Abstract
Reduced neprilysin (NEP), a cell surface metallopeptidase, which cleaves and inactivates pro-inflammatory and vasoactive peptides, predisposes the lung vasculature to exaggerated remodeling in response to hypoxia. We hypothesize that loss of NEP in pulmonary artery smooth muscle cells (PASMCs) results in increased migration and proliferation.
PASMCs isolated from NEP−/− mice exhibited enhanced migration and proliferation in response to serum and PDGF, which was attenuated by NEP replacement. Inhibition of NEP by overexpression of a peptidase dead mutant or knockdown by siRNA in NEP+/+ cells increased migration and proliferation. Loss of NEP led to an increase in Src kinase activity and phosphorylation of PTEN resulting in activation of the PDGF receptor (PDGFR). Knockdown of Src kinase with siRNA or inhibition with PP2 a src kinase inhibitor decreased PDGFRY751 phosphorylation and attenuated migration and proliferation in NEP−/− SMCs.
NEP substrates, endothelin-1(ET-1) or fibroblast growth factor-2 (FGF2), increased activation of Src and PDGFR in NEP+/+ cells, which was decreased by an ETAR antagonist, neutralizing antibody to FGF2 and Src inhibitor.
Similar to the observations in PASMCs levels of p-PDGFR, p-Src and p-PTEN were elevated in NEP−/− lungs. ETAR antagonist also attenuated the enhanced responses in NEP−/−PASMCs and lungs. Taken together our results suggest a novel mechanism for regulation of PDGFR signaling by NEP substrates involving Src and PTEN. Strategies that increase lung NEP activity/expression or target key downstream effectors, like Src, PTEN or PDGFR, may be of therapeutic benefit in pulmonary vascular disease.
Keywords: Neprilysin, smooth muscle cell, migration, PDGFR, Src, PTEN
INTRODUCTION
Neprilysin (NEP) is a cell-surface peptidase expressed in vascular cells, cardiac myocytes, lung, brain, renal epithelial cells and fibroblasts1, 2. It catalyzes the degradation of various neuropeptides implicated in migration, proliferation and contraction, including atrial naturetic peptide (ANP), enkephalins, substance P, bradykinin, endothelin-1 and angiotensin II1. Among growth factors, fibroblast growth factor (FGF) is cleaved by NEP but PDGF and transforming growth factor (TGFβ) are not3. In addition to its peptidase activity basic amino acid residues in the tail of NEP contribute to protein–protein interactions resulting in modulation of signaling factors in prostate cancer cell lines4.
NEP deficient mice have an exaggerated response to inflammatory stimuli with colitis, septic shock and pancreatitis induced lung injury5, 6,7. We have shown that NEP null mice develop exaggerated pulmonary hypertension accompanied by increased muscularization of distal pulmonary arteries (PAs) and medial and adventitial thickening in response to hypoxia8.
In systemic circulation inhibitors of NEP have a protective role and prevent fatty streak formation and atherosclerotic plaques in animal models of hypertension9. It has been observed that increase in NEP activity correlated with increased risk of hypertension, insulin resistance and obesity in human subjects10. Studies addressing mechanisms by which NEP regulates pulmonary vascular function could be important in determining the potential use of vasopeptidase inhibitors for systemic hypertension.
METHODS
NEP−/− mice
C57BL/6 NEP null mice were routinely used for isolation of PASMCs. In vivo studies were performed on NEP null mice on both C57BL/6 and FVB/n background.
Isolation and characterization of PASMC from adult mice
PASMCs were isolated from proximal medial tissue of the pulmonary artery from individual age matched 13–17wk old NEP+/+ and NEP−/− littermate mice as described8. In all experiments `n' represents population of cells each isolated from different matched NEP+/+ and NEP−/−mice.
Boyden chamber assay
Migration was determined by a modified Boyden chamber assay using a polycarbonate filter with 8μM pore diameter obtained from Neuroprobe (Gaithersburg, MD)11. The cells that migrated to the lower surface were counted (five random 20× fields/well).
Scratch assay
PASMCs were grown to confluence on 60 mm plates12. The cells were serum starved in DMEM-F-12 medium with 0.2% serum for 24 h. Quantitation of migration was done by counting the number of PASMCs in a 5 cm2 area of the scratch after 6h. Average from 3 different populations was used for statistical analysis.
3[H] Thymidine incorporation
The effect of growth factors and neuropeptides on DNA synthesis was evaluated as previously reported8.
Western blotting
Cell lysates were prepared, proteins separated on SDS-PAGE and transferred to nitrocellulose. Membranes were incubated with primary antibody and protein bands visualized by chemiluminescence8. GAPDH was used as a loading control. A Bio-Rad gel scanner and densitometer (Gel DocXR with Quantity 1 program) were used to assess the intensity of the bands obtained by Western blots.
Flow Cytometry
PASMCs were fixed with paraformaldehyde 2% and methanol and stained with antibodies to PDGFRα and PDGFRβ. Cells were analyzed using a Gallios flow cytometer (Beckman Coulter) and Summit 4.3 software® (Beckman Coulter). Ten thousand events were obtained and analyzed for % gated singlets and mean intensity. Overlay plots were obtained using Kaluza software®. Four different paired cell isolates were used for analysis.
Lentivirus infection
PASMCs were transfected with lentivirus expressing human full length NEP or peptidase dead mutant NEP (NEPX) at an MOI of 10 as described previously8.
SiRNA transfection
PASMC were transfected with mouse specific siRNA (10nM) or universal siRNA from Sigma Aldrich, using Dharmafect Reagent® from Dharmacon (Denver, CO) as per manufacturer recommendations. SiRNA for NEP, PDGFRα, PDGFRβ and Src were obtained from Sigma. Cells were used 48h after transfection for migration and proliferation assays.
ETAR antagonist treatment
PASMCs were treated with ETAR antagonist Ambrisentan (10 μM). NEP+/+ and NEP−/− mice on FVB/n background were treated with the ETA receptor antagonist Atrasentan (10mg/Kg) for 7 days in drinking water.
Statistical analysis
Data were analyzed using GraphPad Prism 4.02 for Windows (GraphPad Software for Science Inc., San Diego, CA). Results are presented as mean ± SEM. The significance of differences between two measurements was determined by unpaired, two-tailed t-tests; one-way analysis of variance was used for multiple comparisons followed by Fishers least significance analyses. P<0.05 was considered statistically significant. The `n' for each experiment represents the number of cell isolates each obtained from a different mouse.
RESULTS
Loss of NEP leads to increased migration and proliferation of mouse PASMC
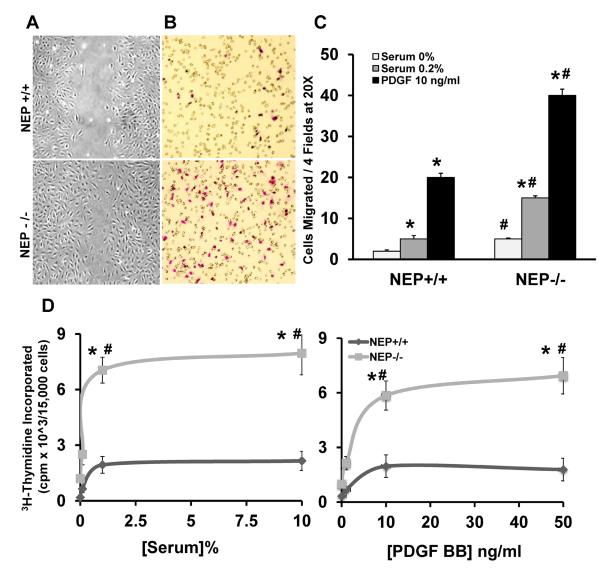
Migration and proliferation of PASMCs are important mechanisms contributing to pulmonary vascular remodeling8, 13. PASMCs from NEP−/− mice exhibited increased migration in the presence of serum (0.2%) and PDGF-BB (10ng/mL) compared to wild type cells assessed by wound healing and Boyden chamber assays (Figure 1A–C). Proliferation was measured by 3H-thymidine incorporation at three different doses of serum and PDGF and showed 3–4 fold higher incorporation in NEP−/− PASMC compared to NEP+/+ cells (Figure 1D and E).
Figure 1. Loss of NEP leads to increased migration and proliferation of murine PASMCs.
Migration of PASMCs isolated from NEP+/+ and NEP−/− mice was measured by scratch and Boyden chamber assays and proliferation by 3H-thymidine incorporation as described in Methods. Panel A shows a representative scratch assay and Panel B a stained filter of migrated cells from a Boyden chamber assay. Number of cells migrated in 20× field from three different populations of PASMCs in response to serum (0.2%) and PDGF (10ng/ml) using Boyden chamber assay is shown in Panel C. Thymidine incorporation in the presence of increasing concentrations of serum (0, 0.2, 1 and 10%) and PDGF (0, 1, 10 and 50ng/ml) from 3 different populations of PASMCs is shown in Panels D and E. *p≤ 0.05 for comparison of control saline and treatment group. #p≤ 0.05 for comparisons between NEP +/+ and −/− PASMCs.
Twelve of 15 different matched pairs of NEP+/+ and −/− PASMCs showed a major difference in proliferation on initial screening; these cell lines were used for further study. Enhanced SMC outgrowth was also observed from NEP−/− PA tissue cultured ex vivo compared to +/+ control (data not shown), suggesting that the differences observed in phenotype were intrinsic to the PASMC and not acquired over time in culture. Scratch assays performed in the presence of mitomycin (10μM) had no effect on the enhanced migration of NEP−/− PASMCs suggesting that proliferation did not contribute (Supplement Figure1A).
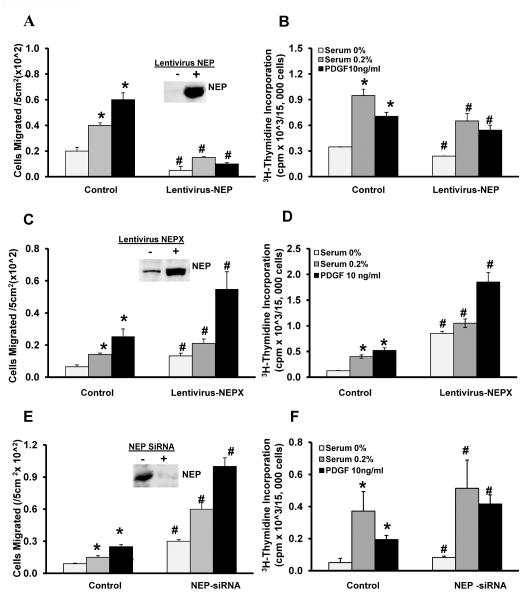
Altering NEP levels modifies migration and proliferation of PASMCs
NEP−/− PASMCs were infected with lentiviral vector expressing full length human NEP. Migration and proliferation were measured after 48h. As seen in Figure 2A–B, replacing NEP in null cells attenuated migration and proliferation in the presence of serum (0.2%) and PDGF. Insert in Figure 2A shows levels of NEP expressed.
Figure 2. Manipulating NEP levels affect migration and proliferation in PASMCs.
NEP−/− PASMCs were infected with lentivirus expressing full length human NEP. NEP+/+ PASMCs were infected with lentivirus expressing a peptidase dead mutant (NEPX) or transfected with NEP siRNA (10nMole/L). Migration and proliferation were assessed after 48h. Panel A shows the number of cells migrated/5 cm2 area from 3 different isolates along with a representative blot showing NEP levels after infection with lentivirus in null cells. Thymidine incorporation is shown in Panel B. The effect of expression of NEPX on migration is shown in Panel C along with a representative blot of NEPX levels. Panel D shows the effect on thymidine incorporation in NEP +/+ PASMCs. The effect of NEP knockdown with siRNA on migration along with a representative blot showing a decrease in NEP levels is shown in Panel E and Panel F shows effect on thymidine incorporation. *p≤ 0.05 for comparison of saline and treatment group. # p≤ 0.05 for comparisons between control and lentivirus or siRNA treatments.
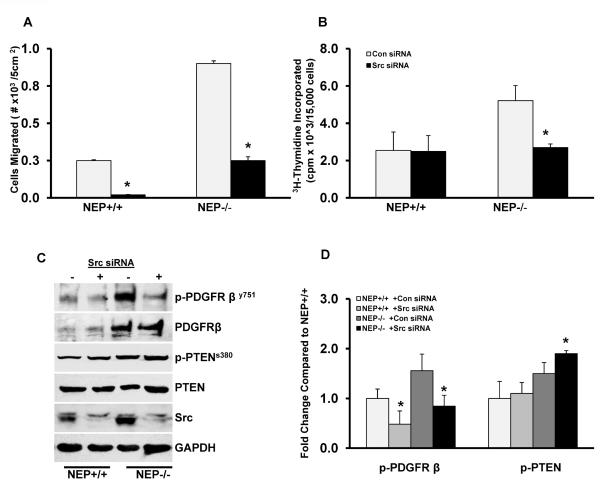
Inactivation of NEP in +/+ cells with either a lentivirus expressing a peptidase dead mutant of human NEP (NEPX), or with the inhibitor phosphoramidon, or knockdown of expression with siRNA to mouse NEP caused increased migration and proliferation in response to serum and PDGF similar to that observed with NEP−/− cells (Figure 2C–F). There was a 4-fold increase in NEPX levels as shown in Figure 2C. There was a 98 ± 2% decrease in NEP expression after treatment with siRNA as shown in Figure 2E. Inhibition of NEP with phosphoramidon enhanced migration and proliferation in NEP+/+ PASMCs similar to that observed with mutant NEPX (Supplement Figure 1B and C). NEPX had no effect on migration and proliferation in NEP−/−cells (data not shown) suggesting that the peptidase activity of NEP is required to inhibit the exaggerated responses in null cells and that substrates on NEP are mediating the effect.
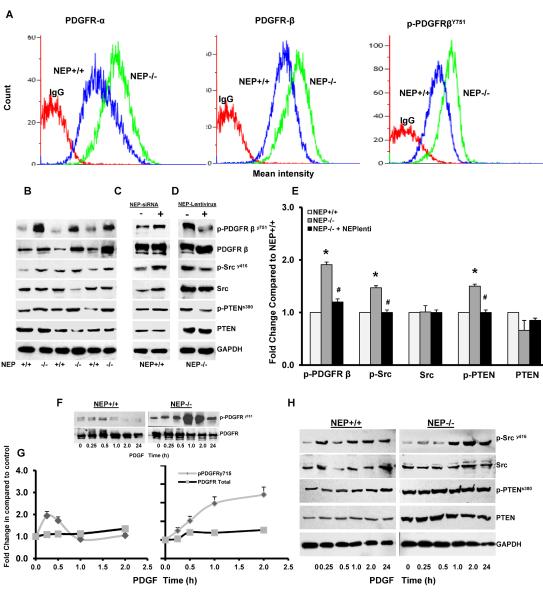
Loss of NEP increases PDGFR expression and signaling in PASMCs
To determine whether the enhanced migratory and proliferative responses to PDGF were due to an increase in receptor expression, we measured PDGFRα, β and βY751 levels by flow cytometry (Figure 3A) and Western blot (Figure 3B and Supplement Figure 2A). Protein levels of PDGFRα, β and activated βY751 were significantly higher in NEP−/− cells (Supplement Table 1). A decrease in mRNA for both receptors PDGFRα (0.67 fold) and β (0.56 fold) was observed in NEP null PASMCs, perhaps representing an autoregulatory mechanism to downregulate receptor expression (Supplement Figure 2B).
Figure 3. Loss of NEP increases PDGFR expression and signaling in NEP−/− PASMCs.
Flow cytometry analysis was performed on NEP+/+ and NEP−/− PASMCs to assess levels of PDGFRα, β and p-PDGFR751 expression. An overlay from a representative paired isolate is shown in Panel A. Lysates were analyzed for levels of phospho- and total PDGFR, Src and PTEN at baseline in NEP+/+ and NEP−/− PASMCs by Western blot shown in Panel B. Effects of NEP siRNA (10nMole/L) in NEP+/+ PASMCs, and NEP lentivirus in NEP−/− PASMCs on expression of phospho- and total PDGFR, Src and PTEN are shown in Panels C and D, respectively. Panel E shows the fold change in expression levels of the different kinases in NEP−/− PASMCs compared to matched NEP+/+ cells for 6 different paired isolates. Data was normalized to GAPDH used as a loading control. Panel F shows time dependent changes (0–24h) in levels of p-PDGFRy715 and total PDGFR levels in response to PDGF(10ng/ml) in NEP+/+ and NEP−/− cells and Panel G a comparison plot of change with time. Panel H shows change in levels of p-Src and p-PTEN in response to PDGF (10ng/ml) in NEP+/+ and NEP−/− cells. *p≤ 0.05 for comparison between NEP+/+ and −/− PASMC. # p≤ 0.05 for comparisons between NEP−/− with and without lentivirus treatment.
Src and PTEN influence PDGFR signaling by phosphorylation dependent mechanisms and are also regulated by NEP14,15. As shown in Figure 3B, levels of SrcY416 and PTENS380 were higher in NEP−/− PASMCs. Total PTEN levels trended lower (0.6 fold) in NEP−/− compared to +/+ cells but were variable and did not reach statistical significance.
SiRNA-mediated knock down of NEP in wild type PASMCs caused a 1.8-fold increase in p- PDGFRY751, a 1.5-fold increase in p-Src and a 1.2-fold increase in pPTEN similar to that seen in null cells (Figure 3C). Conversely, lentiviral expression of NEP in −/− PASMCs attenuated PDGFR, Src and PTEN phosphorylation (Figure 3D and 3E).
We compared effect of PDGF treatment on phosphorylation of PDGFR, Src and PTEN in NEP+/+ and −/− PASMCs and is shown in Figure 3F and G. PDGFR phosphorylation in NEP+/+ cells was transient and increased at 15 and 30 min, while a more sustained rise is seen in NEP−/− PASMCs. Total levels of PDGFR were not affected by the treatment. Figure 3H shows that the level of p-Src was 2 fold higher and total PTEN lower at 24h in NEP−/− PASMCs.
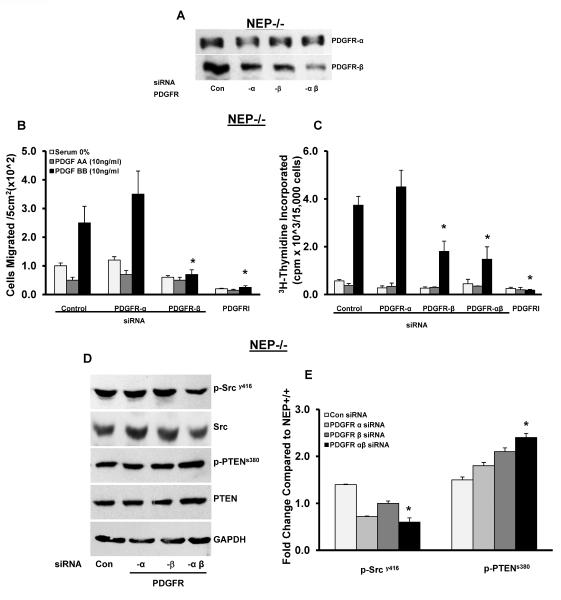
Inhibition of PDGFR attenuates migration and proliferation in PASMCs
PASMCs treated with siRNA to PDGFRα, β and αβ were used to assess the contribution of the two receptors to migration and proliferation. As shown in Figure 4A partial knockdown of each of the receptors was observed at 48h. Average expression levels from 3 different isolates were 55 ± 15% for PDGFRα and 45 ± 15% for β and 25 ±20% for αβ siRNA. We cannot rule out some degree of antibody cross reactivity to the two receptors contributing to the observed magnitude of each knockout.
Figure 4. Knockdown of PDGFR attenuates migration and proliferation and normalizes PDGFR signaling in NEP−/− PASMCs.
PASMCs were treated with either control siRNA or siRNA to PDGFRα, β or αβ and migration and proliferation were measured after 48h. Panel A shows levels of PDGFRα and –β receptors in the presence of siRNA. PDGF AA (10 ng/ml; ligand specific for PDGFRα) and PDGF BB (10 ng/ml; specific for PDGFR β) were used to assess the contribution of each receptor to migration and proliferation in siRNA or PDGFR kinase inhibitor III (PDGFRI, 500 nM/L for 24h) treated NEP −/− cells shown in Panels B and C from three different isolates. NEP−/− PASMCs treated with PDGFRα, β and αβ siRNA were analyzed for p-PDGFR, p-Src and p-PTEN and levels are shown in Panel D. Panel E shows average levels from 3 different isolates. *p≤ 0.05 for comparisons between control siRNA and PDGFR siRNA treatment (n=3).
We found that selective PDGFRβ, but not α, activation stimulated migration and proliferation in both cell types (Figure 4B–C, Supplement Figure 3A–B). Results obtained from siRNA-mediated knockdown of PDGFRα, β and αβ show that PDGFRβ was the predominant receptor contributing to migration and proliferation of PASMCs (Figures 4B–C). Cells treated with siRNA to PDGFRα maintained their migratory and proliferative response to PDGF-BB (Figure 4B–C).
We assessed levels of phospho and total Src and PTEN in PASMCs treated with siRNA to PDGFRα, β and αβ. As seen in Figure 4D, knockdown of both PDGFRα and β caused a significant decrease in p-Src with total levels remaining unchanged. In the case of PTEN, knockdown of PDGFR increased both p-PTEN and total PTEN levels. Total PTEN levels were 1.8 fold higher in cells treated with PDGFR αβ siRNA (Figure 4E).
We found that knockdown of PDGFR αβ had significant effects on Src kinase activation in PASMCs suggesting both receptors contribute to its activation (Figure 4D–E). We think the residual p-Src is due to PDGFR-independent and neuropeptide-dependent activation, because PDGFRβ or αβ siRNA completely inhibited basal p-Src phosphorylation in NEP+/+ cells (data not shown). It is interesting that PDGFRα associated Src activity does not influence migratory and proliferative responses whereas PDGFRβ does, suggesting that the two receptors couple to different downstream effectors.
Pharmacologic inhibition of the PDGFR kinase (PDGFR inhibitor III) attenuated migration and proliferation of NEP−/− PASMCs (Figure 4A and B, Supplement Figure 3A–B). Inhibition of PDGFR kinase activity did not show significant effects on Src phosphorylation in NEP−/− PASMCs suggesting that activity of PDGFR was not required for Src activation and may be mediated by the NEP substrates (Supplement Figure 3C and D). We observed reduced p-PTEN and total PTEN in cells treated with PDGFRI. Additional studies are required to understand the regulation of PTEN by PDGFR.
Inhibition of Src kinase attenuates migration and proliferation in mouse PASMC
To determine the role of activated Src, we used siRNA to knockdown Src and PP2, a Src kinase inhibitor, and measured effects on migration and proliferation. Western analysis confirmed a 90% ±10 decrease of Src protein with no significant changes in Fyn and Lyn (data not shown). As seen in Figure 5A–B, treatment of PASMCs with Src siRNA inhibited migration and decreased proliferation to baseline levels in NEP −/− cells. Similarly, PASMCs treated with the inhibitor, PP2 (10μM), had decreased migration and proliferation (Supplement Figure 4A and B). Knockdown of Src with siRNA reduced PDGFRY751 phosphorylation to baseline levels and increased PTEN phosphorylation (Figure 5C and D). Src siRNA also increased total PTEN levels. In comparison, inhibition of Src kinase with PP2 treatment abolished PDGFRY751 phosphorylation but did not have significant effects on p-PTEN (Supplement Figure 4C and D).
Figure 5. SiRNA mediated knockdown of Src kinase attenuates migration and proliferation in NEP−/− PASMCs.
NEP+/+ and −/− PASMCs were treated with Src siRNA (10nM for 48h), and effects on migration and proliferation was measured (Panels A and B). Effect of Src kinase knockdown on phospho- and total levels of PDGFR, Src and PTEN at baseline is shown in Panel C. Panel D shows quantitation of p-PDGFR and p-PTEN in NEP−/− PASMCs compared to +/+ cells from 3 different paired isolates at baseline. *p≤ 0.05 for comparisons of control to Src siRNA (n=3).
NEP substrates enhance PDGFR induced migration and proliferation and signaling in NEP+/+ cells
ET-1 and FGF2 are representative substrates of NEP and have been demonstrated to play a role in the pathogenesis of pulmonary hypertension16. Levels and stability of ET-1 in cell culture supernatants and FGF2 in cell lysates were measured at baseline and was found to be higher in NEP−/− PASMCs (Supplement Figure 5).
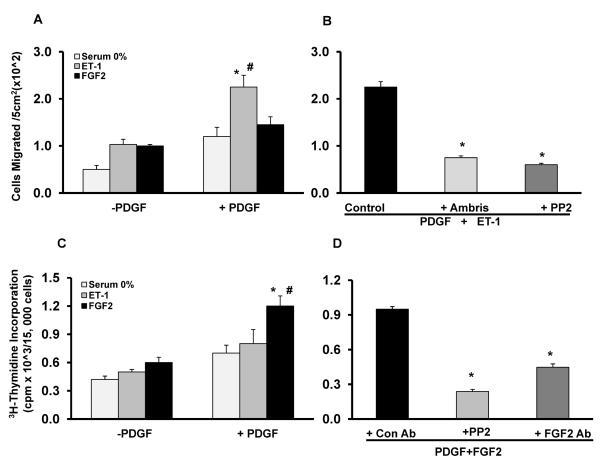
NEP+/+ PASMCs were treated with PDGF in the presence or absence of ET-1 or FGF2. ET-1 enhanced PDGF induced migration significantly, which was attenuated by Ambrisentan, an ET receptor antagonist (Figure 6A–B). FGF2 had more of a potentiating effect on the proliferative response of PDGF, which was attenuated by neutralizing antibody to FGF2 (Figure 6C–D). Inhibition of Src kinase with PP2 also attenuated the enhanced migratory response to PDGF plus ET-1 and the proliferative response to PDGF plus FGF2 (Figure 6B and D).
Figure 6. NEP substrates augment PDGF-induced migration and proliferation in NEP+/+ PASMCs.
NEP+/+ PASMCs were treated with ET-1(10nM) and FGF2 (10ng/ml) in the presence or absence of PDGF (10ng/ml) and effects on migration and proliferation were assessed. Panel A shows effects of ET-1 and FGF2 on PDGF induced migration in a scratch assay. Panel B shows effect of ETAR antagonist Ambrisentan (10μM) and PP2 (10μM) on PDGF+ET-1 induced migration. Panel C shows the effect of ET-1 and FGF2 on PDGF-induced thymidine incorporation. Panel D shows the effect of PP2 (10μM) and neutralizing FGF2 antibody (1μg/ml) on PDGF+FGF2 induced thymidine incorporation. *p≤ 0.05 for comparisons of control saline to PDGF (n=3). # p≤ 0.05 for comparisons of −PDGF treatment to +PDGF.
Neutralizing antibody to FGF2 inhibited proliferation but not migration of NEP−/− cells. In contrast, neutralizing antibody to PDGF attenuated migration of NEP null cells. There was also a trend toward an inhibitory effect on growth (Supplement Figure 5A–D).
ET-1 and FGF2 enhance PDGF signaling in NEP+/+ PASMCs by a Src dependent Mechanism
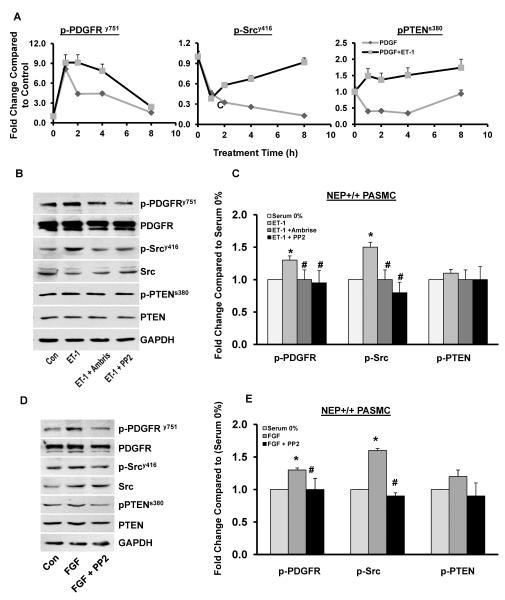
To further analyze the synergy between representative NEP substrates and PDGF signaling, NEP+/+ PASMCs were treated with PDGF in the presence or absence of ET-1 for 1, 2, 4, and 8h and levels of phospho and total PDGFR, Src and PTEN were measured. The magnitude of p-PDGFR, p-Src and p-PTEN activation was 1.3–1.5 fold higher and sustained with PDGF in the presence of ET-1(Figure 7A).
Figure 7. NEP substrates enhance PDGF signaling in NEP+/+ PASMCs.
NEP+/+ PASMCs were treated with PDGF in the presence or absence ET-1 for 1, 2, 4, and 8h and lysates were analyzed for levels of phospho and total PDGFR, Src and PTEN. Panel A shows graphical representation of change in p-PDGFR, p-Src and p-PTEN levels with time (n=3). NEP+/+ PASMCs were treated with ET-1 (10nM) for 8h in the presence or absence of Ambrisentan (10 μM) or PP2 (10μM) and lysates analyzed for levels of phospho and total PDGFR, Src and PTEN shown in Panel C. Panel D shows a graphical representation of effects of Ambrisentan and PP2 on the phospho levels from 3 different isolates. Panel E shows effect of PP2 (10μM) on FGF2 (10 ng/ml) induced levels of phospho and total PDGFR, Src and PTEN. Graphical representation from 3 different isolates is shown in Panel F. *p≤ 0.05 for comparisons of control to treated (n=3). # p≤ 0.05 for comparisons of ET-1/FGF ± inhibitor treatment.
Treatment of NEP+/+ PASMCs with ET-1 increased phosphorylation of PDGFRy751 by 1.3 fold and Src by 1.5 fold, and was inhibited by Ambrisentan and PP2 (Figure 7B–C). A time course of activation of PDGFR and Src by ET-1 is shown in Supplement Figure 7A. Treatment of NEP+/+ cells with FGF2 for 24h increased PDGFR phosphorylation by 1.3-fold and Src by 1.5-fold (Figure 6B and Supplement Figure7B). Inhibition of Src eliminated FGF2-induced PDGFR phosphorylation (Figure 7D–E).
ETAR antagonist attenuates PDGF responses in NEP−/− PASMCs and lungs
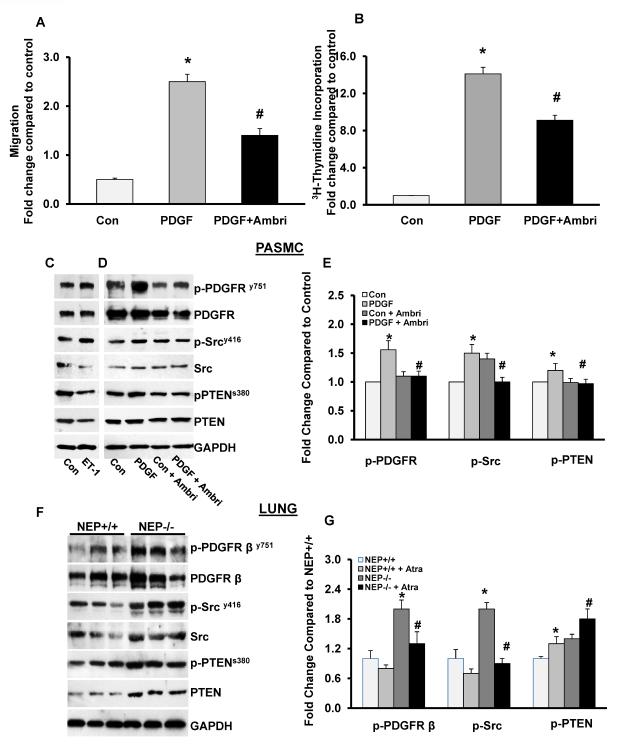
ETA receptor antagonist Ambrisentan attenuated both migration and proliferation of PDGF-stimulated NEP−/−cells (Figure 8A–B). Addition of ET-1 (10 nM) further increased phosphorylation of Src and decreased PTEN levels (Figure 8C). Ambrisentan decreased PDGFR and PTEN phosphorylation both at baseline and in response to PDGF (Figure 8D and E). Src activity was inhibited only in response to PDGF. Analysis of lung lysates from NEP−/− vs +/+ mice showed that p-PDGFR and p-Src and p-PTEN were higher in the null tissue, suggesting that the pathway is activated in vivo. Treatment with ETAR antagonist Atrasentan attenuated the baseline phosphorylation of PDGFR and Src and increased levels of PTEN (Figure 8F and G).
Figure 8. ETA antagonist attenuates PDGF responses in NEP−/− PASMCs and lungs.
NEP−/− PASMCs were treated with PDGF with and without Ambrisentan. Migration and proliferation was measured as shown in Panels A and B. Lysates from cells treated with ET-1 or PDGF with and without Ambrisentan were analyzed for levels of phospho and total PDGFR, Src and PTEN. Effect of ET-1 alone is shown in a representative Western blot in Panel C and the effect of Ambrisentan in Panel D. Panel E shows average data from 5 different populations. Lysates from lungs of NEP+/+ and −/− mice were analyzed for levels of phospho and total PDGFR, Src and PTEN shown in Panel F. Panel G shows effect of ETAR antagonist Atrasentan (10mg/Kg/ for 7d) on phosphorylation. *p≤ 0.05 for comparisons of control to PDGF (n=5). # p≤ 0.05 for comparisons of PDGF ± ETAR antagonist in Panel E and NEP+/+ to NEP−/− in Panel G (n=6).
DISCUSSION
In this study we show that loss of the endopeptidase activity of NEP in PASMCs leads to increased migration and proliferation to growth stimuli. A peptidase-inactive mutant of NEP or knockdown with siRNA showed similar effects in wild type cells suggesting a role for NEP substrates, which include vasoconstrictors and dilators belonging to the GPCR family of ligands.
Our results demonstrate a mechanism by which NEP contributes to pulmonary vascular remodeling involving neuropeptide-mediated transactivation of growth factor receptor and signaling intermediates17. In NEP−/− cells, increased local concentrations of NEP substrates cause phosphorylation of Src kinase, inactivation of PTEN and constitutive activation of PDGFR, which is partially reversed by the ETA receptor antagonist Ambrisentan (Figure 8). Enhanced PDGFR signaling was observed in NEP−/− lungs, and was attenuated by a ETAR antagonist.
Representative NEP substrates, ET-1 and FGF2, synergize with PDGF in wild type PASMCs and increase phosphorylation of Src kinase and PDGFR. This phosphorylation is inhibited by Ambrisentan and neutralizing antibody to FGF2 (Figure 7). This study provides new insights into how ET receptor antagonists may exert some of their attenuating effects on vascular cell migration and growth (Figure 7).
PDGF and PDGFR are overexpressed in rodent models of pulmonary hypertension and in PA of patients with idiopathic pulmonary hypertension18,19. In experimental models it has been shown that PDGFR signaling is suppressed by BMPR2, PPARγ and PTEN, and expression of these proteins is frequently lost in pulmonary hypertension20, 21, 22. Our results suggest a novel mechanism by which a peptidase suppresses PDGF signaling and maintains SMC in a quiescent state. Loss of NEP in PASMCs leads to activation of Src resulting in phosphorylation and expression of PDGFR (Figures 3 and 4).
A potential role for Src kinase has been described in the BMPR2 kinase deficient model of pulmonary hypertension23. In NEP null PASMCs increased levels of NEP substrates, ET-1 and FGF2, caused Src activation and enhance PDGFR signaling24. Inhibition or knockdown of Src decreases phosphorylation of PDGFRY751 and attenuates migration and proliferation (Figure 5C–E).
PTEN plays an important role in vascular biology and its loss in mice causes pulmonary hypertension25. We have found that PTEN is inactivated by phosphorylation and expressed at lower levels in NEP−/− PASMCs compared to wild type cells. Expression of NEP in null cells attenuated the increased phosphorylation of PTEN (Figure 3D). In addition, siRNA mediated knockdown of PDGFR or Src increases PTEN levels whereas the kinase inhibitors decrease phosphorylation suggesting an indirect regulatory mechanism (Figures 4E and F and 5C–D Supplement Figures 4 and 5).
Vasopeptidase inhibitors that target NEP have been shown to be beneficial in systemic hypertension but were associated with increased risk of angioedema26, 27. Our results suggest that NEP inhibition in the pulmonary circulation could predispose the lung vasculature to injury. The role of NEP in different tissues may depend on local substrates available and resident cell composition and phenotype. These factors may vary in different vascular beds, emphasizing the need for understanding the mechanism of NEP action in the lung.
We demonstrate here for the first time that NEP activity is required to maintain a quiescent PASMC phenotype. This is consistent with our recent observations that NEP null mice have enhanced hypoxia-induced pulmonary vascular remodeling and expression of NEP is decreased in lungs of patients with chronic pulmonary obstructive disease (COPD) and pulmonary vascular remodeling28, 8. We also show that ETA antagonist effects may be mediated at least in part by inhibition of PDGFR responses. Strategies to increase NEP activity/expression or directly inhibit Src and PDGFR in lung may be of therapeutic benefit for prevention and treatment of pulmonary hypertension.
PERSPECTIVES
NEP is a cell surface enzyme that cleaves and inactivates both vasodilator and vasoconstrictor neuropeptides important for vascular function. Loss of NEP in PASMCs activates an integrated signaling network involving neuropeptides and growth factors with Src kinase playing a central role. This cascade leads to increased migration and growth of PASMCs, an important component of vascular remodeling in pulmonary hypertension. Although NEP inhibitors may have protective effects in the systemic circulation, our observations also suggest that these drugs could predispose the lung vasculature to injury. Future studies will determine whether vascular bed specific functions of NEP are due to intrinsic differences in cell types or to differences in substrates of NEP.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
-
…
Loss of NEP leads to integrated signaling of NEP substrates, Src, PTEN and PDGFR and enhanced migration and proliferation of PASMCs. This unexpected finding explains why reduced NEP predisposes to exaggerated pulmonary hypertension.
What is relevant?
-
…
NEP is required for maintenance of neuropeptide levels, regulation of vascular tone and keeping PASMCs in a quiescent state.
-
…
Loss of NEP leads to increased migration and proliferation of PASMCs, important contributors to vascular remodeling in pulmonary hypertension.
-
…
This study suggests new therapeutic approaches for the treatment of pulmonary hypertension.
Summary
This study demonstrates how loss of NEP activity leads to increased migration and proliferation of PASMCs involving cross talk between GPCRs and growth factors, and increase in Src kinase and PDGFR signaling. Substrates of NEP such as ET-1 and FGF are currently therapeutic targets for the treatment of pulmonary hypertension. Our results suggest that NEP inhibitors could predispose the lung vasculature to injury by increasing levels of these peptides. Future studies will determine whether vascular bed specific functions of NEP are due to intrinsic differences in cell types or differences in substrates of NEP.
ACKNOWLEDGEMENTS
We thank Marian Maslak for expert general administrative and technical assistance and Dr Hanjun Guan for lentiviral constructs.
SOURCES OF FUNDING The work was supported by funding from NHLBI - HL078927, HL014985 and HL095439 and VA Merit Review.
ABREVIATIONS
- NEP
Neprilysin
- PDGF
platelet derived growth factor
- PASMC
pulmonary artery smooth muscle cell
Footnotes
DISCLOSURES None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- 1.Turner AJ. Exploring the structure and function of zinc metallopeptidases: Old enzymes and new discoveries. Biochem Soc Trans. 2003;31:723–727. doi: 10.1042/bst0310723. [DOI] [PubMed] [Google Scholar]
- 2.Fitzpatrick PA, Guinan AF, Walsh TG, Murphy RP, Killeen MT, Tobin NP, Pierotti AR, Cummins PM. Down-regulation of neprilysin (ec3.4.24.11) expression in vascular endothelial cells by laminar shear stress involves nadph oxidase-dependent ros production. Int J Biochem Cell Biol. 2009;41:2287–2294. doi: 10.1016/j.biocel.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Horiguchi A, Chen DY, Goodman OB, Jr, Zheng R, Shen R, Guan H, Hersh LB, Nanus DM. Neutral endopeptidase inhibits prostate cancer tumorigenesis by reducing fgf-2-mediated angiogenesis. Prostate Cancer Prostatic Dis. 2008;11:79–87. doi: 10.1038/sj.pcan.4500984. [DOI] [PubMed] [Google Scholar]
- 4.Sumitomo M, Shen R, Nanus DM. Involvement of neutral endopeptidase in neoplastic progression. Biochim Biophys Acta. 2005;1751:52–59. doi: 10.1016/j.bbapap.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Sturiale S, Barbara G, Qiu B, Figini M, Geppetti P, Gerard N, Gerard C, Grady EF, Bunnett NW, Collins SM. Neutral endopeptidase (ec 3.4.24.11) terminates colitis by degrading substance p. Proc Natl Acad Sci U S A. 1999;96:11653–11658. doi: 10.1073/pnas.96.20.11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu B, Gerard NP, Kolakowski LF, Jr, Finco O, Carroll MC, Gerard C. Neutral endopeptidase modulates septic shock. Ann N Y Acad Sci. 1996;780:156–163. doi: 10.1111/j.1749-6632.1996.tb15119.x. [DOI] [PubMed] [Google Scholar]
- 7.Lu B, Figini M, Emanueli C, Geppetti P, Grady EF, Gerard NP, Ansell J, Payan DG, Gerard C, Bunnett N. The control of microvascular permeability and blood pressure by neutral endopeptidase. Nat Med. 1997;3:904–907. doi: 10.1038/nm0897-904. [DOI] [PubMed] [Google Scholar]
- 8.Dempsey EC, Wick MJ, Karoor V, Barr EJ, Tallman DW, Wehling CA, Walchak SJ, Laudi S, Le M, Oka M, Majka S, Cool CD, Fagan KA, Klemm DJ, Hersh LB, Gerard NP, Gerard C, Miller YE. Neprilysin null mice develop exaggerated pulmonary vascular remodeling in response to chronic hypoxia. Am J Pathol. 2009;174:782–796. doi: 10.2353/ajpath.2009.080345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jandeleit-Dahm K, Lassila M, Davis BJ, Candido R, Johnston CI, Allen TJ, Burrell LM, Cooper ME. Anti-atherosclerotic and renoprotective effects of combined angiotensin-converting enzyme and neutral endopeptidase inhibition in diabetic apolipoprotein e-knockout mice. J Hypertens. 2005;23:2071–2082. doi: 10.1097/01.hjh.0000184747.41565.a1. [DOI] [PubMed] [Google Scholar]
- 10.Standeven KF, Hess K, Carter AM, Rice GI, Cordell PA, Balmforth AJ, Lu B, Scott DJ, Turner AJ, Hooper NM, Grant PJ. Neprilysin, obesity and the metabolic syndrome. Int J Obes (Lond) 2011;35:1031–1040. doi: 10.1038/ijo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrington TP, Ishizuka T, Papst PJ, Chayama K, Webb S, Yujiri T, Sun W, Sather S, Russell DM, Gibson SB, Keller G, Gelfand EW, Johnson GL. Mekk2 gene disruption causes loss of cytokine production in response to ige and c-kit ligand stimulation of es cell-derived mast cells. Embo J. 2000;19:5387–5395. doi: 10.1093/emboj/19.20.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuevas BD, Abell AN, Witowsky JA, Yujiri T, Johnson NL, Kesavan K, Ware M, Jones PL, Weed SA, DeBiasi RL, Oka Y, Tyler KL, Johnson GL. Mekk1 regulates calpain-dependent proteolysis of focal adhesion proteins for rear-end detachment of migrating fibroblasts. Embo J. 2003;22:3346–3355. doi: 10.1093/emboj/cdg322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54:S10–19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Lu Y, Yu Q, Liu JH, Zhang J, Wang H, Koul D, McMurray JS, Fang X, Yung WK, Siminovitch KA, Mills GB. Src family protein-tyrosine kinases alter the function of pten to regulate phosphatidylinositol 3-kinase/akt cascades. J Biol Chem. 2003;278:40057–40066. doi: 10.1074/jbc.M303621200. [DOI] [PubMed] [Google Scholar]
- 15.Ganju RK, Shpektor RG, Brenner DG, Shipp MA. Cd10/neutral endopeptidase 24.11 is phosphorylated by casein kinase ii and coassociates with other phosphoproteins including the lyn src-related kinase. Blood. 1996;88:4159–4165. [PubMed] [Google Scholar]
- 16.ten Freyhaus H, Dumitrescu D, Berghausen E, Vantler M, Caglayan E, Rosenkranz S. Imatinib mesylate for the treatment of pulmonary arterial hypertension. Expert Opin Investig Drugs. 2012;21:119–134. doi: 10.1517/13543784.2012.632408. [DOI] [PubMed] [Google Scholar]
- 17.Pyne NJ, Pyne S. Receptor tyrosine kinase-g-protein-coupled receptor signalling platforms: Out of the shadow? Trends Pharmacol Sci. 2011;32:443–450. doi: 10.1016/j.tips.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Katayose D, Ohe M, Yamauchi K, Ogata M, Shirato K, Fujita H, Shibahara S, Takishima T. Increased expression of pdgf a- and b-chain genes in rat lungs with hypoxic pulmonary hypertension. Am J Physiol. 1993;264:L100–106. doi: 10.1152/ajplung.1993.264.2.L100. [DOI] [PubMed] [Google Scholar]
- 19.Dahal BK, Heuchel R, Pullamsetti SS, Wilhelm J, Ghofrani HA, Weissmann N, Seeger W, Grimminger F, Schermuly RT. Hypoxic pulmonary hypertension in mice with constitutively active platelet-derived growth factor receptor-beta. Pulm Circ. 2011;1:259–268. doi: 10.4103/2045-8932.83448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol. 42:482–490. doi: 10.1165/rcmb.2008-0132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, Rabinovitch M. An antiproliferative bmp-2/ppargamma/apoe axis in human and murine smcs and its role in pulmonary hypertension. J Clin Invest. 2008;118:1846–1857. doi: 10.1172/JCI32503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guignabert C, Alvira CM, Alastalo TP, Sawada H, Hansmann G, Zhao M, Wang L, El-Bizri N, Rabinovitch M. Tie2-mediated loss of peroxisome proliferator-activated receptor-gamma in mice causes pdgf receptor-beta-dependent pulmonary arterial muscularization. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1082–1090. doi: 10.1152/ajplung.00199.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong WK, Knowles JA, Morse JH. Bone morphogenetic protein receptor type ii c-terminus interacts with c-src: Implication for a role in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2005;33:438–446. doi: 10.1165/rcmb.2005-0103OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parsons JT, Parsons SJ. Src family protein tyrosine kinases: Cooperating with growth factor and adhesion signaling pathways. Curr Opin Cell Biol. 1997;9:187–192. doi: 10.1016/s0955-0674(97)80062-2. [DOI] [PubMed] [Google Scholar]
- 25.Nemenoff RA, Simpson PA, Furgeson SB, Kaplan-Albuquerque N, Crossno J, Garl PJ, Cooper J, Weiser-Evans MC. Targeted deletion of pten in smooth muscle cells results in vascular remodeling and recruitment of progenitor cells through induction of stromal cell-derived factor-1alpha. Circ Res. 2008;102:1036–1045. doi: 10.1161/CIRCRESAHA.107.169896. [DOI] [PubMed] [Google Scholar]
- 26.Daull P, Jeng AY, Battistini B. Towards triple vasopeptidase inhibitors for the treatment of cardiovascular diseases. J Cardiovasc Pharmacol. 2007;50:247–256. doi: 10.1097/FJC.0b013e31813c6ca5. [DOI] [PubMed] [Google Scholar]
- 27.Dobrek L, Thor P. Neuroendocrine activation as a target of modern chronic heart failure pharmacotherapy. Acta Pol Pharm. 2011;68:307–316. [PubMed] [Google Scholar]
- 28.Wick MJ, Buesing EJ, Wehling CA, Loomis ZL, Cool CD, Zamora MR, Miller YE, Colgan SP, Hersh LB, Voelkel NF, Dempsey EC. Decreased neprilysin and pulmonary vascular remodeling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183:330–340. doi: 10.1164/rccm.201002-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.