Abstract
Respiratory syncytial virus (RSV) infection is an important complication after hematopoietic cell transplantation (HCT), and RSV lower respiratory tract disease (LRD) results in substantial early mortality and late airflow obstruction among survivors. Factors associated with poor outcome are unknown. We evaluated the effect of transplant and treatment factors on overall survival, mortality from respiratory failure, and pulmonary function among 82 HCT recipients who had RSV LRD between 1990 and 2011. All patients received aerosolized ribavirin. In multivariable analyses, only the use of marrow or cord blood as graft source (adjusted hazard ratio [aHR] 4.1, 95% confidence interval [CI] 1.8-9.0, P < 0.001) and oxygen requirement (aHR 3.3, 95% CI 1.5-6.7, P=0.003) remained independently associated with overall mortality and death due to respiratory failure (aHR 4.7, 95% CI 1.8-13, P=0.002 and aHR 5.4, 95% CI 1.8-16, P=0.002, respectively). Antibody-based treatments, including intravenous immunoglobulin and palivizumab, were not independently associated with improved outcome and did not alter the associations of the graft source and oxygen requirements in statistical models. In conclusion, use of peripheral blood stem cells as graft source and lack of oxygen requirement at diagnosis appear to be important factors associated with improved survival of HCT recipients with RSV LRD. These results may explain differences in outcomes reported from RSV infection over time, and may guide the design of future interventional trials.
Keywords: Respiratory syncytial virus, Lower respiratory tract disease, Palivizumab, Pulmonary function, Hematopoietic cell transplantation
INTRODUCTION
Respiratory syncytial virus (RSV), known as a major cause of seasonal respiratory viral infection, can cause lower respiratory tract disease (LRD) in patients after hematopoietic cell transplantation (HCT). RSV infection in this population results in substantial early mortality and has been associated with late airflow decline [1-5]. Outcome studies are often small in size and results are difficult to interpret across sites because factors associated with outcome have not been well defined [6-9]. Candidate variables from smaller series of respiratory virus disease as well as studies with other serious pathogens such as aspergillus suggested a role of lymphopenia, mechanical ventilation, corticosteroids, copathogens and immune-based therapies on the outcome [6, 10, 11].
The purpose of this retrospective study was to examine the impact of transplant- and treatment-related factors on overall survival, mortality from respiratory failure, and the degree of pulmonary function decline in a large cohort of HCT recipients who received aerosolized ribavirin for RSV disease.
METHODS
Study design
This was a retrospective cohort study in which patients with RSV LRD during pre-transplant conditioning or following HCT at the Fred Hutchinson Cancer Research Center (FHCRC) from January 1990 to April 2011 were evaluated. RSV LRD were defined as detection of RSV by shell vial (SV) centrifugation culture, direct fluorescent antibody (DFA) tests, or conventional culture using a bronchoalveolar lavage (BAL) specimen (N= 79), lung biopsy sample (N= 2), or tracheal mucus (N= 1), accompanied with lower respiratory tract symptoms and/or abnormal radiographic findings [7]. More recent patients were also positive by PCR. All patients were treated with aerosolized ribavirin (6 g given as a single dose over 16 hours or 2 g three times daily over 2 hours) [12]. Patients who did not receive ribavirin (N=17), who received oral or intravenous ribavirin (N=12), or RSV-specific polyclonal immunoglobulin (N=5) were excluded. Intravenous immunoglobulin (IVIG) for treatment (500 mg/kg 3 times weekly) was given at the attending physician’s discretion. Replacement IVIG was given when the level of IgG dropped to less than 400 mg/dl. Palivizumab was given to 12 subjects as part of a research study prior to FDA approval [13]. After the drug became commercially available (1999), we instituted a guideline to routinely administer palivizumab for RSV LRD in combination with aerosolized ribavirin (starting 2002). A single dose of palivizumab (15 mg/kg ideal body weight) was administered intravenously, except in one patient who received 2 doses. This study was approved by the Institutional Review Board at the FHCRC.
Definitions
Underlying disease risk groups at transplantation were classified as either standard or high risk. The high-risk group was defined as acute leukemia, chronic lymphoid leukemia, lymphoma, multiple myeloma, and solid tumor not in remission, chronic myeloid leukemia in blast crisis, and all relapsed diseases after HCT. All other stages in any disease as well as myelodysplastic syndrome were categorized into the standard risk group. Myeloablative conditioning regimens mainly consisted of high-dose cyclophosphamide and busulfan or fractionated total body irradiation (TBI) (12.0 or 13.2 Gy). Reduced intensity conditioning regimens consisted of fludarabine with a single fraction of TBI (2 Gy). A copathogen was defined as a significant pathogen detected by culture or direct staining methods obtained from BAL specimens, or lung biopsy samples. Death due to respiratory failure was defined as any death caused exclusively or predominantly by respiratory failure.
Pulmonary function testing and bronchiolitis obliterans syndrome
All but five patients had undergone pulmonary function testing (PFT) prior to the diagnosis of RSV LRD and these values, mostly prior to HCT, were used as baseline values. PFT values after LRD included PFTs obtained at day 60 ± 25 days (early post LRD) and day 365 ± 90 days (late post LRD). All PFTs were performed in accordance with American Thoracic Society guidelines [14]. Predicted values of forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), total lung capacity (TLC), and diffusion capacity of the lung for carbon monoxide (DLCO) were determined using published equations for children and adults. All DLCO measurements were corrected for hemoglobin values. Based on the modified National Institutes of Health (NIH) guidelines, airflow obstruction was defined as FEV1/FVC < 0.7, FEV1 < 75%, and FEV1 decrease of ≥ 10% from pre-RSV LRD value [15, 16].
Patients with bronchiolitis obliterans syndrome (BOS) were identified according to the modified NIH diagnostic criteria: (1) FEV1/FVC < 0.7, (2) FEV1 < 75%, (3) FEV1 decrease of ≥ 10% from pre-transplant value, (4) residual volume > 120% of predicted normal or evidence of air trapping, and (5) absence of respiratory tract infection or pathologic confirmation [15-17].
Statistical analysis
Patients were analyzed based on their first episode of RSV LRD. The Wilcoxon rank sum test was used to compare the change of PFT values before and after RSV LRD. The probability of overall survival was estimated with the use of the Kaplan-Meier method. The probability of mortality due to respiratory failure was estimated by cumulative incidence curves, with death from other reasons as a competing risk. The log-rank test was used for the comparison between curves. Cox proportional hazards models were used to evaluate unadjusted and adjusted hazard ratios (HR) for overall mortality and mortality due to respiratory failure as well as associated 95% confidence intervals (CI). Use of palivizumab or IVIG was treated as a time-dependent variable. Covariates evaluated as candidates for risk factors in the multivariable models included transplant year, stem cell source, donor type, conditioning regimen, white blood cell count, copathogens, oxygen use at diagnosis, ribavirin use pre diagnosis, and antibody-based treatment. Variables with p ≤ 0.1 in the univariate models were candidates for multivariable models. Antibody-based treatments were included into the final models regardless of level of significance in the univariate analysis. Two-sided p values < 0.05 were considered to be statistically significant. All statistical analyses were performed using SAS 9.2 for Windows (SAS Institute, Inc., Cary, NC).
RESULTS
Patient characteristics
A total of 82 patients with RSV LRD who received aerosolized ribavirin were evaluated. Characteristics of all patients and the patients receiving PFTs are outlined in Table 1. The median time to LRD after HCT was 54.5 days (range, -1 to 2058 days) and the median recipient age was 43 years (range, 3 to 68).
Table 1.
Characteristics of patients with RSV LRD
| All patients (n=82) | Patients receiving PFT (n=28) | |
|---|---|---|
| Gender | ||
| Male | 48 (59) | 14 (50) |
| Female | 34 (41) | 14 (50) |
| Age at RSV LRD, year | ||
| ≤ 20 | 6 (7) | 2 (7) |
| 21-60 | 68 (83) | 25 (89) |
| > 60 | 8 (10) | 1 (4) |
| Days between transplant and RSV LRD | ||
| ≤ 30 | 34 (41) | 14 (50) |
| 31-365 | 39 (48) | 13 (46) |
| > 365 | 9 (11) | 1 (4) |
| Transplant year | ||
| 1989-1996 | 24 (29) | 5 (18) |
| 1997-2001 | 33 (40) | 15 (53) |
| 2002-2010 | 25 (31) | 8 (29) |
| Disease risk | ||
| Standard | 48 (59) | 16 (57) |
| High | 34 (41) | 12 (43) |
| Stem cell source | ||
| Peripheral blood stem cell | 37 (45) | 15 (54) |
| Bone marrow | 41 (50) | 11 (39) |
| Cord blood | 4 (5) | 2 (7) |
| Donor type | ||
| Autologous | 15 (18) | 2 (6) |
| HLA-matched related | 25 (30) | 9 (32) |
| HLA-matched unrelated | 21 (26) | 8 (29) |
| HLA-mismatched | 21 (26) | 9 (32) |
| Conditioning regimen | ||
| MA + TBI (> 12Gy) | 45 (55) | 19 (68) |
| MA ± low TBI | 23 (28) | 4 (14) |
| Reduced intensity conditioning | 14 (17) | 5 (18) |
| %FEV1/FVC pre RSV LRD | ||
| ≥ 70 | 60 (78) | 22 (79) |
| < 70 | 17 (22) | 6 (21) |
| %TLC pre RSV LRD | ||
| ≥ 80 | 66 (90) | 26 (96) |
| < 80 | 7 (10) | 1 (4) |
| White blood cell counts | ||
| > 1000 ×106/L | 51 (62) | 19 (68) |
| ≤ 1000 ×106/L | 31 (38) | 9 (32) |
| Lymphocyte count | ||
| > 500 ×106/L | 19 (23) | 7 (25) |
| 100-500 ×106/L | 38 (46) | 14 (50) |
| < 100 ×106/L | 25 (31) | 7 (25) |
| Co-pathogen | ||
| None | 54 (66) | 20 (71) |
| Any pathogen | 23 (28) | 6 (22) |
| Multiple pathogens | 5 (6) | 2 (7) |
| Oxgen at diagnosis | ||
| None | 33 (42) | 12 (44) |
| ≤ 2L/minute | 12 (15) | 7 (26) |
| > 2L/minute | 26 (33) | 7 (26) |
| Mechanical ventilation | 8 (10) | 1 (4) |
| Steroid use at diagnosis | ||
| None | 38 (46) | 12 (43) |
| < 1 mg/kg | 25 (30) | 9 (32) |
| ≥ 1mg/kg | 19 (23) | 7 (25) |
| Ribavirin use pre diagnosis | ||
| None | 56 (68) | 15 (53) |
| < 5 days | 10 (12) | 5 (18) |
| ≥ 5 days | 16 (20) | 8 (29) |
| Intravenous immunoglobulin | ||
| None | 43 (53) | 16 (57) |
| Low-dose* | 19 (23) | 7 (25) |
| High-dose | 20 (24) | 5 (18) |
| Palivizumab | ||
| Yes | 38 (46) | 15 (54) |
| No | 44 (54) | 13 (46) |
All values are indicated as the number (percentage).
To maintain levels of > 400 mg/dl, as needed
Abbreviations; MA: myeloablative conditioning, TBI: total body irradiation, FEV1: foeced expiratory volume in 1 second, FVC: forced vital capacity %TLC: percentage of predicted total lung capacity
Outcomes
Among all 82 patients, 26 (32%) and 35 (43%) died from any cause by day 30 and day 100 after RSV LRD, respectively. Deaths due to respiratory failure by day 30 and day 100 after RSV LRD were observed in 18 (22%) and 24 (29%) patients, respectively. The probabilities of overall survival and of death due to respiratory failure at day 100 post RSV LRD according to transplant year are shown in Figure 1A and B. Patients undergoing HCT in and after 1997 showed an improved outcome, although the differences did not reach statistical significance (overall survival: p = 0.074, death due to respiratory failure: p = 0.283) (Figure 1A and B).
Figure 1.
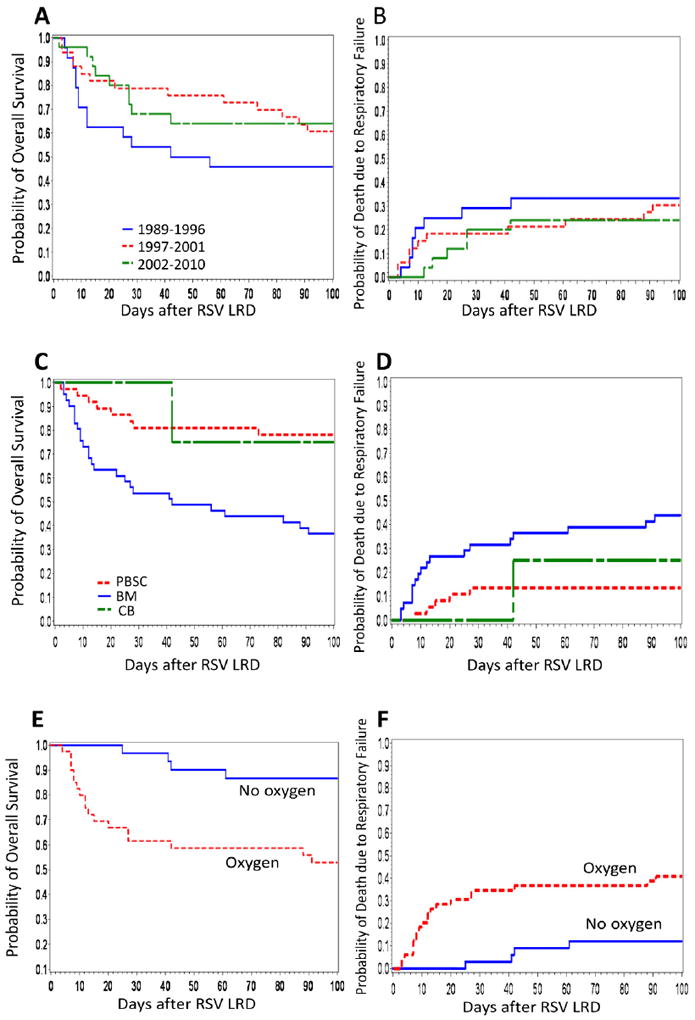
(A) Kaplan-Meier estimate of overall survival according to transplant year in HCT recipients after RSV LRD (p = 0.256, for three group comparison). (B) Cumulative incidence of death due to respiratory failure according to transplant year (p = 0.605). (C) Kaplan-Meier estimate of overall survival according to stem cell source in HCT recipients after RSV LRD (p < .001). (D) Cumulative incidence of death due to respiratory failure according to stem cell source (p = 0.006). (E) Kaplan-Meier estimate of overall survival according to the oxygen requirement at diagnosis in HCT recipients after RSV LRD (p = 0.001). (F) Cumulative incidence of death due to respiratory failure according to the oxygen requirement at diagnosis (p = 0.002).
Risk factors for mortality from all causes or respiratory failure by 100 days post RSV LRD
Univariate analyses of risk factors for overall mortality identified that the use of bone marrow (BM) as stem cell source, baseline oxygen requirement of more than 2L per minute, and white blood cell count of 1000 ×106/L or less at diagnosis are significantly correlated with high mortality (Table 2). The results for death due to respiratory failure were similar. Multivariable analyses demonstrated that only the use of BM or Cord blood (CB) as stem cell source and oxygen requirement remained associated with increased overall mortality and death due to respiratory failure (Table 3), confirmed in the cohort excluding the four patients receiving CB (data not shown). Overall survival and mortality due to respiratory failure according to these two factors are shown in Figure 2. Day-100 mortality due to respiratory failure among peripheral blood stem cell transplantation (PBSCT) recipients without oxygen was 0%, while among BM or CB transplantation (BMT/CBT) recipients who received oxygen, overall mortality was 58% (Figure 2B). A total of 24 patients required mechanical ventilation during the clinical course of RSV LRD (including eight at the time of diagnosis) and 15 of them died from respiratory failure by 100 days after RSV LRD. All of the four BMT/CBT recipients requiring mechanical ventilation at diagnosis died, compared to one of four PBSCT recipients (Figure 2C and D). To examine whether the use of antibody-based treatments were independently associated with these two outcomes and/or whether they altered the effect of the stem cell source and oxygen requirements we fit several multivariable models (Table 3). None of these models showed an independent effect of antibody-based treatments or a significant change in the effect size of the two major risk factors. Additional models were fit including oxygen levels >2L or mechanical ventilation and mechanical ventilation alone, none of which showed qualitatively different results (data not shown). Subset analyses restricting the patients transplanted between 1997 and 2010, which would decrease the effect of a time bias, also did not reveal different results (Table 4). The effect of the receipt of peripheral blood stem cells (PBSC) and lack of oxygen requirement at diagnosis on overall survival and death due to respiratory failure are shown in Figures 1C-F.
Table 2.
Univariate analysis of risk factors for mortality from all causes or respiratory failure by day 100 after RSV LRD
| Overall mortality | Mortality from respiratory failure | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Transplant year | ||||||
| 1989-1996 | 1.00 | 1.00 | ||||
| 1997-2001 | 0.59 | 0.3-1.3 | 0.180 | 0.75 | 0.3-1.9 | 0.548 |
| 2002-2010 | 0.54 | 0.2-1.3 | 0.155 | 0.59 | 0.2-1.7 | 0.327 |
| Stem cell source | ||||||
| Peripheral blood stem cell | 1.00 | 1.00 | ||||
| Bone marrow | 3.94 | 1.8-8.7 | <.001 | 4.31 | 1.6-12 | 0.004 |
| Cord blood | 1.07 | 0.1-8.6 | 0.947 | 1.71 | 0.2-15 | 0.626 |
| Donor type | ||||||
| Autologous | 1.00 | 1.00 | ||||
| Allogeneic | 1.91 | 0.7-5.4 | 0.222 | 5.60 | 0.8-42 | 0.092 |
| Conditioning regimen | ||||||
| MA + TBI (> 12Gy) | 1.00 | 1.00 | ||||
| MA ± low TBI | 0.78 | 0.4-1.7 | 0.524 | 0.34 | 0.1-1.1 | 0.081 |
| Reduced intensity conditioning | 0.50 | 0.2-1.4 | 0.199 | 0.64 | 0.2-1.9 | 0.424 |
| White blood cell counts | ||||||
| > 1000 ×106/L | 1.00 | 1.00 | ||||
| ≤ 1000 ×106/L | 2.29 | 1.2-4.4 | 0.015 | 2.16 | 1.0-4.8 | 0.060 |
| Co-pathogen | ||||||
| None | 1.00 | 1.00 | ||||
| Any pathogen | 1.36 | 0.7-2.8 | 0.406 | 0.80 | 0.3-2.2 | 0.671 |
| Multiple pathogens | 1.83 | 0.5-6.1 | 0.331 | 2.35 | 0.7-8.1 | 0.177 |
| Oxygen at diagnosis | ||||||
| None | 1.00 | 1.00 | ||||
| ≤ 2L/minute | 0.71 | 0.2-3.3 | 0.656 | 1.58 | 0.3-8.7 | 0.596 |
| > 2L/minute | 3.13 | 1.4-7.1 | 0.006 | 5.15 | 1.7-16 | 0.005 |
| Mechanical ventilation | 6.21 | 2.2-18 | <.001 | 11.2 | 3.0-42 | <.001 |
| Ribavirin use pre diagnosis | ||||||
| None | 1.00 | 1.00 | ||||
| < 5 days | 0.67 | 0.2-1.9 | 0.450 | 0.23 | 0.0-1.7 | 0.148 |
| ≥ 5 days | 0.43 | 0.2-1.2 | 0.120 | 0.44 | 0.1-1.5 | 0.191 |
| Ab-based treatment as time dependent | ||||||
| None/Low-dose | 1.00 | 1.00 | ||||
| High-dose | 1.17 | 0.5-2.8 | 0.727 | 1.41 | 0.5-3.9 | 0.510 |
| Palivizumab* | 0.74 | 0.3-1.6 | 0.450 | 0.76 | 0.3-2.0 | 0.567 |
All variables in Table 1 were used for the univariate analysis. Only variables with p < 0.2 in any analysis, except for Ab-based treatment are shown in this table. The following parameters were not significantly associated; gender, age at RSV LRD, days between transplant and RSV LRD, diagnosis, disease risk at transplantation, %FEV1/FVC pre RSV LRD, %TLC pre RSV LRD, lymphocyte count, steroid use at diagnosis, GVHD prophylaxis, recipient CMV serostatus, and GVHD in lung at RSV LRD.
This includes two patients who received both palivizumab and high-does intravenous immunoglobulin.
Abbreviations; MA: myeloablative conditioning, TBI: total body irradiation, Ab: antibody, FEV1: forced expiratory volume in 1 second, FVC: forced vital capacity %TLC: percentage of predicted total lung capacity, GVHD: graft versus host disease, CMV: cytomegalovirus
Table 3.
Multivariable analysis of risk factors and treatment efficacy for mortality from all causes or respiratory failure by day 100 after RSV LRD
| Overall mortality | Mortality from respiratory failure | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Model1 | ||||||
| Stem cell source | ||||||
| Peripheral blood stem cell | 1.00 | 1.00 | ||||
| Bone marrow/Cord blood | 4.08 | 1.8-9.0 | <.001 | 4.73 | 1.8-13 | 0.002 |
| Oxygen at diagnosis | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 3.12 | 1.5-6.7 | 0.003 | 5.39 | 1.8-16 | 0.002 |
| Model 2 | ||||||
| Ab-based treatment as time dependent variable | ||||||
| None/Low-dose | 1.00 | 1.00 | ||||
| High-dose | 1.75 | 0.7-4.2 | 0.216 | 2.15 | 0.8-6.1 | 0.148 |
| Palivizumab* | 1.04 | 0.5-2.3 | 0.920 | 1.10 | 0.4-2.9 | 0.843 |
| Stem cell source | ||||||
| Peripheral blood stem cell | 1.00 | 1.00 | ||||
| Bone marrow/Cord blood | 3.86 | 1.7-8.7 | 0.001 | 4.44 | 1.6-12 | 0.004 |
| Model 3 | ||||||
| Ab-based treatment as time dependent variable | ||||||
| None/Low-dose | 1.00 | 1.00 | ||||
| High-dose | 1.75 | 0.7-4.3 | 0.221 | 2.42 | 0.9-6.9 | 0.097 |
| Palivizumab* | 0.99 | 0.4-2.2 | 0.974 | 1.07 | 0.4-2.8 | 0.888 |
| Oxygen at diagnosis | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 2.94 | 1.3-6.5 | 0.008 | 5.39 | 1.8-16 | 0.003 |
The factors with p < 0.1 in Table 2 were evaluated in the multivariable models; Ab-based treatments were included regardless of level of significance in the univariate models.
This includes two patients who received both palivizumab and high-does intravenous immunoglobulin.
Abreviation; Ab: antibody.
Figure 2.
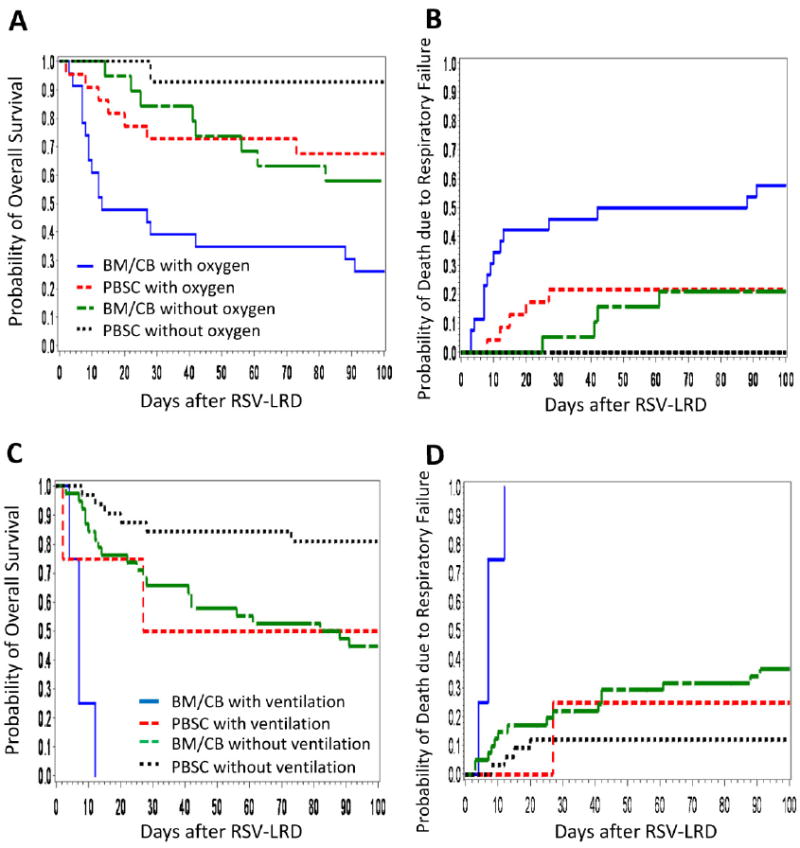
(A) Kaplan-Meier estimate of overall survival according to stem cell source and the oxygen requirement at diagnosis (p = <.0001, for four group comparison). (B) Cumulative incidence of death due to respiratory failure according to stem cell source and the oxygen requirement at diagnosis (p = <.0001). (C) Kaplan-Meier estimate of overall survival according to stem cell source and mechanical ventilation requirement at diagnosis (p = <.0001). (D) Cumulative incidence of death due to respiratory failure according to stem cell source and mechanical ventilation requirement at diagnosis (p = <.0001).
Table 4.
Risk factors for mortality from all causes or respiratory failure by day 100 after RSV LRD in patients transplanted between 1997 and 2010 (n=58)
| Overall mortality | Mortality from respiratory failure | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk factors | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Transplant year | ||||||||||||
| 1997-2001 | 1.00 | 1.00 | ||||||||||
| 2002-2010 | 0.95 | 0.4-2.2 | 0.907 | 0.81 | 0.3-2.2 | 0.686 | ||||||
| Stem cell source | ||||||||||||
| Peripheral blood stem cell | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Bone marrow | 3.77 | 1.5-9.4 | 0.004 | 4.71 | 1.9-12 | 0.001 | 5.16 | 1.6-16 | 0.005 | 7.76 | 2.4-25 | <.001 |
| Cord blood | 1.06 | 0.1-8.6 | 0.956 | 1.85 | 0.2-17 | 0.581 | ||||||
| Conditioning regimen | ||||||||||||
| MA + TBI (> 12Gy) | 1.00 | 1.00 | ||||||||||
| MA ± low TBI | 0.83 | 0.3-2.3 | 0.725 | 0.20 | 0.0-1.5 | 0.118 | ||||||
| Reduced intensity conditioning | 0.78 | 0.3-2.4 | 0.670 | 0.91 | 0.3-2.9 | 0.867 | ||||||
| White blood cell counts | ||||||||||||
| > 1000 ×106/L | 1.00 | 1.00 | ||||||||||
| ≤ 1000 ×106/L | 1.86 | 0.8-4.3 | 0.147 | 1.76 | 0.7-4.7 | 0.262 | ||||||
| Co-pathogen | ||||||||||||
| None | 1.00 | 1.00 | ||||||||||
| Any pathogen | 1.10 | 0.4-2.9 | 0.843 | 0.71 | 0.2-2.6 | 0.601 | ||||||
| Multiple pathogens | 2.22 | 0.6-7.8 | 0.214 | 2.81 | 0.8-10 | 0.118 | ||||||
| Oxygen at diagnosis* | ||||||||||||
| None | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| ≤ 2L/minute | 0.51 | 0.1-4.2 | 0.532 | 1.19 | 0.1-12 | 0.878 | ||||||
| > 2L/minute | 3.24 | 1.2-8.8 | 0.020 | 3.57 | 1.4-9.0 | 0.007 | 6.57 | 1.7-25 | 0.006 | 7.98 | 2.2-29 | 0.001 |
| Mechanical ventilation | 3.58 | 0.9-14 | 0.065 | 5.59 | 0.9-34 | 0.060 | ||||||
| Steroid use at diagnosis | ||||||||||||
| None | 1.00 | 1.00 | ||||||||||
| < 1 mg/kg | 0.80 | 0.3-2.1 | 0.645 | 0.94 | 0.3-3.1 | 0.913 | ||||||
| ≥ 1 mg/kg | 2.00 | 0.7-5.9 | 0.208 | 3.19 | 1.0-11 | 0.057 | ||||||
| Ribavirin use pre diagnosis | ||||||||||||
| None | 1.00 | |||||||||||
| < 5 days | 0.37 | 0.1-2.8 | 0.338 | |||||||||
| ≥ 5 days | 0.25 | 0.0-1.9 | 0.180 | |||||||||
| Ab-based treatment as time-dependent | ||||||||||||
| None/Low-dose | 1.00 | 1.00 | ||||||||||
| High-dose | 1.49 | 0.4-6.0 | 0.575 | 1.19 | 0.3-5.3 | 0.824 | ||||||
| Palivizumab** | 1.11 | 0.4-3.4 | 0.856 | 0.77 | 0.2-2.5 | 0.671 | ||||||
All variables in Table 1 were used for the univariate analysis. Only variables with p < 0.2 in any analysis are shown in this table. The following parameters were not significantly associated; gender, age at RSV LRD, days between transplant and RSV LRD, donor type, diagnosis, disease risk at transplantation, GVHD prophylaxis, recipient CMV serostatus, and GVHD in lung at RSV LRD, %FEV1/FVC pre RSV LRD, %TLC pre RSV LRD, lymphocyte count. The factors with p < 0.1 were evaluated in the multivariable models.
In the multivariate analysis, oxgen at diagnosis were categorized into two groups; none + ≤ 2L/minute and > 2L/minute + mechanical ventilation.
This includes two patients who received both palivizumab and high-dose intravenous immunoglobulin.
Abbreviations; MA: myeloablative conditioning, TBI: total body irradiation, FEV1: foeced expiratory volume in 1 second, FVC: forced vital capacity %TLC: percentage of predicted total lung capacity, GVHD: graft versus host disease, CMV: cytomegalovirus
Changes in pulmonary function and bronchiolitis obliterans syndrome
Among the 82 patients, a total of 28 were examined for pulmonary function at 60 days and/or 1 year after RSV LRD. Most of the PFT values declined within 2 months after infection and for some parameters, the decline continued for 1 year (Figure 3). In %DLco, the decrease was significant at 60 days after RSV LRD (p = 0.003). The modified NIH criteria for airflow obstruction were met in 2 of 21 patients at 60 days after RSV LRD, and in two of 16 patients at 1 year, respectively. Among the 82 patients, five were diagnosed as BOS according to the modified NIH diagnostic criteria. Four of them had BOS before RSV LRD, while only one developed BOS nine months after RSV LRD.
Figure 3.
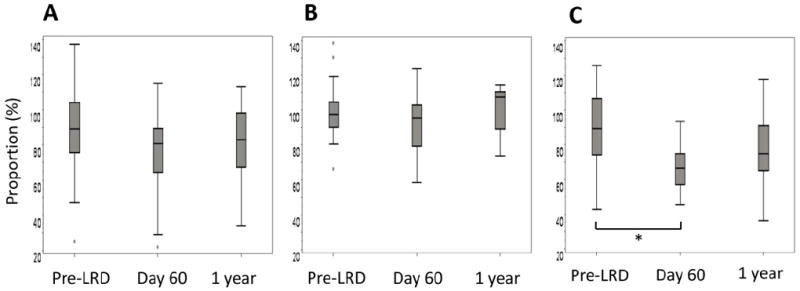
Percentages of FEV1 (A), TLC (B), and DLCO (C) for predicted each value were shown at the time point of pre-LRD, day 60, and 1 year after RSV LRD. P-values for three parameters in %FEV1, %TLC, and %DLco were 0.249, 0.260, and 0.001, respectively. *, p < 0.05, by Wilcoxon rank sum test.
DISCUSSION
This retrospective cohort study of RSV LRD found that a favorable outcome is significantly associated with the use of PBSC as a graft source and the absence of requiring oxygen at the time of diagnosis of RSV LRD. The importance of respiratory failure at the time of diagnosis on subsequent clinical outcome has been suggested by other studies [11]. Since oxygen use at diagnosis may serve as a surrogate indicator of acute lung injury, this observation is plausible. Perhaps the most important and novel finding in this report is that patients after PBSCT had a lower mortality and death from respiratory failure than those who had received a BMT or CBT. Previous studies demonstrated that patients after PBSCT have higher numbers of adoptively transferred lymphocytes and earlier immune reconstitution, resulting in fewer infections compared with those after BMT [10, 18-20]. Although only very few CBT recipients were included in this analysis, CBT is also known to have late immune reconstitution compared with PBSCT or BMT [21-24]. Our observation that PBSC transplant recipients have improved survival after documented RSV LRD suggests that the degree of immunosuppression is a key factor in the recovery from the disease.
Interestingly, other factors such as lymphopenia at the time of diagnosis, the presence of copathogens, baseline lung function and antibody treatments did not appear to be important in this study. Although palivizumab has shown a proven prophylactic effect in infants at high risk for bronchiolitis, little is known about the treatment efficacy of palivizumab in adults and HCT recipients with RSV LRD. Some studies have suggested an improved outcome of RSV infection after palivizumab treatment, however, the drug is not approved for this indication and most studies have been small and non-randomized, thereby limiting the ability to perform multivariable analyses [3, 11, 25-28]. The current study suggests that PBSC and more frequent diagnosis of RSV LRD at a stage before oxygen dependency, rather than palivizumab, was likely the important factor in the improved outcome of RSV disease in recent years. Although Ottolini et al. demonstrated that palivizumab can reduce viral replication in lung in an animal model, the complete control of viral replication required sequential use of multiple doses of palivizumab [29]. Likewise, intubated infants with RSV infection showed reduced RSV titers in tracheal aspirates after palivizumab treatment, however, clinical outcomes were not affected [30]. Based on these data, we speculate that once RSV-attributed airway inflammation begins [5], palivizumab alone may not be able to interfere with the inflammatory cascade. In the setting of prophylaxis of RSV LRD, palivizumab reduced respiratory symptoms including wheezing in premature infants [31]. Therefore, the use of palivizumab before progression to LRD might theoretically be beneficial to prevent pulmonary function decline. At this point, such an effect is hypothetical and randomized trials would be needed to answer these questions. The development of newer, more potent RSV monoclonal antibodies such as motavizumab could potentially be of interest in future studies, although this new monoclonal was not approved by the Food and Drug Administration following trials in high-risk infants [32, 33] and a recent study suggested no benefit in the treatment setting [34].
The decline in pulmonary function is one of the important features of RSV LRD [5]. Previous studies have suggested that respiratory virus infections after lung transplantation stimulate the incidence of BOS [35-38]. Compared to the incidence rates of BOS (2-6%) in recent studies based on the new NIH diagnostic criteria [17, 39], the incidence in our study was relatively high (10%; 5 among 50 allogeneic HCT recipients surviving more than 100 days after HCT). However, considering that our sample size is small and most patients were diagnosed with BOS before RSV LRD, we have insufficient information to make definitive conclusions about the association between BOS and RSV LRD.
Our study has strengths and limitations. It includes the largest outcome cohort of RSV LRD thus far with standardized diagnostic criteria (virologically proven LRD by conventional methods) and uniform antiviral treatment. Antibody-based treatments were protocol-based for palivizumab but given according to the attending physician’s discretion for IVIG. The non-randomized nature of the study is the most important limitation. However, conducting randomized trials is very difficult for rare diseases such as RSV LRD in HCT recipients and has failed in several instances even when a fully funded multicenter protocol was available (clinicaltrials.gov NCT00014391). Although this is the only outcome study of RSV LRD that has performed multivariable modeling, the statistical power to detect or rule out the possibility of smaller effects of antibody-based treatments such as palivizumab was limited. Based on post-hoc calculations the minimum detectable effect size in our study for antibody-based treatments would have been a 50% reduction of mortality. Thus, very large multicenter retrospective studies would be needed to evaluate an effect size of less than 10% as suggested by our multivariable models. Whether such relatively small effect would be deemed clinically significant is unclear given the enormous cost of the treatment (approximately US $32,000/75kg body weight for drug acquisition). Our study also included only a small number of CBT recipients, which might represent a distinct clinical subgroup [11]. We analyzed CBT and BMT recipients together in this study because both groups have comparatively late immune reconstitution. However, the impact of CB is still unclear and requires larger numbers of patients. The exclusion of CBT recipients from the major analyses did not change the key conclusions of this paper. Another limitation is that the cases occurred over two decades, which may have led to differences in supportive care practices and the timing of bronchoscopic evaluation. We accounted for this by including the year of transplantation and the degree of lung injury in our models.
In conclusion, our study identified two significant factors that are associated with improved outcome of RSV LRD in HCT recipients; the use of PBSC as the graft source and the lack of oxygen requirement at the time of diagnosis of LRD. Patients who have both factors at the time of diagnosis at RSV LRD had very favorable outcome with aerosolized ribavirin treatment, which was administered to all patients (Figures 1C-F). These data could explain differences in outcome data reported over time and from different centers around the world. They could also serve as adjustment factors in multivariable models in future outcome analyses or to stratify patients in future randomized treatment trials. Antibody therapies had no apparent effect on these associations, but our sample size prevented us from ruling out possible small effects. Multicenter studies are needed to validate these findings. Overall, our data suggest that the observed improvement in outcome of RSV disease in recent years is likely due to increased use of PBSC as graft source and the associated improved immune reconstitution, and to the increased early initiation of antiviral treatment before the onset of lung injury.
Acknowledgments
We thank Deborah L Reyes and Chris Davis for assisting with data collection and Margaret Green, MD, for suggestions regarding the analysis.
Financial Support. This work was partially supported by NIH grants CA18029, CA15704, HL081595, HL93294, K23HL091059, and L40AI071572. S.S. is a recipient of a fellowship from The Mochida Memorial Foundation for Medical and Pharmaceutical Research. A.P.C. also received support from the Seattle Children’s Center for Clinical and Translational Research and CTSA grant ULI RR025014.
J.A.E. received research funding from Novartis and MedImmune. M.B. served as a consultant for Novartis and Gilead Sciences. J.W.C. is an employee of Gilead Sciences.
Footnotes
Potential conflicts of interest. All other authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martino R, Porras RP, Rabella N, et al. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biology of blood and marrow transplantation. 2005;11:781–796. doi: 10.1016/j.bbmt.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichols WG, Gooley T, Boeckh M. Community-acquired respiratory syncytial virus and parainfluenza virus infections after hematopoietic stem cell transplantation: the Fred Hutchinson Cancer Research Center experience. Biology of blood and marrow transplantation. 2001;7(Suppl):11S–15S. doi: 10.1053/bbmt.2001.v7.pm11777098. [DOI] [PubMed] [Google Scholar]
- 3.Khanna N, Widmer AF, Decker M, et al. Respiratory syncytial virus infection in patients with hematological diseases: single-center study and review of the literature. Clinical infectious diseases. 2008;46:402–412. doi: 10.1086/525263. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy AJ, Kingman HM, Kelly C, et al. The outcome of 26 patients with respiratory syncytial virus infection following allogeneic stem cell transplantation. Bone marrow transplantation. 1999;24:1315–1322. doi: 10.1038/sj.bmt.1702078. [DOI] [PubMed] [Google Scholar]
- 5.Erard V, Chien JW, Kim HW, et al. Airflow decline after myeloablative allogeneic hematopoietic cell transplantation: the role of community respiratory viruses. The Journal of infectious diseases. 2006;193:1619–1625. doi: 10.1086/504268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood. 2011;117:2755–2763. doi: 10.1182/blood-2010-08-263400. [DOI] [PubMed] [Google Scholar]
- 7.Boeckh M, Englund J, Li Y, et al. Randomized controlled multicenter trial of aerosolized ribavirin for respiratory syncytial virus upper respiratory tract infection in hematopoietic cell transplant recipients. Clinical infectious diseases. 2007;44:245–249. doi: 10.1086/509930. [DOI] [PubMed] [Google Scholar]
- 8.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biology of blood and marrow transplantation. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Community-acquired respiratory viruses. American journal of transplantation. 2004;4(Suppl 10):105–109. doi: 10.1111/j.1600-6135.2004.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clinical infectious diseases. 2007;44:531–540. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- 11.de Fontbrune FS, Robin M, Porcher R, et al. Palivizumab treatment of respiratory syncytial virus infection after allogeneic hematopoietic stem cell transplantation. Clinical infectious diseases. 2007;45:1019–1024. doi: 10.1086/521912. [DOI] [PubMed] [Google Scholar]
- 12.Chemaly RF, Torres HA, Munsell MF, et al. An adaptive randomized trial of an intermittent dosing schedule of aerosolized ribavirin in patients with cancer and respiratory syncytial virus infection. The Journal of infectious diseases. 2012;206:1367–1371. doi: 10.1093/infdis/jis516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boeckh M, Berrey MM, Bowden RA, Crawford SW, Balsley J, Corey L. Phase 1 evaluation of the respiratory syncytial virus-specific monoclonal antibody palivizumab in recipients of hematopoietic stem cell transplants. The Journal of infectious diseases. 2001;184:350–354. doi: 10.1086/322043. [DOI] [PubMed] [Google Scholar]
- 14.Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. The American review of respiratory disease. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 15.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biology of blood and marrow transplantation. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Williams KM, Chien JW, Gladwin MT, Pavletic SZ. Bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. JAMA. 2009;302:306–314. doi: 10.1001/jama.2009.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biology of blood and marrow transplantation. 2011;17:1072–1078. doi: 10.1016/j.bbmt.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storek J, Dawson MA, Storer B, et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood. 2001;97:3380–3389. doi: 10.1182/blood.v97.11.3380. [DOI] [PubMed] [Google Scholar]
- 19.Talmadge JE, Reed E, Ino K, et al. Rapid immunologic reconstitution following transplantation with mobilized peripheral blood stem cells as compared to bone marrow. Bone marrow transplantation. 1997;19:161–172. doi: 10.1038/sj.bmt.1700626. [DOI] [PubMed] [Google Scholar]
- 20.Ottinger HD, Beelen DW, Scheulen B, Schaefer UW, Grosse-Wilde H. Improved immune reconstitution after allotransplantation of peripheral blood stem cells instead of bone marrow. Blood. 1996;88:2775–2779. [PubMed] [Google Scholar]
- 21.Jacobson CA, Turki AT, McDonough SM, et al. Immune Reconstitution after Double Umbilical Cord Blood Stem Cell Transplantation: Comparison with Unrelated Peripheral Blood Stem Cell Transplantation. Biology of blood and marrow transplantation. 2012;18:565–574. doi: 10.1016/j.bbmt.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Arena G, Musto P, Cascavilla N, et al. Flow cytometric characterization of human umbilical cord blood lymphocytes: immunophenotypic features. Haematologica. 1998;83:197–203. [PubMed] [Google Scholar]
- 23.Komanduri KV, St John LS, de Lima M, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110:4543–4551. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruggeri A, Peffault de Latour R, Carmagnat M, et al. Outcomes, infections, and immune reconstitution after double cord blood transplantation in patients with high-risk hematological diseases. Transplant infectious disease. 2011;13:456–465. doi: 10.1111/j.1399-3062.2011.00632.x. [DOI] [PubMed] [Google Scholar]
- 25.Banna GL, Aversa SM, Cattelan AM, Crivellari G, Monfardini S. Respiratory syncytial virus-related pneumonia after stem cell transplantation successfully treated with palivizumab and steroid therapy. Scandinavian journal of infectious diseases. 2004;36:155–157. doi: 10.1080/00365540410019282. [DOI] [PubMed] [Google Scholar]
- 26.McCoy D, Wong E, Kuyumjian AG, Wynd MA, Sebti R, Munk GB. Treatment of respiratory syncytial virus infection in adult patients with hematologic malignancies based on an institution-specific guideline. Transplant infectious disease. 2011;13:117–121. doi: 10.1111/j.1399-3062.2010.00561.x. [DOI] [PubMed] [Google Scholar]
- 27.Tsitsikas DA, Oakervee H, Cavenagh JD, Gribben J, Agrawal SG, Mattes FM. Treatment of respiratory syncytial virus infection in haemopoietic stem cell transplant recipients with aerosolized ribavirin and the humanized monoclonal antibody palivizumab: a single centre experience. British journal of haematology. 2009;146:574–576. doi: 10.1111/j.1365-2141.2009.07763.x. [DOI] [PubMed] [Google Scholar]
- 28.Chavez-Bueno S, Mejias A, Merryman RA, Ahmad N, Jafri HS, Ramilo O. Intravenous palivizumab and ribavirin combination for respiratory syncytial virus disease in high-risk pediatric patients. The Pediatric infectious disease journal. 2007;26:1089–1093. doi: 10.1097/INF.0b013e3181343b7e. [DOI] [PubMed] [Google Scholar]
- 29.Ottolini MG, Curtis SR, Mathews A, Ottolini SR, Prince GA. Palivizumab is highly effective in suppressing respiratory syncytial virus in an immunosuppressed animal model. Bone marrow transplantation. 2002;29:117–120. doi: 10.1038/sj.bmt.1703326. [DOI] [PubMed] [Google Scholar]
- 30.Malley R, DeVincenzo J, Ramilo O, et al. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. The Journal of infectious diseases. 1998;178:1555–1561. doi: 10.1086/314523. [DOI] [PubMed] [Google Scholar]
- 31.Simoes EA, Groothuis JR, Carbonell-Estrany X, et al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. The Journal of pediatrics. 2007;151:34–42. 42–e31. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 32.Carbonell-Estrany X, Simoes EA, Dagan R, et al. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trial. Pediatrics. 2010;125:e35–51. doi: 10.1542/peds.2008-1036. [DOI] [PubMed] [Google Scholar]
- 33.Lagos R, DeVincenzo JP, Munoz A, et al. Safety and antiviral activity of motavizumab, a respiratory syncytial virus (RSV)-specific humanized monoclonal antibody, when administered to RSV-infected children. The Pediatric infectious disease journal. 2009;28:835–837. doi: 10.1097/INF.0b013e3181a165e4. [DOI] [PubMed] [Google Scholar]
- 34.Octavio RSJ, Wang K, Jensen K, Harris B, Losonsky G, Griffin P. Motavizumab treatment of children hospitalized with RSV lower respiratory tract infection does not decrease viral load or severity of illness. 8th Annual Respiratory Syncytial Virus Symposium; Santa Fe, New Mexico. 2012. [Google Scholar]
- 35.Magnusson J, Westin J, Andersson LM, Brittain-Long R, Riise GC. The Impact of Viral Respiratory Tract Infections on Long-Term Morbidity and Mortality following Lung Transplantation: A Retrospective Cohort Study Using a Multiplex PCR Panel. Transplantation. 2012 doi: 10.1097/tp.0b013e318271d7f0. in press. [DOI] [PubMed] [Google Scholar]
- 36.Vu DL, Bridevaux PO, Aubert JD, Soccal PM, Kaiser L. Respiratory viruses in lung transplant recipients: a critical review and pooled analysis of clinical studies. American journal of transplantation. 2011;11:1071–1078. doi: 10.1111/j.1600-6143.2011.03490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khalifah AP, Hachem RR, Chakinala MM, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. American journal of respiratory and critical care medicine. 2004;170:181–187. doi: 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]
- 38.Gottlieb J, Schulz TF, Welte T, et al. Community-acquired respiratory viral infections in lung transplant recipients: a single season cohort study. Transplantation. 2009;87:1530–1537. doi: 10.1097/TP.0b013e3181a4857d. [DOI] [PubMed] [Google Scholar]
- 39.Nakaseko C, Ozawa S, Sakaida E, et al. Incidence, risk factors and outcomes of bronchiolitis obliterans after allogeneic stem cell transplantation. International journal of hematology. 2011;93:375–382. doi: 10.1007/s12185-011-0809-8. [DOI] [PubMed] [Google Scholar]


