Abstract
A concise and highly enantioselective total synthesis of the akuammiline alkaloid (−)-vincorine has been accomplished. A key element of the synthesis is a stereoselective organocatalytic Diels–Alder, iminium cyclization cascade sequence, which serves to construct the tetracyclic alkaloid core architecture in one step from simple achiral precursors. The challenging seven-membered, azepanyl ring system is installed by way of a single electron-mediated cyclization event initiated from an acyl telluride precursor. The total synthesis of (−)-vincorine is achieved in nine steps and 9% overall yield from commercially available starting materials.
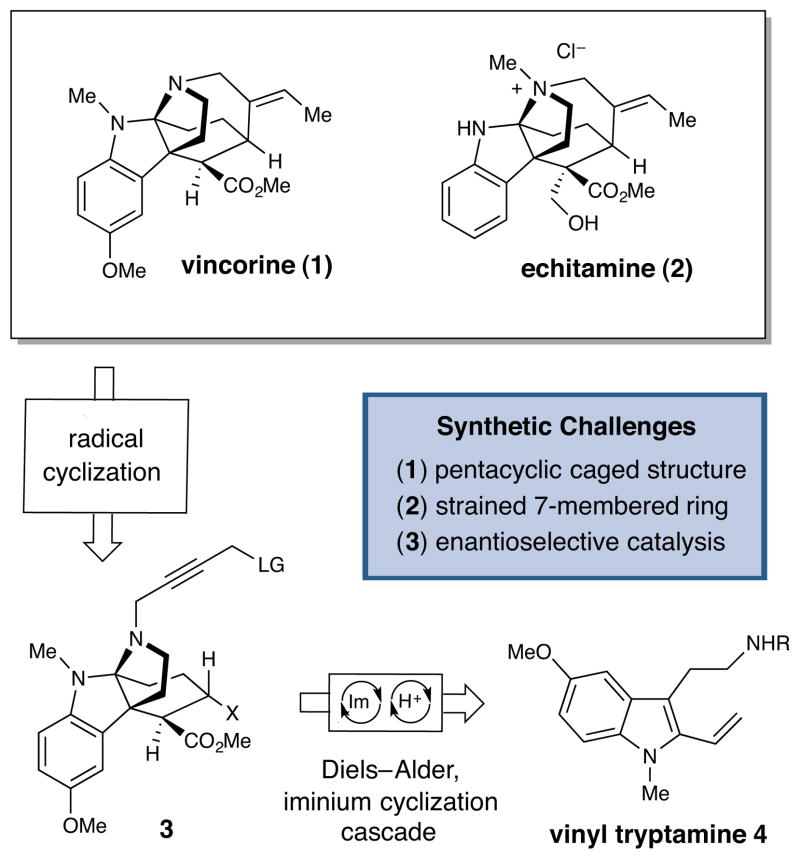
The Vinca alkaloid natural products have historically served as valuable lead candidates in the development of anti-cancer agents (vinblastine),1a,b vasodilators (vincamine),1c anti-psychotics and anti-hypertensives (reserpine).1d Recently, members of the biologically active akuammiline family of Vinca alkaloids have emerged as high-profile targets for chemical synthesis and medicinal chemistry studies.2 Vincorine (1) is the parent compound of an akuammiline alkaloid subclass characterized by a synthetically challenging tetracyclic core that incorporates a strained seven-membered, azepanyl ring and a pyrroloindoline motif. In preliminary assays, the related alkaloids echitamine (2) and corymine (not shown) were found to exhibit anti-cancer activity3 and glycine receptor antagonism,4 respectively. With the goal of devising a concise synthesis of this common core structure for further biological investigation, a number of research labs have undertaken the synthesis of vincorine.5 Indeed, recent total syntheses by the Qin group6a (35 steps, racemic) and the Ma group6b (18 steps, 64% ee) have served to highlight the significant structural challenges posed by this cage-like system. In this communication, we disclose a highly enantioselective 9-step total synthesis of (−)-vincorine, which employs organocascade catalysis7 as a central enabling feature. We anticipate that the general synthetic strategy described herein will prove readily adaptable to other bioactive akuammiline alkaloid natural products and analogs thereof.
Design Plan
From a retrosynthetic standpoint, we envisioned the disconnection of vincorine via two key steps (Scheme 1). While previous syntheses of vincorine have relied on C–N bond formation to forge the strained seven-membered azepanyl system, we reasoned that an intramolecular single electron-mediated C–C bond formation (initiated from compound 3) might offer additional strategic advantages in that the requisite allylic methine stereochemistry would be produced along with the embedded heterocycle. The remaining structural and stereochemical elements of the akuammiline core would arise from a single organocatalytic Diels–Alder,8 iminium cyclization cascade event beginning from simple, achiral starting materials (4 and 5). We have previously demonstrated the complexity-generating capacity of other organocatalytic9,10 cascade mechanisms in the recent total syntheses of strychnine, minfiensine,11a and additional selected alkaloids of the Strychnos, Aspidosperma, and Kopsia families.11b
Scheme 1.
Akuammiline Alkaloids; Retrosynthesis of Vincorine
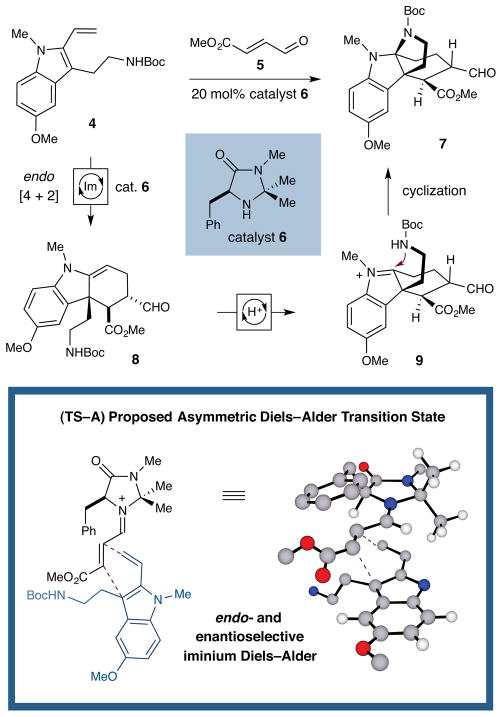
In the specific context of this vincorine synthesis program, we envisioned employing a catalytic cascade sequence to effect the overall conversion of tryptamine 4 to the tetracyclic adduct 7 in one step. As outlined in Scheme 2, the sequence would begin with condensation of secondary amine catalyst 6 onto enal 5 to form an activated iminium ion. In the proposed transition state (TS–A), orientation of the reactive π-system away from the catalyst gem-dimethyl group would facilitate effective shielding of one π-face by the benzyl group, thereby enabling a highly stereoselective endo Diels–Alder reaction with diene 4, to deliver enamine cycloadduct 8. Brønsted acid-catalyzed interconversion of enamine 8 and iminium ion 9 by the ammonium salt of 6 would precipitate intramolecular 5-exo amine cyclization via the pendent carbamate group to generate the tetracyclic product 7.12 Notably, the core structure 7 would emerge from this transformation bearing three of the four vincorine stereocenters – including the all-carbon quaternary center C(8) – with the correct relative and absolute configuration.
Scheme 2.
Enantioselective Organocatalytic Cascade Sequence
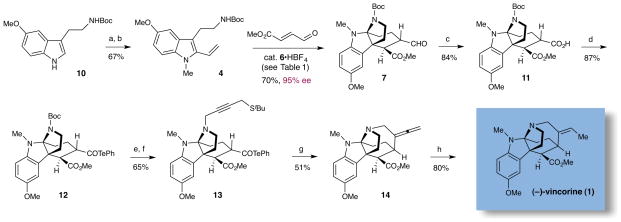
The total synthesis of vincorine was initiated with the preparation of diene 4 in two steps from commercially available 5-methoxy-N′-Boc tryptamine (10), via methylation then directed metalation/Negishi coupling13 (Scheme 3). For the key Diels–Alder/cyclization cascade, a survey of chiral secondary amines revealed that the first-generation Diels–Alder imidazolidinone catalyst 6 was both highly efficient and stereoselective. Using optimized reaction conditions (6•HBF4, MeCN, −20 °C), the tetracyclic vincorine core system 7 was generated in 70% yield and 95% ee (Table 1, entry 6).
Scheme 3.
Nine-Step Enantioselective Catalytic Total Synthesis of Vincorinea
aReagents and conditions: (a) NaH, DMF, 0 °C; MeI. (b) n-BuLi, DME, −40 °C; ZnCl2, −78 °C to rt; XPhos precatalyst, vinyl iodide. (c) NaClO2, 2-methyl-2-butene, THF, t-butanol, H2O, 0 °C. (d) isobutyl chloroformate, N-methylmorpholine, THF; diphenyl ditelluride, NaBH4, THF/MeOH, rt. (e) TFA, rt. (f) 4-(t-butylthio)but-2-ynal, NaBH(OAc)3, CH2Cl2, rt. (g) 1,2-dichlorobenzene, 200 °C. (h) Pd/C, H2, THF, −15 °C.
Table 1.
Organocatalytic Diels–Alder/Cyclization Cascade
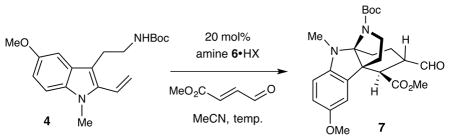
| |||||
|---|---|---|---|---|---|
| entry | HX | temp (°C) | time (h) | % yielda | % eeb |
| 1 | HClO4 | 0 | 4 | 38 | 88 |
| 2 | HCl | 0 | 4 | 25 | 73 |
| 3 | HBF4 | 0 | 4 | 71 | 93 |
| 4 | HBF4 | −10 | 6 | 75 | 94 |
| 5c | HBF4 | −10 | 6 | 62 | 94 |
| 6 | HBF4 | −20 | 6 | 73 (70)d | 95 |
Yield based on SFC analysis relative to an internal standard.
Determined by SFC analysis.
Reaction run with 5% v/v water.
Isolated yield.
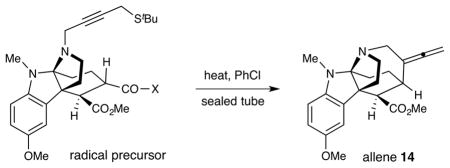
With the majority of the requisite vincorine architecture in hand, we next sought to rapidly install the final 7-membered azepanyl ring by way of a 7-exo-dig radical cyclization. As shown in Scheme 3, Pinnick oxidation14 served to convert aldehyde 7 to carboxylic acid 11, prior to the formation of an alkyl radical precursor in the form of an acyl telluride. Specifically, the telluride 12 was generated in a sequence involving formation of a mixed anhydride followed by displacement with sodium phenyl telluride in good yield for the overall transformation. Installation of a suitable π-system to enable radical-mediated C–C bond cyclization was accomplished via Boc-deprotection and subsequent nitrogen alkylation using 2-butynal-4-tert-butyl sulfide in a reductive amination step. Notably, the acyl telluride moiety was successfully maintained through multiple transformations, readily withstanding exposure to neat trifluoroacetic acid and sodium triacetoxyborohydride.
The suitability of the acyl telluride unit as the preferred alkyl radical precursor was determined by comparison to a range of alternative acyl-X fragmentation partners in the key cyclization event (13→14, see Table 2).15 Attempts to achieve useful isolated yields with a thiohydroxamic acid derivative (Barton ester)16 were largely unsuccessful, due to competitive formation of a thiopyridyl byproduct via radical recombination (Table 2, entry 1, 18%). Similarly disappointing results were obtained using a thermally stable acyl selenide17 precursor with hexabutyl ditin radical initiator. Although the stannyl radical – formed from hexabutyl ditin via photolysis with a high pressure mercury lamp – was able to successfully initiate the desired cyclization, extensive decomposition of the allene product under the reaction conditions led to low isolated yields of the desired adduct (entries 2 and 3). We ultimately achieved success with a thermally-initiated alkyl radical precursor, namely acyl telluride 13.18 Under optimal conditions (200 °C, 0.5 mM), radical cyclization of the acyl telluride precursor provided the allene product 14 in 51% isolated yield (entry 6). We postulate that the transformation of telluride 13 to the desired azepanyl allene 14 proceeds through carbon–Te bond homolysis, followed by loss of carbon monoxide to generate an alkyl radical. This result is notable given the difficulty of building seven-membered azepanes in comparison with the corresponding six-membered alkaloid analogs. Moreover, to our knowledge, this example represents the first use of an acyl telluride as an effective alkyl radical precursor.
Table 2.
Evaluation of Radical Cyclization Substrates
| |||||
|---|---|---|---|---|---|
| entry | CO–X | conditions | conc (mM) | conversion (%) | yielda (%) |
| 1 |
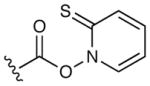
|
120 °C, 1 h | 1 | 100 | 18 |
|
| |||||
| 2 |
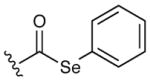
|
120 °C, 3 h Bu6Sn6, hν |
5 | 38 | 17 |
| 3 | 120 °C, 5 h Bu6Sn6, hν |
5 | 85 | 17 | |
|
| |||||
| 4 |
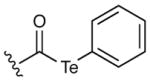
|
120 °C, 12 h | 5 | 100 | 8 |
| 5 | 160 °C, 12 h | 5 | 100 | 31 | |
| 6b | 200 °C, 10 h | 0.5 | 100 | 51c | |
Yield based on 1H NMR analysis.
1,2-Dichlorobenzene used as solvent.
Isolated yield.
In the final step of the synthesis, the terminal unsaturation of the allene functionality underwent selective hydrogenation from the less hindered face to provide (−)-vincorine in 80% yield as a single olefin isomer in nine steps and 9% overall yield from the commercially available indole 10. The product generated was identical in all spectroscopic respects to the natural isolate (see Supporting Information).
In conclusion, we have developed a nine-step synthetic route to (−)-vincorine from commercially available starting materials. Key features of the synthesis include an organocatalytic Diels–Alder/amine cyclization cascade and a 7-exo-dig radical cyclization initiated from an unusual acyl telluride precursor. The utility of this new cascade catalysis strategy is now being explored in the production of a large collection of natural and unnatural vincorine analogs including echitamine. Findings from these studies will be reported in due course.
Supplementary Material
Acknowledgments
Financial support was provided by the NIHGMS (R01 GM078201-07) and kind gifts from Merck, Amgen and Abbott. B.D.H. thanks Eli Lilly and Bristol-Myers Squibb for graduate fellowships. Special thanks to Dr. Christoph Grondal for work on related earlier studies.
Footnotes
Supporting Information Available. Experimental procedures and spectral data are provided. This information of available free of charge via the internet at “http://pubs.acs.org.”
References
- 1.(a) Neuss N, Neuss MN. In: The Alkaloids. Brossi A, Suffness M, editors. Vol. 37. Academic; San Diego: 1990. p. 229. [Google Scholar]; (b) Jordan MA, Wilson L. Nat Rev Cancer. 2004;4:253. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 2.For a review on akuammiline alkaloids, see: Ramirez A, Garcia-Rubio S. Curr Med Chem. 2003;10:1891. doi: 10.2174/0929867033457016.For recent syntheses of akuammiline alkaloids, see: Adams GL, Carroll PJ, Smith AB., III J Am Chem Soc. 2013;135:519. doi: 10.1021/ja3111626.Zu L, Boal BW, Garg NK. J Am Chem Soc. 2011;133:8877. doi: 10.1021/ja203227q.
- 3.(a) Kamarajan P, Sekar N, Mathuram V, Govindasamy S. Biochem Int. 1991;25:491. [PubMed] [Google Scholar]; (b) Kamarajan P, Ramamoorthy N, Govindasamy S. J Clin Biochem Nutr. 1995;18:65. [Google Scholar]; (c) Saraswathi V, Ramamoorthy N, Subramanian S, Gunasekaran P, Govindasamy S. Chemotherapy. 1998;44:198. doi: 10.1159/000007115. [DOI] [PubMed] [Google Scholar]
- 4.Leewanich P, Tohda M, Matsumoto K, Subhadhirasukul S, Takayama H, Aimi N, Watanabe H. Eur J Pharmacol. 1997;332:321. doi: 10.1016/s0014-2999(97)01097-2. [DOI] [PubMed] [Google Scholar]
- 5.(a) Mokíý J, Dúbravková L, Šefčovič P. Experientia. 1962;18:564. doi: 10.1007/BF02172182. [DOI] [PubMed] [Google Scholar]; (b) Kamatas SM, Sevenet T, Thal C, Potier P. Phytochemistry. 1975;14:1637. [Google Scholar]
- 6.(a) Zhang M, Huang X, Shen L, Qin Y. J Am Chem Soc. 2009;131:6013. doi: 10.1021/ja901219v. [DOI] [PubMed] [Google Scholar]; (b) Zi W, Xie W, Ma D. J Am Chem Soc. 2012;134:9126. doi: 10.1021/ja303602f. [DOI] [PubMed] [Google Scholar]
- 7.(a) Huang Y, Walji AM, Larsen CH, MacMillan DWC. J Am Chem Soc. 2005;127:15051. doi: 10.1021/ja055545d. [DOI] [PubMed] [Google Scholar]; (b) Simmons B, Walji AM, MacMillan DWC. Angew Chem Int Ed. 2009;48:4349. doi: 10.1002/anie.200900220. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Walji AM, MacMillan DWC. Synlett. 2007;10:1477. [Google Scholar]
- 8.(a) Ahrendt KA, Borths CJ, MacMillan DWC. J Am Chem Soc. 2001;123:4243. [Google Scholar]; (b) Northrup AB, MacMillan DWC. J Am Chem Soc. 2002;124:2458. doi: 10.1021/ja017641u. [DOI] [PubMed] [Google Scholar]; (c) Wilson RM, Jen WS, MacMillan DWC. J Am Chem Soc. 2005;127:11616. doi: 10.1021/ja054008q. [DOI] [PubMed] [Google Scholar]
- 9.Lelais G, MacMillan DWC. Aldrichimica Acta. 2006;39:79. [Google Scholar]
- 10.MacMillan DWC. Nature. 2008;455:304. doi: 10.1038/nature07367. [DOI] [PubMed] [Google Scholar]
- 11.(a) Jones SB, Simmons B, MacMillan DWC. J Am Chem Soc. 2009;131:13606. doi: 10.1021/ja906472m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jones SB, Simmons B, Mastracchio A, MacMillan DWC. Nature. 2011;475:183. doi: 10.1038/nature10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austin JF, Kim SG, Sinz CJ, Xiao WJ, MacMillan DWC. Proc Nat Acad Sci USA. 2004;101:5482. doi: 10.1073/pnas.0308177101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milne JE, Buchwald SL. J Am Chem Soc. 2004;126:13028. doi: 10.1021/ja0474493. [DOI] [PubMed] [Google Scholar]
- 14.Bal BS, Childers WE, Pinnick HW. Tetrahedron. 1981;37:2091. [Google Scholar]
- 15.For a related radical addition/fragmentation utilizing a sulfide fragmenting group, see: Barton DHR, Crich D. J Chem Soc, Perkin Trans 1. 1986;0:1613.
- 16.Barton DHR, Crich D, Motherwell WB. J Chem Soc, Chem Commun. 1983;0:939. [Google Scholar]
- 17.For an excellent review on acyl radical chemistry, see: Chatgilialoglu C, Crich D, Komatsu M, Ryu I. Chem Rev. 1999;99:1991. doi: 10.1021/cr9601425.
- 18.Chen C, Crich D, Papadatos A. J Am Chem Soc. 1992;114:8313.. It is noteworthy that in this report, acyl tellurides derived from aliphatic carboxylic acids are found to be unreactive under both thermal and photochemical conditions.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.