased expression and genetic polymorphisms of SREBP genes have been linked to cardiovascular disease, consistent with the role of hypertriglyceridemia and hypercholesterolemia as important risk factors[25,30–32]. In addition, SREBP-1c participates in the hepatic steatosis or fatty liver observed in humans related to alcohol consumption[33]. Besides their roles in metabolic diseases, SREBPs also play important roles in cancer. For example, SREBP activation mediates Akt-dependent cell growth[34], and SREBP-1a, which can activate both fatty acid and cholesterol biosynthesis[36], is highly expressed in cancer cells[36,37]. As a result, the SREBP target gene fatty acid synthase is significantly up regulated in various types of cancers, and blocking fatty acid synthase gene expression or activity results in apoptosis of tumor cells[37].
The Mediator Complex
In eukaryotes, the transcription of protein-coding genes is controlled by specific transcription factors and the RNA polymerase II (Pol-II), together with general transcription factors (GTFs: TFIIA, TFIIB, TFIID, TFIIE, TFIIF and TFIIH)[38,39]. Research in the past two decades has demonstrated that transcriptional cofactors provide a critical layer of regulation on gene expression. One such cofactor is the highly conserved Mediator complex, which is recruited by specific transcription factors and coordinates the Pol-II recruitment, modification of the Pol-II-CTD tail, enhancer-promoter communication and post-initiation regulation[40].
The Mediator was initially purified from yeast as a multi-subunit complex required for Pol-II-dependent transcription in vitro[41–43]. Similar complexes were later purified from mammalian cells as key cofactors for transcription factors, including SREBPs and nuclear hormone receptors (NRs)[44–46]. To date, there are more than 30 distinct subunits of the Mediator complexes identified in mammals and most of them are conserved among various species. Biochemical analyses have identified at least two distinct Mediator complexes: a small one consisting of ~20 peptides that stimulates transcription of Pol-II-dependent genes in vitro, and a large one with four additional subunits that are cyclin-dependent kinase 8 (CDK8), Cyclin C (CycC), MED12, and MED13[47]. The CDK8-containing large Mediator complex generally represses transcription in vitro [48–52]. Since the Mediator interacts with Pol-II, it is believed that the Mediator complexes regulate most of Pol-II-dependent gene expression. However, accumulating data support a model that, besides some core subunits, most subunits are only involved in regulating specific pathways[40]. This is largely because specific transcription factors can only interact with specific subunits in addition to the availability of those transcription factors in the nucleus. For example, the MED1 subunit physically interacts with NRs, such as thyroid hormone receptors, peroxisome proliferator-activated receptors and estrogen receptors, through a LXXLL motif in MED1 [53]. Functionally, loss of MED1 results in deficiency of the NR-mediated biological functions. Thus, MED1 specifically controls the NR pathways.
Besides the roles in transcriptional initiation, recent studies also show that the Mediator complexes play a role in transcriptional elongation. The CDK8 subunit has been shown to activate transcriptional elongation of serum response factor-target genes in cancer cells[54]. Recently, MED26 has been reported to recruit the transcriptional elongation complex and thus regulates transcriptional elongation[55].
Regulation of SREBP Functions by MED15
The MED15 subunit of the Mediator (previously named as ARC105 or PCQAP) is highly conserved from yeast (Gal11 as the ortholog of MED15) to mammals. Accumulating evidences indicate that MED15 plays an important role in regulating lipid metabolism [56,57] and multi-drug resistance[58]. MED15 was identified as an interacting protein of the trans-activation domains of SREBPs[56]. Further biochemical analysis mapped the interaction domain at the N-terminus of MED15, which has a structure similar to the KIX domains in CBP/p300. Through the interaction between the SREBP trans-activation domains and the MED15-KIX domain, SREBPs recruit the Mediator complex to activate the target gene transcription. The MED15-KIX domain is highly selective in associating with transcription factors. Unlike the CBP-KIX domain, which interacts several transcription factors including SREBPs, c-Myb and CREB, the MED15-KIX domain only interacts with SREBPs[56]. Functionally, loss of MED15 resulted in a decrease in SREBP-target gene expression in mammalian cells and in C. elegans. Multiple lines of evidence suggest that the major target genes of MED15 ortholog MDT-15 in C. elegans are stearoyl-CoA desaturases, which are coregulated by SBP-1 (the worm ortholog of SREBP)[56] and the worm nuclear receptor NHR-49[57]. As a result, MDT-15 knockdown in C. elegans decreased oleate production, which subsequently led to a decrease of lipid accumulation, body size and physical activity. These phenotypes were highly similar to SBP-1 knockdown. Moreover, supplementation with oleic acid rescues most of the phenotypes caused by MDT-15 knockdown. In addition, MDT-15 is the only mediator subunit that causes a decrease in lipid accumulation in C. elegans when knocked down. However, the patho-physiological significance of MED 15 regulation on SREBPs is unclear and the in vivo functions of MED15 in mammals remain to be investigated.
Regulation of Nuclear SREBP Protein Stability by GSK-3β and CDK8
It has been shown that phosphorylation of nuclear SREBPs within a conserved domain controls the protein stability[59,60]. GSK-3β, which functions downstream of the insulin signaling, was reported to be responsible for the phosphorylation of SREBPs at several conserved sites, including Threonine-426 of SREBP-1a (corresponding to Threonine-402 in SREBP-1c) [59,60]. Phosphorylation of SREBP-1a at the Threonine-426 residue has been shown to facilitate its binding to the E3 ligase, SCFFbm7b, thus controlling its ubiquitination and subsequent degradation[59].
Recently, we have identified a novel and highly conserved role for the CDK8 subunit of the Mediator complexes and its activator CycC in the control of nuclear SREBP protein stability (Zhao et al. unpublished data). Biochemical analyses reveal that SREBP-1c can be directly phosphorylated by CDK8 at the conserved Threonine-402 residue in vitro and in cultured mammalian cells. As a result, CDK8 promotes ubiquitination and subsequent degradation of nuclear SREBP-1c without affecting the level of precursor SREBP-1c. It is not uncommon that the same threonine or serine residue of a protein can be phosphorylated by multiple kinases. However, it is currently unclear whether CDK8 crosstalks with GSK-3β, and whether CDK8 regulation of SREBPs depends on the mediator complexes.
Consistent with the function of CDK8 on nuclear SREBP protein stability, loss of CDK8 or CycC increases SREBP-dependent lipid accumulation and SREBP-target gene expression in both Drosophila and mammalian cells. Moreover, knockdown of CDK8 in mouse liver in vivo caused a fatty liver-like phenotype along with increased SREBP-target gene expression and de novo lipogenesis. Interestingly, CDK8 and CycC protein levels are negatively regulated by food intake in mouse liver and by insulin in hepatocyte cultures. Thus, a simple model is proposed: insulin stimulates de novo lipogenesis by down-regulating CDK8 and CycC proteins, which normally inhibits de novo lipogenesis through promoting nuclear SREBP-1c degradation. This new mechanism of insulin-induced lipogenesis is in addition to the well- documented function of insulin in stimulating SREBP-1c at the mRNA level. The recent findings establish a physiological role of CDK8 in regulating fatty acid biosynthesis.
Regulation of SREBPs by Acetylation and Deacetylation
SREBPs are known to interact with CBP/p300, which are histone acetyltransferases. Such interaction not only facilitates the chromatin accessibility of SREBPs at the promoters of target genes by acetylating histone tails, but also causes acetylation at the conserved lysine residues of SREBPs [61]. Acetylation at the Lysine-324 and Lysine-333 residues of SREBP-1a, which located in the DNA-binding domain, inhibits its ubiquitination and thus proteasome-mediated degradation[61]. Therefore, CBP/p300 regulate SREBPs by increasing both their transcriptional activity and nuclear protein stability.
The SIRT1 member of the class III NAD+-dependent family of protein deacetylases[62] not only remove acetyl groups from histone tails, but can also deacetylate transcription factors such as p53, NF-κB, and E2F1 [63–65]. SIRT1 is highly conserved. Importantly, SIRT1 protein levels are elevated in livers of fasted mice, and decreased in re-feeding state[66]. In mammals, SREBPs are highly abundant and active in liver in the fed state to promote the expression of lipogenic and cholesterogenic genes. During fasting, nuclear SREBP proteins are lower and SREBP-dependent lipid biosynthesis is rapidly diminished in mouse liver[67]. This inverse relationship between SREBP and SIRT1 levels in response to food availability suggests a link between SIRT1 and nuclear SREBP functions. In fact, SIRT1 can directly deacetylate SREBPs, and modulation of SIRT1 activity results in changes in SREBP ubiquitination, protein stability, and target gene expression[67]. As a result, during fasting SIRT1 or its orthologs down-regulates SREBPs in mouse[67,68], Drosophila[67] and C. elegans[67], resulting in inhibition of lipid synthesis and fat storage. Consistent with the regulation of SIRT1 on SREBPs, knockdown or knockout of SIRT1 in mouse liver or transgenic over-expression of SIRT1 in mice result in altered triglyceride and cholesterol levels in serum and liver [66,69–72]. Thus, SIRT1 orthologs play a conserved role in regulating lipid homeostasis by inhibiting SREBP-dependent gene expression during fasting.
Regulation of SREBP Transcription Factors by Oxidative Stress
Oxidative stress is common in metabolic disorders such as obesity, diabetes, fatty liver diseases and cardiovascular disease[73]. Nutrient overload and aging cause mitochondrial dysfunction, resulting in overproduction of reactive oxygen species (ROS) and depletion of intracellular antioxidants[73,74]. Extracellular signals, such as inflammation and insulin, can also increase the production of ROS[75,76]. Consistent with the increased lipid accumulation in non-adipose tissues, such as liver, SREBPs are often dysregulated in metabolic diseases and during aging[11]. Thus, oxidative stress may regulate SREBPs. Indeed, it has been reported that oxidative stress increases the levels of nuclear SREBPs and lipid accumulation in cultured hepatocytes[77]. Interestingly, it has been shown that SIRT1 can be regulated by oxidative stress in mammalian cells[78], and the protein levels of CycC can be modulated by oxidative stress in yeast[79]. Thus, oxidative stress regulation on SIRT1 and CycC/CDK8 provides a molecular link between over-nutrition and nuclear SREBP protein levels.
Conclusions
The SREBP transcription factors are regulated at multiple levels. In addition to the well-known mechanism of SREBP maturation by proteolytic processing, the nuclear forms of SREBPs are also regulated through recruiting transcriptional cofactors, such as CBP/p300 and the Mediator complexes. By interacting with these cofactors, the transcriptional activity of nuclear SREBPs is modulated. Moreover, nuclear SREBP protein stability is regulated by post-translational modifications, including GSK-3β and CDK8-mediated phosphorylation, CBP/p300-mediated acetylation and SIRT1-mediated deacetylation (Fig. 1). Since the abundance of CDK8 and SIRT1 is regulated by environmental factors, such as nutrient levels, oxidative stress and aging, the post-translational modifications provides a molecular connection between extracellular signals and the nuclear SREBPs. In future studies, it would be interesting to explore how CDK8 and CycC are regulated in pathological states and whether nuclear SREBPs are regulated by additional mechanisms. Due to the pivotal roles of SREBPs in controlling lipid homeostasis, these novel regulators of SREBPs may provide new targets for fighting human diseases, such as obesity, diabetes, cardiovascular disease and fatty liver diseases.
Fig. 1. Regulation of nuclear SREBP transcription factors (nSREBP).
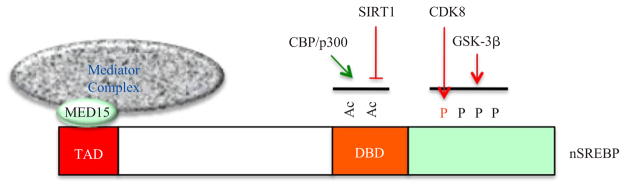
In the nucleus, through interacting with the KIX domains in CBP/p300 and the MED 15 subunit, the trans-activation domain (TAD) of SREBPs recruits cofactors CBP/p300 and the Mediator complex to control their transcriptional activity. CBP/p300 can also acetylate the lysine residues in the DNA-binding domain (DBD) of SREBPs and thus increases nSREBP stability by blocking ubiquitination. During fasting, nSREBP acetylation can be reversed by SIRT1-mediated deacetylation, resulting in nSREBP degradation. Phosphorylation by CDK8 and/or GSK-3β establishes a docking site for the E3 ligase of SREBPs, SCFFbw7b, and therefore stimulates the proteasome-dependent degradation of nSREBPs
 1
1
 SREBP
SREBP
 , SREBP
, SREBP
 TAD
TAD
 CBP/p300
CBP/p300
 MED15
MED15
 CBP/p300
CBP/p300
 SREBP-DBD
SREBP-DBD
 ,
,
 SREBP
SREBP
 , SIRT1
, SIRT1
 SREBP
SREBP
 ,
,
 SREBP
SREBP
 CDK8
CDK8
 GSK-3β
GSK-3β
 SREBP
SREBP
 E3
E3
 (SCFFbw7b)
(SCFFbw7b)
 ,
,
 SREBP
SREBP

Acknowledgments
This work was supported by grants from National Institutes of Health (DK093623), American Diabetes Association (7-11-BS-173), and Einstein Diabetes Research Center (P60-DK020541)
Dr. Fajun Yang thanks Professors Wenjuan Xin and Baolu Zhao for their mentorship and inspiration in his research career. We apologize to our colleagues for being unable to cite all appropriate references owing to space limitations.
References
- 1.Reaven GM. Insulin resistance: The link between obesity and cardiovascular disease. Med Clin North Am. 2011;95(5):875–892. doi: 10.1016/j.mcna.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Kaidar-Person O, Bar-Sela G, Person B. The two major epidemics of the twenty-first century: Obesity and cancer. Obes Surg. 2011;21(11):1792–1797. doi: 10.1007/s11695-011-0490-2. [DOI] [PubMed] [Google Scholar]
- 3.Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- 4.Van Herpen NA, Schrauwen-Hinderling VB. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav. 2008;94(2):231–241. doi: 10.1016/j.physbeh.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 5.Cheung O, Sanyal AJ. Abnormalities of lipid metabolism in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28(4):351–359. doi: 10.1055/s-0028-1091979. [DOI] [PubMed] [Google Scholar]
- 6.Shimomura I, Bashmakov Y, Ikemoto S, Horton JD, Brown MS, Goldstein JL. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc Natl Acad Sci USA. 1999;96 (24):13656–13661. doi: 10.1073/pnas.96.24.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimano H, Yahagi N, Amemiya-Kudo M, Hasty AH, Osuga J, Tamura Y, Shionoiri F, Iizuka Y, Ohashi K, Harada K, Gotoda T, Ishibashi S, Yamada N. Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J Biol Chem. 1999;274(50):35832–35839. doi: 10.1074/jbc.274.50.35832. [DOI] [PubMed] [Google Scholar]
- 8.Liang G, Yang J, Horton JD, Hammer RE, Goldstein JL, Brown MS. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1 c. J Biol Chem. 2002;277(11):9520–9528. doi: 10.1074/jbc.M111421200. [DOI] [PubMed] [Google Scholar]
- 9.Amemiya-Kudo M, Shimano H, Hasty AH, Yahagi N, Yoshikawa T, Matsuzaka T, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, Osuga J, Harada K, Gotoda T, Sato R, Kimura S, Ishibashi S, Yamada N. Transcriptional activities of nuclear SREBP-1a, -1c, and -2 to different target promoters of lipogenic and cholesterogenic genes. J Lipid Res. 2002;43(8):1220–1235. [PubMed] [Google Scholar]
- 10.Ferre P, Foufelle F. SREBP-1c transcription factor and lipid homeostasis: Clinical perspective. Horm Res. 2007;68(2):72–82. doi: 10.1159/000100426. [DOI] [PubMed] [Google Scholar]
- 11.Jeon TI, Osborne TF. SREBPs: Metabolic integrators in physiology and metabolism. Trends Endocrinol Metab. 2012;23(2):65–72. doi: 10.1016/j.tem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osborne TF, Espenshade PJ. Evolutionary conservation and adaptation in the mechanism that regulates SREBP action: What a long, strange tRIP it’s been. Genes Dev. 2009;23(22):2578–2591. doi: 10.1101/gad.1854309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eberle D, Hegarty B, Bossard P, Ferre P, Foufelle F. SREBP transcription factors: Master regulators of lipid homeostasis. Biochimie. 2004;86(11):839–848. doi: 10.1016/j.biochi.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Ferre P, Foufelle F. SREBP-1c transcription factor and lipid homeostasis: Clinical perspective. Horm Res. 2007;68(2):72–82. doi: 10.1159/000100426. [DOI] [PubMed] [Google Scholar]
- 15.Hua X, Yokoyama C, Wu J, Briggs MR, Brown MS, Goldstein JL, Wang X. SREBP-2, a second basic-helix-loop-helix-leucine zipper protein that stimulates transcription by binding to a sterol regulatory element. Proc Natl Acad Sci USA. 1993;90(24):11603–11607. doi: 10.1073/pnas.90.24.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoyama C, Wang X, Briggs MR, Admon A, Wu J, Hua X, Goldstein JL, Brown MS. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell. 1993;75(1):187–197. [PubMed] [Google Scholar]
- 17.Wang X, Sato R, Brown MS, Hua X, Goldstein JL. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell. 1994;77(1):53–62. doi: 10.1016/0092-8674(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 18.Hua X, Wu J, Goldstein JL, Brown MS, Hobbs HH. Structure of the human gene encoding sterol regulatory element binding protein-1 (SREBF1) and localization of SREBF1 and SREBF2 to chromosomes 17p11. 2 and 22q13. Genomics. 1995;25(3):667–673. doi: 10.1016/0888-7543(95)80009-b. [DOI] [PubMed] [Google Scholar]
- 19.Shimano H, Horton JD, Shimomura I, Hammer RE, Brown MS, Goldstein JL. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J Clin Invest. 1997;99(5):846–854. doi: 10.1172/JCI119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J Clin Invest. 1997;99(5):838–845. doi: 10.1172/JCI119247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verges B. New insight into the pathophysiology of lipid abnormalities in type 2 diabetes. Diabetes Metab. 2005;31(5):429–439. doi: 10.1016/s1262-3636(07)70213-6. [DOI] [PubMed] [Google Scholar]
- 22.Laudes M, Barroso I, Luan J, Soos MA, Yeo G, Meirhaeghe A, Logie L, Vidal-Puig A, Schafer AJ, Wareham NJ, O’Rahilly S. Genetic variants in human sterol regulatory element binding protein-1c in syndromes of severe insulin resistance and type 2 diabetes. Diabetes. 2004;53(3):842–846. doi: 10.2337/diabetes.53.3.842. [DOI] [PubMed] [Google Scholar]
- 23.Eberle D, Clement K, Meyre D, Sahbatou M, Vaxillaire M, Le Gall A, Ferre P, Basdevant A, Froguel P, Foufelle F. SREBF-1 gene polymorphisms are associated with obesity and type 2 diabetes in French obese and diabetic cohorts. Diabetes. 2004;53(8):2153–2157. doi: 10.2337/diabetes.53.8.2153. [DOI] [PubMed] [Google Scholar]
- 24.Felder TK, Oberkofler H, Weitgasser R, Mackevics V, Krempler F, Paulweber B, Patsch W. The SREBF-1 locus is associated with type 2 diabetes and plasma adiponectin levels in a middle-aged Austrian population. Int J Obes (Lond) 2007;31(7):1099–1103. doi: 10.1038/sj.ijo.0803505. [DOI] [PubMed] [Google Scholar]
- 25.Mingrone G, Rosa G, Greco AV, Manco M, Vega N, Nanni G, Castagneto M, Vidal H. Intramyocitic lipid accumulation and SREBP-1c expression are related to insulin resistance and cardiovascular risk in morbid obesity. Atherosclerosis. 2003;170(1):155–161. doi: 10.1016/s0021-9150(03)00254-5. [DOI] [PubMed] [Google Scholar]
- 26.Kakuma T, Lee Y, Higa M, Wang Z, Pan W, Shimomura I, Unger RH. Leptin, troglitazone, and the expression of sterol regulatory element binding proteins in liver and pancreatic islets. Proc Natl Acad Sci USA. 2000;97(15):8536–8541. doi: 10.1073/pnas.97.15.8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999;274(42):30028–30032. doi: 10.1074/jbc.274.42.30028. [DOI] [PubMed] [Google Scholar]
- 28.Tobe K, Suzuki R, Aoyama M, Yamauchi T, Kamon J, Kubota N, Terauchi Y, Matsui J, Akanuma Y, Kimura S, Tanaka J, Abe M, Ohsumi J, Nagai R, Kadowaki T. Increased expression of the sterol regulatory element-binding protein-1 gene in insulin receptor substrate-2(−/−) mouse liver. J Biol Chem. 2001;276(42):38337–38340. doi: 10.1074/jbc.C100160200. [DOI] [PubMed] [Google Scholar]
- 29.Deng QG, She H, Cheng JH, French SW, Koop DR, Xiong S, Tsukamoto H. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology. 2005;42(4):905–914. doi: 10.1002/hep.20877. [DOI] [PubMed] [Google Scholar]
- 30.Vedie B, Jeunemaitre X, Megnien JL, Atger V, Simon A, Moatti N. A new DNA polymorphism in the 5′ untranslated region of the human SREBP-1a is related to development of atherosclerosis in high cardiovascular risk population. Atherosclerosis. 2001;154(3):589–597. doi: 10.1016/s0021-9150(00)00569-4. [DOI] [PubMed] [Google Scholar]
- 31.Salek L, Lutucuta S, Ballantyne CM, Gotto AM, Jr, Marian AJ. Effects of SREBF-1a and SCAP polymorphisms on plasma levels of lipids, severity, progression and regression of coronary atherosclerosis and response to therapy with fluvastatin. J Mol Med. 2002;80(11):737–744. doi: 10.1007/s00109-002-0381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinet P, Vedie B, Chironi G, Gariepy J, Simon A, Moatti N, Paul JL. Characterization of polymorphic structure of SREBP-2 gene: Role in atherosclerosis. Atherosclerosis. 2003;168(2):381–387. doi: 10.1016/s0021-9150(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 33.You M, Fischer M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP) J Biol Chem. 2002;277(32):29342–29347. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 34.Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8(3):224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimano H, Horton JD, Shimomura I, Hammer RE, Brown MS, Goldstein JL. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J Clin Invest. 1997;99(5):846–854. doi: 10.1172/JCI119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J Clin Invest. 1997;99(5):838–845. doi: 10.1172/JCI119247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cance. 2007;7(10):763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 38.Woychik NA, Hampsey M. The RNA polymerase II machinery: Structure illuminates function. Cell. 2002;108(4):453–463. doi: 10.1016/s0092-8674(02)00646-3. [DOI] [PubMed] [Google Scholar]
- 39.Gill G. Regulation of the initiation of eukaryotic transcription. Essays Biochem. 2001;37:33–43. doi: 10.1042/bse0370033. [DOI] [PubMed] [Google Scholar]
- 40.Borggrefe T, Yue X. Interactions between subunits of the Mediator complex with gene-specific transcription factors. Semin Cell Dev Biol. 2011;22(7):759–768. doi: 10.1016/j.semcdb.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 41.Kelleher RJ, 3rd, Flanagan PM, Kornberg RD. A novel mediator between activator proteins and the RNA polymerase II transcription apparatus. Cell. 1990;61 (7):1209–1215. doi: 10.1016/0092-8674(90)90685-8. [DOI] [PubMed] [Google Scholar]
- 42.Flanagan PM, Kelleher RJ, 3rd, Sayre MH, Tschochner H, Kornberg RD. A mediator required for activation of RNA polymerase II transcription in vitro. Nature. 1991;350(6317):436–438. doi: 10.1038/350436a0. [DOI] [PubMed] [Google Scholar]
- 43.Kim YJ, Bjorklund S, Li Y, Sayre MH, Kornberg RD. A multiprotein mediator of transcriptional activation and its interaction with the C-terminal repeat domain of RNA polymerase II. Cell. 1994;77(4):599–608. doi: 10.1016/0092-8674(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 44.Naar AM, Beaurang PA, Zhou S, Abraham S, Solomon W, Tjian R. Composite co-activator ARC mediates chromatin-directed transcriptional activation. Nature. 1999;398(6730):828–832. doi: 10.1038/19789. [DOI] [PubMed] [Google Scholar]
- 45.Fondell JD, Ge H, Roeder RG. Ligand induction of a transcriptionally active thyroid hormone receptor coactivator complex. Proc Natl Acad Sci USA. 1996;93 (16):8329–8333. doi: 10.1073/pnas.93.16.8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rachez C, Lemon BD, Suldan Z, Bromleigh V, Gamble M, Naar AM, Erdjument-Bromage H, Tempst P, Freedman LP. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature. 1999;398(6730):824–828. doi: 10.1038/19783. [DOI] [PubMed] [Google Scholar]
- 47.Taatjes DJ, Naar AM, Andel F, 3rd, Nogales E, Tjian R. Structure, function, and activator-induced conformations of the CRSP coactivator. Science. 2002;295 (5557):1058–1062. doi: 10.1126/science.1065249. [DOI] [PubMed] [Google Scholar]
- 48.Mittler G, Kremmer E, Timmers HT, Meisterernst M. Novel critical role of a human mediator complex for basal RNA polymerase II transcription. EMBO Rep. 2001;2(9):808–813. doi: 10.1093/embo-reports/kve186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malik S, Roeder RG. Dynamic regulation of pol II transcription by the mammalian mediator complex. Trends Biochem Sci. 2005;30(5):256–263. doi: 10.1016/j.tibs.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 50.Naar AM, Lemon BD, Tjian R. Transcriptional coactivator complexes. Annu Rev Biochem. 2001;70:475–501. doi: 10.1146/annurev.biochem.70.1.475. [DOI] [PubMed] [Google Scholar]
- 51.Myers LC, Kornberg RD. Mediator of transcriptional regulation. Annu Rev Biochem. 2000;69:729–749. doi: 10.1146/annurev.biochem.69.1.729. [DOI] [PubMed] [Google Scholar]
- 52.Bourbon HM, Aguilera A, Ansari AZ, Asturias FJ, Berk AJ, Bjorklund S, Blackwell TK, Borggrefe T, Carey M, Carlson M, Conaway JW, Conaway RC, Emmons SW, Fondell JD, Freedman LP, Fukasawa T, Gustafsson CM, Han M, He X, Herman PK, Hinnebusch AG, Holmberg S, Holstege FC, Jaehning JA, Kim YJ, Kuras L, Leutz A, Lis JT, Meisteremest M, Naar AM, Nasmyth K, Parvin JD, Ptashne M, Reinberg D, Ronne H, Sadowski I, Sakurai H, Sipiczki M, Sternberg PW, Stillman DJ, Strich R, Struhl K, Svejstrup JQ, Tuck S, Winston F, Roeder RG, Kornberg RD. A unified nomenclature for protein subunits of mediator complexes linking transcriptional regulators to RNA polymerase II. Mol Cell. 2004;14(5):553–557. doi: 10.1016/j.molcel.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 53.Yuan CX, Ito M, Fondell JD, Fu ZY, Roeder RG. The TRAP220 component of a thyroid hormone receptor-associated protein (TRAP) coactivator complex interacts directly with nuclear receptors in a ligand-dependent fashion. Proc Natl Acad Sci USA. 1998;95 (14):7939–7944. doi: 10.1073/pnas.95.14.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donner AJ, Ebmeier CC, Taatjes DJ, Espinosa JM. CDK8 is a positive regulator of transcriptional elongation within the serum response network. Nat Struct Mol Biol. 2010;17(2):194–201. doi: 10.1038/nsmb.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takahashi H, Parmely TJ, Sato S, Tomomori-Sato C, Banks CA, Kong SE, Szutorisz H, Swanson SK, Martin-Brown S, Washburn MP, Florens L, Seidel CW, Lin C, Smith ER, Shilatifard A, Conaway RC, Conaway JW. Human mediator subunit MED26 functions as a docking site for transcription elongation factors. Cell. 2011;146(1):92–104. doi: 10.1016/j.cell.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang F, Vought BW, Satterlee JS, Walker AK, Jim Sun ZY, Watts JL, DeBeaumont R, Saito RM, Hyberts SG, Yang S, Macol C, Iyer L, Tjian R, Van den Heuvel S, Hart AC, Wagner G, Naar AM. An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature. 2006;442(7103):700–704. doi: 10.1038/nature04942. [DOI] [PubMed] [Google Scholar]
- 57.Taubert S, Van Gilst MR, Hansen M, Yamamoto KR. A Mediator subunit, MDT-15, integrates regulation of fatty acid metabolism by NHR-49-dependent and -independent pathways in C. elegans. Genes Dev. 2006;20 (9):1137–1149. doi: 10.1101/gad.1395406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thakur JK, Arthanari H, Yang F, Pan SJ, Fan X, Breger J, Frueh DP, Gulshan K, Li DK, Mylonakis E, Struhl K, Moye-Rowley WS, Cormack BP, Wagner G, Naar AM. A nuclear receptor-like pathway regulating multidrug resistance in fungi. Nature. 2008;452(7187):604–609. doi: 10.1038/nature06836. [DOI] [PubMed] [Google Scholar]
- 59.Sundqvist A, Bengoechea-Alonso MT, Ye X, Lukiyanchuk V, Jin J, Harper JW, Ericsson J. Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF(Fbw7) Cell Metab. 2005;1(6):379–391. doi: 10.1016/j.cmet.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 60.Bengoechea-Alonso MT, Ericsson J. A phosphorylation cascade controls the degradation of active SREBP1. J Biol Chem. 2009;284(9):5885–5895. doi: 10.1074/jbc.M807906200. [DOI] [PubMed] [Google Scholar]
- 61.Giandomenico V, Simonsson M, Gronroos E, Ericsson J. Coactivator-dependent acetylation stabilizes members of the SREBP family of transcription factors. Mol Cell Biol. 2003;23(7):2587–2599. doi: 10.1128/MCB.23.7.2587-2599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444:868–874. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- 63.Feige JN, Auwerx J. Transcriptional targets of sirtuins in the coordination of mammalian physiology. Curr Opin Cell Biol. 2008;20(3):303–309. doi: 10.1016/j.ceb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haigis MC, Guarente LP. Mammalian sirtuins—Emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20(21):2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 65.Saunders LR, Verdin E. Sirtuins: Critical regulators at the crossroads between cancer and aging. Oncogene. 2007;26(37):5489–5504. doi: 10.1038/sj.onc.1210616. [DOI] [PubMed] [Google Scholar]
- 66.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci USA. 2007;104(31):12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walker AK, Yang F, Jiang K, Ji JY, Watts JL, Purushotham A, Boss O, Hirsch ML, Ribich S, Smith JJ, Israelian K, Westphal CH, Rodgers JT, Shioda T, Elson SL, Mulligan P, Najafi-Shoushtari H, Black JC, Thakur JK, Kadyk LC, Whetstine JR, Mostoslavsky R, Puigserver P, Li X, Dyson NJ, Hart AC, Naar AM. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010;24(13):1403–1417. doi: 10.1101/gad.1901210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ponugoti B, Kim DH, Xiao Z, Smith Z, Miao J, Zang M, Wu SY, Chiang CM, Veenstra TD, Kemper JK. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem. 2010;285(44):33959–33970. doi: 10.1074/jbc.M110.122978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Erion DM, Yonemitsu S, Nie Y, Nagai Y, Gillum MP, Hsiao JJ, Iwasaki T, Stark R, Weismann D, Yu XX, Murray SF, Bhanot S, Monia BP, Horvath TL, Gao Q, Samuel VT, Shulman Gl. SirT1 knockdown in liver decreases basal hepatic glucose production and increases hepatic insulin responsiveness in diabetic rats. Proc Natl Acad Sci USA. 2009;106(27):11288–11293. doi: 10.1073/pnas.0812931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28(1):91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 71.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA. 2008;105(28):9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9(4):327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hopps E, Noto D, Caimi G, Averna MR. A novel component of the metabolic syndrome: The oxidative stress. Nutr Metab Cardiovasc Dis. 2010;20(1):72–77. doi: 10.1016/j.numecd.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 74.Mammucari C, Rizzuto R. Signaling pathways in mitochondrial dysfunction and aging. Mech Ageing Dev. 2010;131(7–8):536–543. doi: 10.1016/j.mad.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21(1):103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50(5):567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sekiya M, Hiraishi A, Touyama M, Sakamoto K. Oxidative stress induced lipid accumulation via SREBP1c activation in HepG2 cells. Biochem Biophys Res Commun. 2008;375(4):602–607. doi: 10.1016/j.bbrc.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 78.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 79.Cooper KF, Mallory MJ, Strich R. Oxidative stress-induced destruction of the yeast C-type cyclin Ume3p requires phosphatidylinositol-specific phospholipase C and the 26S proteasome. Mol Cell Biol. 1999;19(5):3338–3348. doi: 10.1128/mcb.19.5.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]


