Abstract
Chronic allograft vasculopathy (CAV) in murine heart allografts can be elicited by adoptive transfer of donor specific antibody (DSA) to class I MHC antigens and is independent of complement. Here we address the mechanism by which DSA causes CAV. B6.RAG1−/− or B6.RAG1−/−C3−/− (H-2b) mice received B10.BR (H-2k) heart allografts and repeated doses of IgG2a, IgG1 or F(ab’)2 fragments of IgG2a DSA (anti-H-2k). Intact DSA regularly elicited markedly stenotic CAV in recipients over 28 days. In contrast, depletion of NK cells with anti-NK1.1 reduced significantly DSA-induced CAV, as judged morphometrically. Recipients genetically deficient in mature NK cells (γ-chain knock out) also showed decreased severity of DSA-induced CAV. Direct NK reactivity to the graft was not necessary. F(ab’)2 DSA fragments, even at doses twofold higher than intact DSA, were inactive. Graft microvascular endothelial cells responded to DSA in vivo by increased expression of phospho-extracellular signal-regulated kinase (pERK), a response not elicited by F(ab’)2 DSA. We conclude that antibody mediates CAV through NK cells, by an Fc dependent manner. This new pathway adds to the possible mechanisms of chronic rejection and may relate to the recently described C4d-negative chronic antibody-mediated rejection in humans.
Keywords: Antibody-mediated rejection, chronic allograft vasculopathy, Fc receptors, heart transplantation, NK cells
Introduction
Chronic allograft vasculopathy (CAV) remains the major factor limiting long-term survival of heart allografts in humans (1,2) despite advances therapy. Mechanistic studies in mouse heart allografts have demonstrated three distinct pathways to CAV: T cells with minor antigen mismatch (3,4), natural killer (NK) cells with parent to F1 grafts (5,6) and MHC antibody with adoptive transfer of donor specific antibody (DSA) (4,7,8). Antibody-mediated CAV was demonstrated initially by adoptive transfer of polyclonal or monoclonal DSA in normal and immunodeficient recipients of heart allografts (8) and in human vascular allografts in mice (9). Uehara et al. showed that adoptive transfer of monoclonal DSA to class I MHC in RAG1−/− mice (4) induced CAV in heart allografts within 28 days. During these studies we appreciated that some noncomplement fixing DSA (IgG1) also produced CAV, which was unexpected because acute antibody-mediated rejection had been shown to be complement-dependent (10–12). Furthermore, CAV occurred even in the absence of C3, one of the crucial components of the complement cascade. The CAV lesions were infiltrated by NK cells and macrophages (7) and we speculated that either or both might be the effector cell.
Several studies point to a potential importance of Fc receptors on either NK cells or macrophages in antibody-mediated rejection (13). In vitro activation of NK cells by FcγRIII leads to production of IFNγ and TNFα among other effects (14,15). Macrophages trigger FcγR dependent chemokine release from cultured endothelial cells and augment the effect of antibody alone (13). However, antibody alone, without cells, elicits strong responses in cultured endothelial cells that include proliferation and activation of intracellular proteins such as extracellular signal-regulated kinase (ERK; Refs. 16–21). It is not clear which, if any, of these three potential pathways is relevant to complement-independent antibody-mediated CAV.
We have, therefore, designed experiments to test several possible mechanisms by which antibody mediates CAV, including NK cells reacting by Fc receptors, NK cells reacting directly to the endothelium with antibody as an accessory and antibody acting on its own independent of Fc receptors on cells.
Materials and Methods
Mice
C57BL/6 (H-2b), B10.BR (H-2k), B6.129S7-Rag1tm1Mom (B6.RAG1−/−, H-2b) and BALB/c (H-2d) mice aged 5–7 weeks were purchased from Jackson Laboratory (Bar Harbor, ME, USA). The (B10.BR × B6.129S7-Rag1−/−)F1 and B10.BR × B6.RAG1−/− F1 mice were bred in our facility. BALB/c.Rag 2−/− and gamma chain knock out (GCKO) B6.Rag2−/− mice were obtained from Taconic Farms (Hudson, NY, USA) and were bred together to create a (CB6F1 GCKO Rag2−/−)F1 according to a cross-breeding agreement with Taconic Farms. C3 deficient B6.RAG1−/−(B6.RAG1−/−C3−/−) male mice were kindly provided by Dr. Michael Carroll, Harvard Medical School and bred as described (7). All mice were maintained under pathogen-free conditions in filter-top cages throughout the experiments with an automatic water system and were cared for according to methods approved by the American Association for the Accreditation of Laboratory Animal Care.
Adoptive transfer of monoclonal antibodies
Anti-H-2Kk IgG1(clone AF 3–12.1.3), anti-H-2Kk IgG2a (clone 36-7-5 or 15-3-1S [HB13]), F(ab’)2 fragment of anti-H-2Kk IgG2a (HB13) and anti-NK1.1mAbs (PK136) were all obtained from BioXCell, Lebanon, NH, USA.
B6.RAG1−/− KO or B6.RAG1−/−C3−/− DKO mice were given repeated injections of mAb at a dose of 30 µg in 200 µL phosphate-buffered saline (PBS) i.p., beginning the day after transplantation and continuing twice a week until completion of the experiments. To delete NK cells from recipient mice, recipientswere pretreated with anti-NK1.1 antibody (PK136) at a dose of 200 µg on day 6 and injected with the same dose on day +1 and once a week until animals were sacrificed. This protocol provided about 70–80% depletion of NK cells from the spleen (data not shown). In the second set of experiments, B6.RAG1−/− recipients were given repeated injections of mAb at a dose of 60 µg of anti-H-2KkIgG2a mAb with or without anti-NK1.1 mAb at a dose of 200 µg. Some recipients were given a dose of 120 µg of the F(ab’) 2 fragment of anti-H-2KkIgG2a mAb.
Murine heterotopic heart transplantation and histological techniques
Hearts were transplanted heterotopically into recipients as previously described (22). Briefly, under 4% chloral hydrate anesthesia, the donor aorta and pulmonary artery were anastomosed to the recipient abdominal aorta and inferior vena cava, respectively. The transplanted hearts were removed on day 28 and the grafts were cross-sectioned into three parts (base, middle and apex). The basal and middle parts of transplanted hearts were embedded and frozen in OCT compound (Sakura Finetek USA Inc., Torrance, CA, USA), and stored at −20°C. The remaining apical blocks were fixed in 10% formalin and embedded in paraffin. Sections including proximal coronary arteries were cut at 4–6 µm and stained using Weigert’s method for elastic fibers to evaluate the severity of coronary lesions of transplanted hearts.
Flow cytometry
Peripheral blood was used to confirm the absence of CD3+ T cells in B6.RAG1−/− and B6.RAG1−/−C3−/− recipients and the effects of anti-NK1.1(PK136) mAb on DX-5+ NK cells. In brief, peripheral blood samples were depleted of erythrocytes by water lysis and resuspended in PBS, 1% w/v BSA and 0.1% w/v sodium azide (FACS media). Cells were incubated for 30 min at 4°C with fluorescein (FITC)-conjugated anti CD3e-FITC (BD Pharmingen), Dx5-PE (BD Biosciences, San Diego, CA, USA). The cells were washed with FACS media twice and fixed in 2% paraformaldehyde solution. Two color FACSCaliber analysis (BD Biosciences) was performed using CellQuest (BD Biosciences) software.
To confirm the H-2k reactivity and the absence of the Fc portion of anti-H-2Kk IgG2a (HB13), FACS analysis was performed. In brief, 0.5 × 106 thymocytes/well from B10.BR mice were incubated for 30 min with either intact anti-H-2Kk IgG 2a mAb (HB13) or the F(ab’)2 fragment of the same antibody. After washing twice with FACS medium, cells were stained with either anti-mouse light chain FITC (BD Biosciences) or anti-mouse IgG2a FITC (BD Biosciences) for 30 min at 4°C. The cells were washed with FACS medium and fixed with 2% paraformaldehyde for 10 min at 4°C.
Immunohistochemistry
To identify infiltrating cells around coronary lesions and in the neointima, antibodies to Ly49g2 (clone 4D11, BD Biosciences) were utilized. Frozen sections were stained with peroxidase-conjugated antibody using standard techniques. Mouse IgG was detected using FITC-conjugated goat anti-mouse IgG (MP Biomedical, Santa Ana, CA, USA; Ref. 8). C4d was detected using a rat monoclonal antibody against mouse C4 that reacts with C4b/C4d (16D2; Abcam Inc., Cambridge, MA, USA, as previously described (4). Immunoperoxidase-stained sections were then developed in a solution of 3-amino-9-ethyl carbazole (Aldrich Chemical Co., Inc., Milwaukee, WI, USA), postfixed in 4% formaldehyde, counterstained with hematoxylin, and mounted in Gelvatol (Monsanto Co., Springfield, MA, USA) as previously described. Formalin-fixed transplanted heart sections were stained with a rabbit polyclonal antibody to phospho-ERK (pERK; Thr202/Try204, #4376, Cell Signaling, Beverly, MA, USA) to detect endothelial cell activation using immunohistochemistry as above.
Morphometric analysis
Morphometric analysis was performed on digital microscopic images of coronary arteries near the ostia on tissue sections stained with Weigert’s elastin stain. This is the preferential and earliest site of transplant arteriopathy in the mouse; these vessels are similar in size to intramyocardial vessels in the human (23). An image of each proximal coronary was captured digitally by light microscopy at 100–200× magnification. Image processing and analysis with ImageJ (NIH) was used to demarcate the borders of the lumen and the intima of the artery. One evaluator, who was blinded as to the diagnosis and treatment of the hearts, demarcated the areas on all the sections. Longitudinal arterial sections were demarcated similarly to cross-sections but the measurement was made on the coronary artery only, at the junction of the coronary artery and the aorta. The software then quantitated the manually demarcated luminal and intimal areas. From these area values the “neointimal index”, defined as neointimal area divided by neointimal area plus luminal area multiplied by 100, was calculated similar to what has been described previously (24). A higher value of the neointimal index indicates a more severe coronary lesion with 100 being complete occlusion.
For pERK analysis, paraffin sections stained for pERK were scored at 400× for positive and negative nuclei, counting separately the capillary and arterial endothelium and also the cardiac myocytes (25–100 nuclei per slide). Results were expressed as %positive nuclei for each cell type.
Statistical analysis
Statistical differences between two groups were analyzed by either the Fisher’s exact test or two-tailed Student’s t-test. In comparison of multiple groups, data were initially assessed for normality with the Bartlett’s test. Because the variables were not normally distributed, nonparametric procedures were used to compare neointimal index. Nonparametric data were expressed as percentile values. In comparisons of two groups, the Mann–Whitney test was performed because the data were skewed. A p value of <0.05 was considered significant. The Excel statistical software for Windows (version 2002, Social Survey Research Information Co. Ltd, Japan) and the Stat View (2002, Japanese version Hulinks Inc., Japan) were used for statistical analysis.
Results
NK depletion prevents CAV in complement deficient recipients
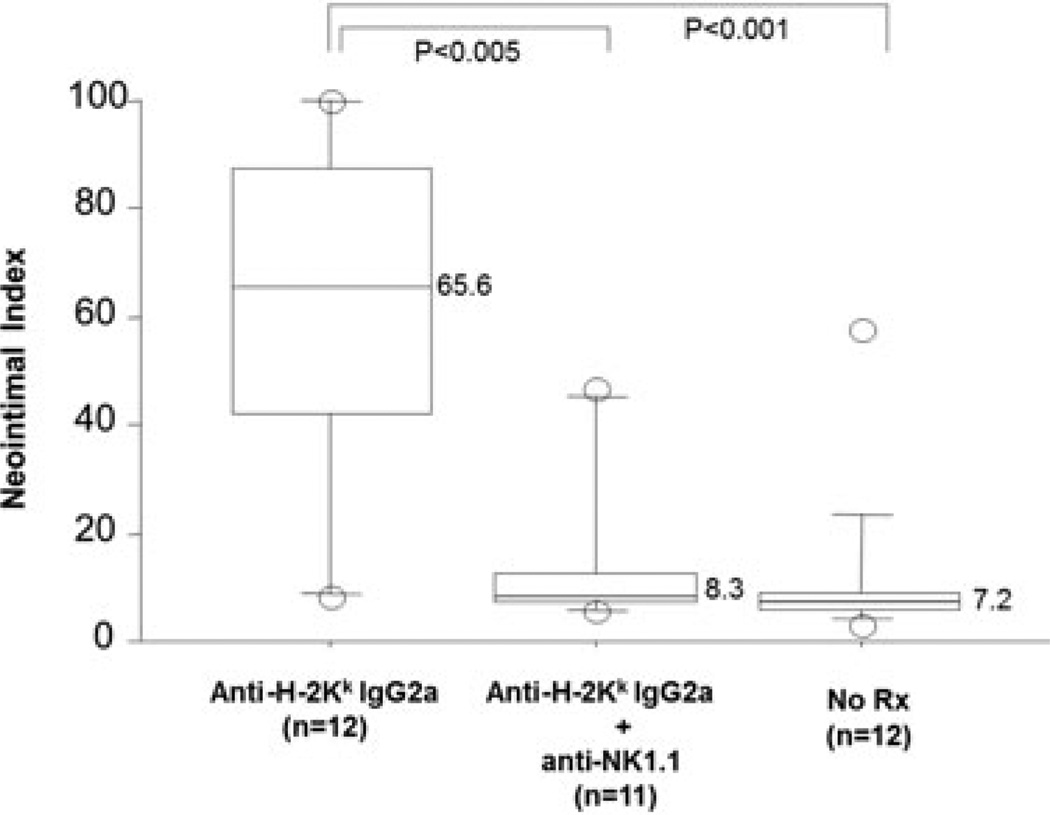
Adoptive transfer of DSAs (36-7-5, IgG2a mAb to H-2Kk) into immunodeficient and complement deficient B6.RAG1−/−C3−/− recipients bearing B10.BR heart allografts (H-2Kk) induced CAV when given as 30 µg twice per week over 28 days. In contrast, similar recipients treated with DSA and the NK depleting antibody PK136 (anti-NK1.1) had a reduced frequency of CAV, 4/12 versus 10/12 in animals treated with DSA, p < 0.05 (Table 1). The difference between the groups was quantitated using morphometric analysis. The CAV lesions were significantly less stenotic in anti-NK1.1 treated recipients of anti-H-2Kk IgG2a mAb (8.3 ± 15.2 vs. 65.6 ± 32.2, p < 0.005, Figure 1), and not distinguishable from the untreated controls, which had little or no CAV and a low overall neointimal index.
Table 1.
Effects of DSA and NK depletion on the frequency of CAV
| Group | Donor | Recipient | Treatment | Frequency of CAV | p-Value |
|---|---|---|---|---|---|
| 1 | B10.BR | B6.RAG1−/−C3−/− | No Rx | 1/12 | |
| 2 | αH-2Kk(IgG2a) | 10/12 | versus group 1 p < 0.0001 | ||
| 3 | αH-2Kk(IgG2a) + αNK1.1 | 4/12 | versus group 2 p < 0.05 | ||
| 4 | B10.BR | B6.RAG1−/− | No Rx | 0/6 | |
| 5 | αH-2Kk(IgG2a) | 9/10 | versus group 3 p < 0.0001 | ||
| 6 | αH-2Kk(IgG2a) + αNK1.1 | 0/6 | vs group 4 p < 0.0001 | ||
| 7 | αH-2Kk(IgG1) | 7/9 | versus group 3 p < 0.001 | ||
| 8 | αH-2Kk(IgG1) + αNK1.1 | 0/3 | versus group 7 p < 0.05 | ||
| 9 | B6.RAG1−/− | B6.RAG1−/− | αH-2Kk(IgG2a) | 0/4 |
Figure 1. NK depletion inhibits DSA induced CAV in B6.RAG1−/−C3−/−recipients.
The severity of coronary lesions in B10.BR heart allografts in B6.RAG1−/− C3−/− recipients given DSA (anti-H-2Kk; clone 36–7–5) is compared with and without anti-NK1.1 mAb treatment. The neointimal index was significantly less in the anti-H-2KkIgG2a mAb treatment group given anti-NK1.1. The severity of coronary lesions in anti-H-2Kk IgG2a plus anti-NK1.1 mAb is similar to that of the no treatment group, 8.3% versus 7.2%. The p values were calculated with the Mann–Whitney U test. The numbers in the parenthesis indicate the numbers of the hearts examined. The number adjacent to the block column indicates the median value of the neointimal index in each group.
NK depletion reduces significantly DSA-mediated CAV in complement sufficient recipients
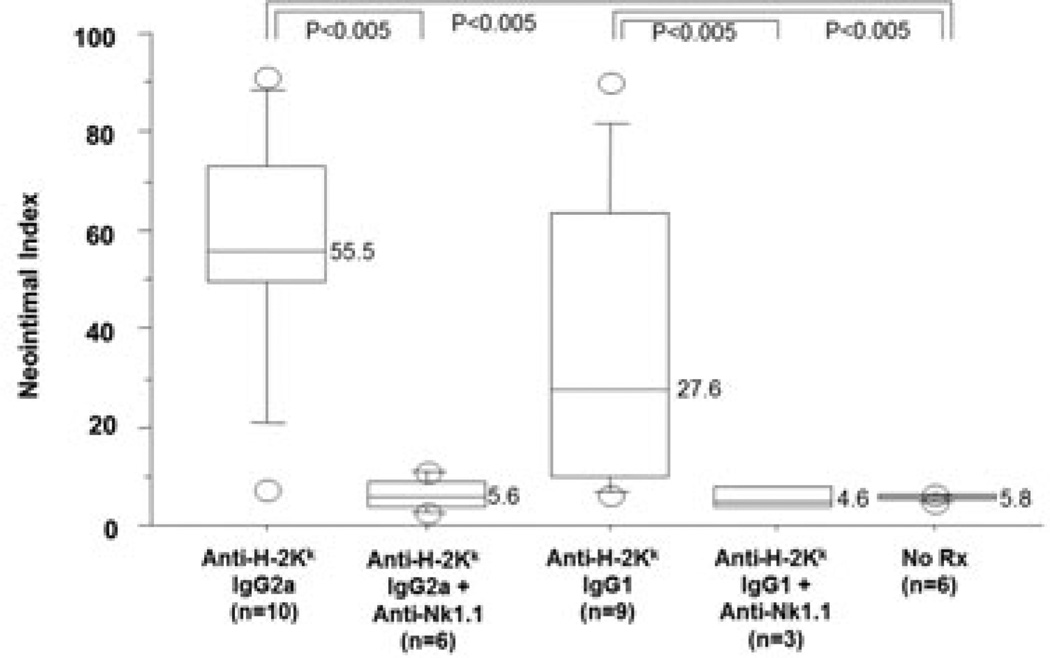
We hypothesized that the NK dependent pathway might be relevant only in the absence of a functional complement system. Therefore, we repeated the experiment in recipients with an intact complement system (B6.RAG1−/−; Table 1). As expected, in the recipients with an intact complement system, IgG2a DSA (36–7–5) elicited CAV in most allografts. However, addition of NK 1.1 mAb significantly reduced the frequency and severity of the CAV lesions (0/6 vs. 9/10, 5.6 ± 3.4 vs. 55.5 ± 24.9, p < 0.0001 and <0.005, respectively; Figure 2 and Figure 3), similar to those levels seen in complement deficient recipients. C4d was present in the myocardial vessels in both groups that received DSA, with or without NK depletion (data not shown).
Figure 2. Effects of NK depletion on IgG2a or IgG1 DSA mediated CAV in complement sufficient recipients.
In B6.RAG1−/− recipients with B10BR hearts, anti-H-2Kk IgG2a mAb and anti-H-2KkIgG1 mAb induced CAV. As in complement deficient B6.RAG1−/− recipients, addition of anti-NK1.1 mAb to either anti-H-2Kk IgG2a mAb or anti-H-2KkIgG1 mAb eliminated the CAV, as judged by the neointimal index (<0.005). Annotations as in Figure 1.
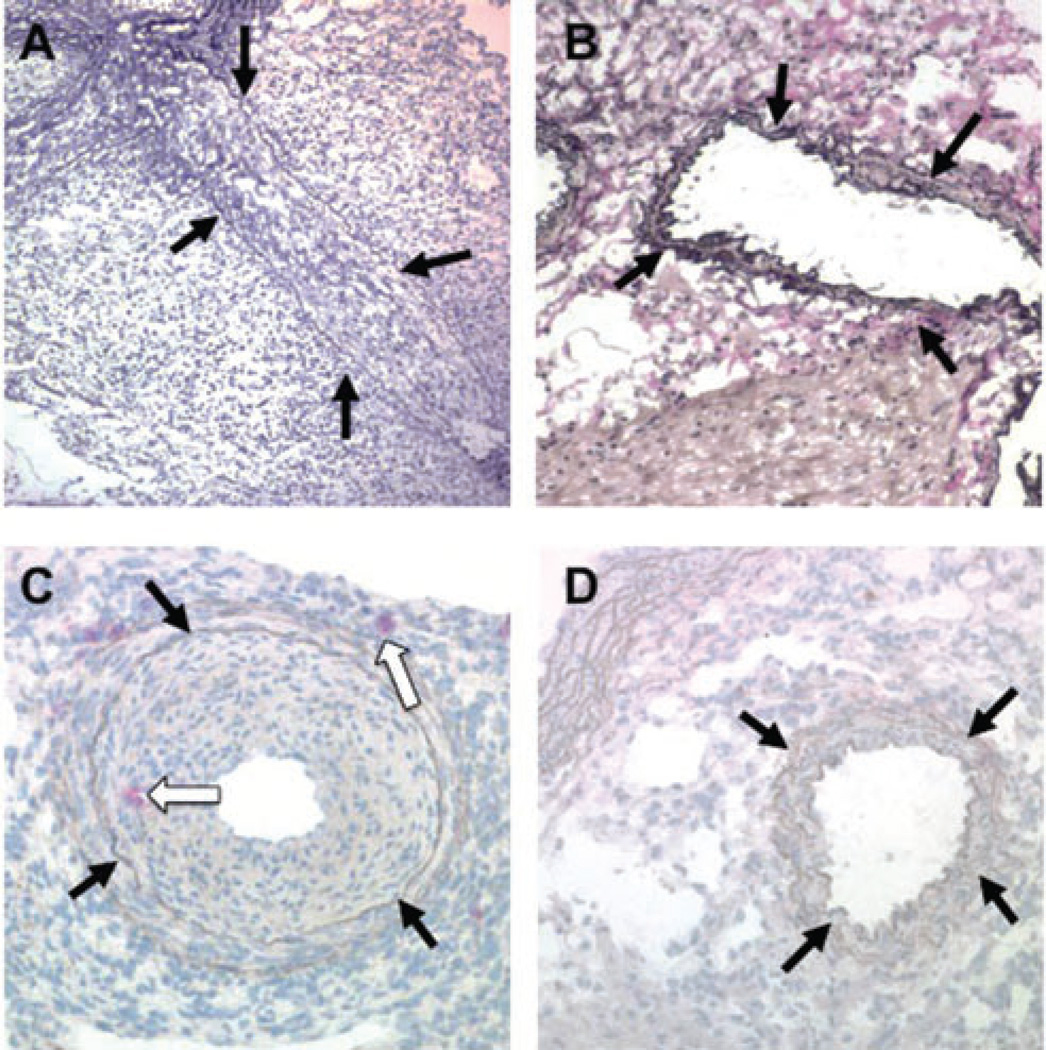
Figure 3. Coronary arteries of B10.BR heart allografts in B6.RAG1−/− complement sufficient recipients after administration of DSA with or without NK depletion.
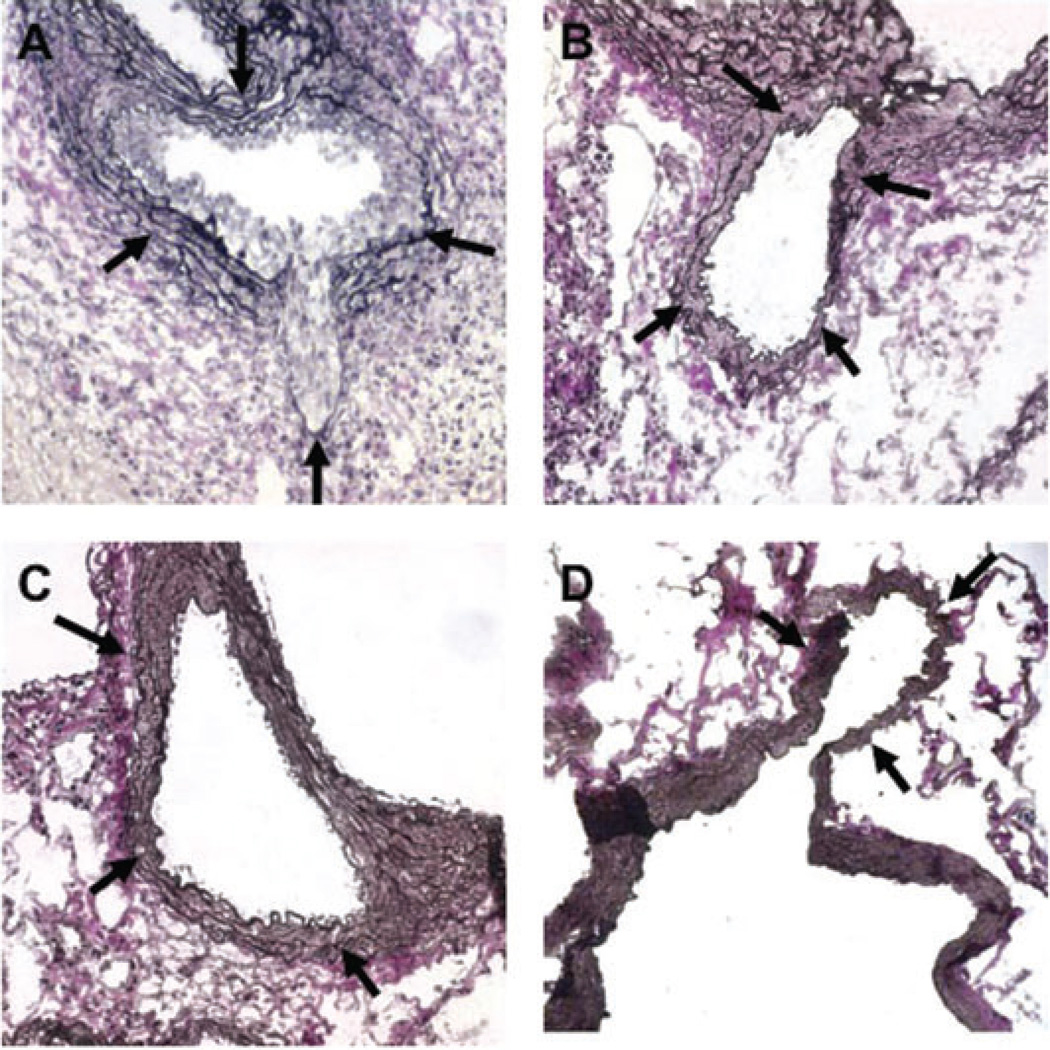
(A) A severely stenotic coronary artery in a B10.BR allograft from a B6.RAG1−/− recipient treated with anti-H-2KkIgG2a mAb is shown, demonstrating severe CAV. (B) Addition of anti-NK1.1 mAb during anti-H-2KkIgG2a mAb administration prevents the CAV lesion. (C) Ly49g2 immunohistochemical stain of a coronary artery with CAV from a B10.BR heart in a B6.RAG1−/− recipient given anti-H-2Kk IgG2a. Red stained NK cells are present in the thickening intima and in the adventitia (white arrows). (D) Same strain combination given anti-NK1.1 in addition to anti-H-2Kk IgG2a. No Ly49g2+ cells or intimal thickening are seen. A–D, elastin stains; black arrows indicate coronary arteries
Histological examination showed that allografts in recipients of DSA had prominent neointimal formation and infiltration of mononuclear cells in the intima and periadventia at 28 days (Figure 3). Cells were present in the intima and adventitia of the coronary arteries that stained for the NK molecule Ly49g2 (Figure 3C). In contrast, anti-NK1.1 treated recipients given DSA had little or no neointimal formation and inflammation or Ly49g2+ cells and were similar to controls (Figure 3D).
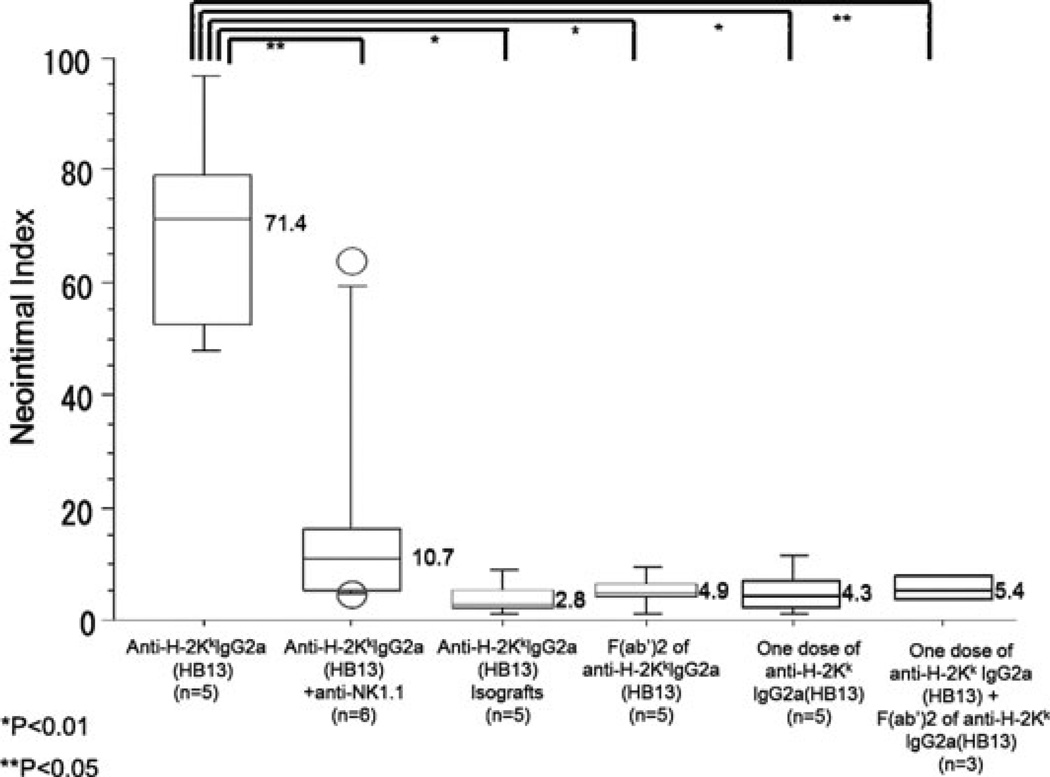
To rule out a peculiarity of the 36–7–5 antibody, we tested a second IgG2a mAb to H-2Kk (HB13; Table 2, Figure 4). B10.BR hearts in B6.RAG1−/− recipients given repeated doses of HB13 (60 µg twice per week for 28 days) all developed CAV (5/5). The severity of vascular lesions using morphometric analysis was similar to that in animals given mAb 36-7-5. When NK cells were depleted from B6.RAG1−/− recipients with B10.BR hearts treated with HB13 mAb, the CAV lesions were quantitatively less severe by morphometric analysis than those treated with HB13 alone (Figure 4, p < 0.05), even though the CAV frequency was not significantly lower (Table 2). Control B6 isografts treated with HB13 had no CAV.
Table 2.
Effects of F(ab’)2 and intact DSA on CAV
| Group | Donor | Recipient | Treatment | Frequency of CAV | p-Value |
|---|---|---|---|---|---|
| 1 | B10.BR | B6.RAG1−/− | αH-2Kk(IgG2a) | 5/5 | |
| 2 | αH-2Kk(IgG2a) + αNK1.1 | 2/5 | versus group 1 NS | ||
| 3 | F(ab’)2 fragment of αH-2Kk(IgG2a) ab + αNK1.1 | 0/5 | versus group 1 p < 0.01 | ||
| 4 | F(ab’)2 fragment of αH-2Kk(IgG2a) ab + αH-2Kk(IgG2a; 1 dose) | 0/3 | |||
| 5 | B6.RAG1−/− | B6.RAG1−/− | αH-2Kk(IgG2a) | 0/6 | versus group 1 p < 0.01 |
Figure 4. Effect of NK depletion on DSA (HB13) induced CAV and lack of CAV with F(ab’)2fragments of HB13.
A second anti-H-2Kk IgG2a DSA (HB13) given to B6.RAG1−/− recipients with B10.BR hearts induces CAV of comparable severity to 36–7–5 DSA, as judged by the neointimal thickness. Addition of anti-NK1.1 mAb during administration of the HB13 DSA eliminates coronary lesions (p < 0.05). The severity of coronary lesions from animals treated with F(ab’)2 fragment of HB13, one dose of anti-H-2Kk IgG2a DSA (HB13), one dose of anti-H-2Kk IgG2a DSA (HB13) plus F(ab’) of anti-H-2Kk IgG2a DSA (HB13) significantly were lower than that of intact HB13 (p < 0.05, p < 0.01 and p < 0.01, respectively). No lesions are seen in B6.RAG1−/− isografts in response to HB13. Annotation as in Figure 1.
IgG1-mediated CAV is NK dependent
NK cells only express Fcγ RIII (CD16; Ref. 12). Fcγ RIII is a low affinity, activating receptor responsible for IgG-mediated cell cytotoxicity and for production of various cytokines and chemokines in response to multimeric IgG (25,26). We reasoned that if NK cells were necessary for CAV, DSA of an IgG1 isotype, which binds only to FcγRIII but not FcγRIIb or FcγRI, would be sufficient to mediate CAV.
A transfer of 30 µg of IgG1 DSA to B6.RAG1−/− recipients of B10.BR hearts induced florid CAV inmost allografts (Table 1 and Figure 2). C4d was not detected in myocardial vessels, confirming the lack of complement fixation in vivo (data not shown). Depletion of NK cells with anti-NK1.1 during the course of administration of the DSA significantly diminished the frequency (p < 0.05) and the severity (p < 0.005) of the CAV (Table 1 and Figure 2).
The next series of experiments addressed the mechanism by which NK cells mediate CAV. Two possibilities were considered. First, NK cells and antibody attack the vessel in parallel: the NK cells recognize the absence of self on the donor heart and the DSA acts separately on the endothelium. We considered this possibility, because under certain circumstances, NK cells can cause CAV in the absence of antibody (5,6). The second, more conventional possibility is that NK cells act through Fc receptors and do not need to recognize the graft as foreign.
Effect of DSA in F1 grafts in parental strain recipients in which NK cells are nonreactive
The (B10.BR × B6.RAG1−/−)F1 hearts were transplanted into B6.RAG1−/− recipients and DSA (HB13) was given according to the standard schedule (60 µg twice per week for 28 days). All recipients (3/3) developed CAV, with an average severity not different from those in five B10.BR grafts in B6.RAG1−/− recipients given DSA (72.9 ± 29.1% vs. 68.6 ± 19.2%, respectively, N.S.). Controls in the same combination not given DSA had no lesions (0/2). We conclude that the NK receptors do not have to interact directly with the graft, since these grafts contain all self antigens (i.e. no “missing self”). Therefore, we tested the second possibility that NK cells interact by Fc receptors.
Comparison of effects of intact IgG and F(ab’)2 DSA on CAV
F(ab’)2 fragments of anti-H-2Kk IgG2a mAb (HB13) were injected at doses of 120 µg (a twofold excess by weight and threefold excess by antigen combining sites, compared with intact antibody). When F(ab’)2 fragments of anti-H-2KkIgG2a mAb were given to recipient animals with B10.BR hearts, none of the five hearts developed coronary lesions (Table 2). The severity of coronary lesions from animals treated with F(ab’)2 fragments of anti-H-2Kk IgG2a mAb was significantly less than that in animals treated with intact HB13 mAb (Figure 4, p < 0.01). Coronary lesions from these experimental groups are shown in Figure 5. C4d deposition was not detected in myocardial vessels, confirming the lack of intact IgG2a in the F(ab’)2 preparation (data not shown).
Figure 5. Comparison of the effects of intact and F(ab’)2DSA on CAV.
(A) A coronary artery from anti-H-2Kk (HB13) mAb treated B6RAG1−/− recipient of a B10.BR heart at day 21 shows marked intimal thickening. (B) Addition of anti-NK1.1 mAb to anti-H-2KkIgG2a mAb eliminated the coronary lesions. (C) Administration of F(ab’)2 fragments of anti-H-2Kk HB13 mAb even in doses 2× that of the intact mAb failed to induce CAV. (D) Control isograft B6RAG1−/− hearts in B6.RAG1−/− recipients given anti-H-2KkIgG2a mAb showed no evidence of vascular lesions. Black arrows indicate coronary arteries.
We observed that donor T cells homeostatically expand in RAG1−/− recipients after B10.BR heart transplantation and are eliminated by IgG2a but not F(ab’)2 DSA (data not shown). Therefore, to eliminate the possibility that these absorbed the F(ab’)2, one dose of anti-H-2KkIgG2a HB13 was given to recipient animals with B10.BR heart grafts on day 1 followed by F(ab’)2 fragment of HB13 twice per week. This eliminated donor T cells (data not shown) but did not lead to CAV (0/3, Table 2 and Figure 4; neointimal index 5.4 ± 2.7%). A single dose of intact HB13 was also insufficient to promote CAV (Figure 4).
NK deficient recipients
As an alternative to antibody-mediated NK depletion, we tested recipients deficient in the common γ-chain, which are deficient in mature NK cells (27). The gene for the common γ-chain is on the X chromosome, therefore, we compared four γ-chain deficient CB6F1 hemizygous males (RAG2−/−γc−/γ) with six control CB6F1 heterozygous females (RAG2−/−γ c−/+), each bearing B10.BR cardiac allografts and given DSA (HB13) by the standard 28 day regimen. CAV severity was significantly less in the γ-chain deficient males than in the heterozygote female controls (43.8 ± 15.7% vs. 65.9 ± 13.3%, respectively; p < 0.05), although all animals in both groups had some degree of CAV. We conclude that antibody-mediated CAV is partially dependent on mature NK cells, although another mechanism, perhaps involving immature NK precursors or macrophages may contribute.
Effect of NK depletion on the activation of ERK in endothelial cells in response to DSA
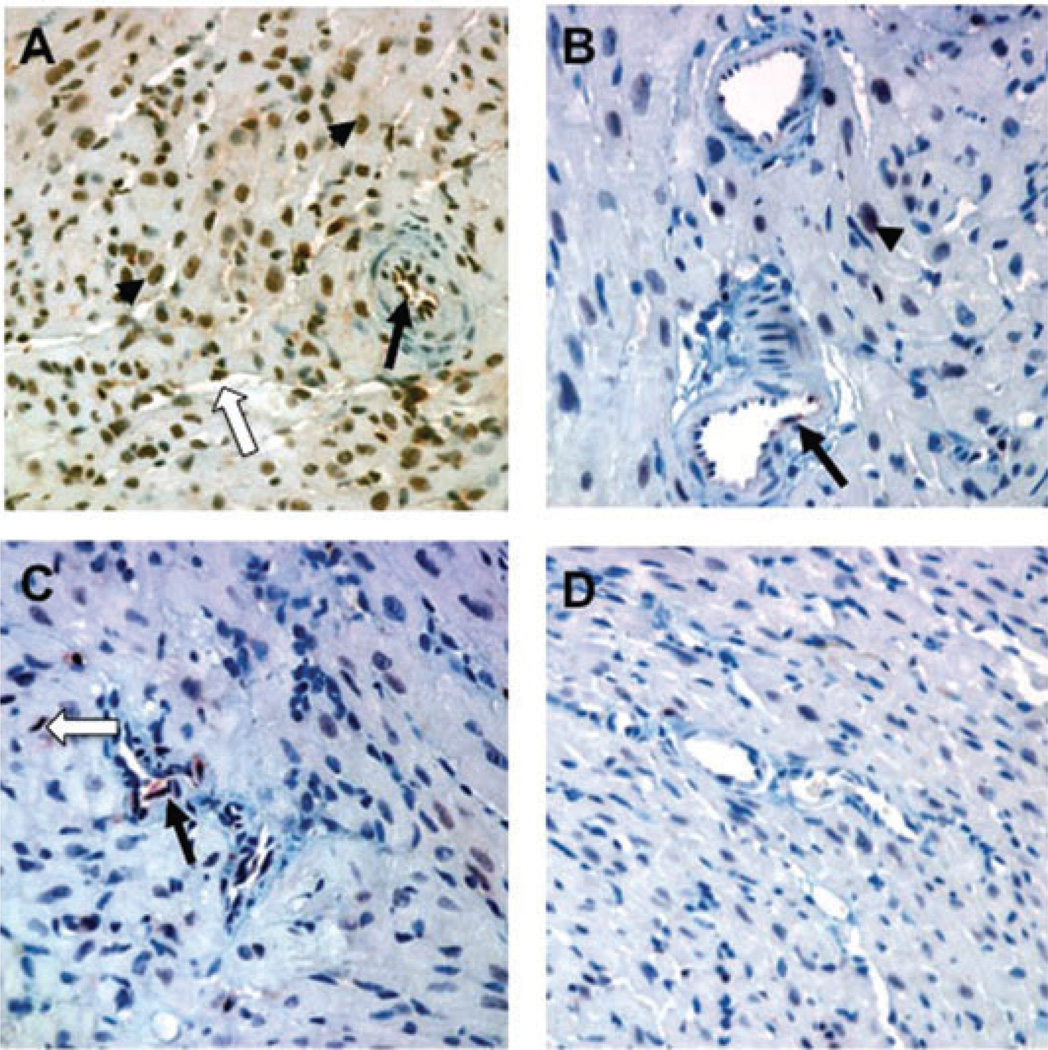
Samples from the various treatment groups were stained for pERK to seek evidence of endothelial activation in vivo. Immunohistochemical analysis showed a marked increase in pERK in myocardial microvascular endothelial cells (both arteries and capillaries) in animals treated with DSA antibody (Figure 6A and Table 3). We observed no appreciable accumulation of leukocytes in the capillaries in the apex of the heart (data not shown). In contrast, treatment with F(ab’)2 fragments of the DSA showed minimal or no pERK staining (Figure 6B; p < 0.01 vs. intact IgG2a), which was similar to the minimal staining seen in isografts treated with the same antibody (Figure 6D). Treatment of mice with anti-H-2Kk Fc and anti-NK1.1 showed less intense staining for pERK than DSA alone (Figure 6C) and a moderately decreased extent, but this difference was not statistically significant compared with DSA alone. Immunohistochemical analysis of native B6.RAG1−/− hearts showed no pERK staining (data not shown). Cardiac myocyte nuclei had a prominent increase in pERK in the DSA group. We conclude that graft endothelial ERK activation was caused by intact DSA by a mechanism that was dependent on the Fc portion of the antibody.
Figure 6. Effects of DSA on pERK expression in heart allograft endothelial cells on day 28.
(A) Intact anti-H-2Kk DSA (HB13) elicits a strong arterial (black arrow) and capillary (white arrow) endothelial pERK response in B10.BR allografts in B6.RAG1−/− recipients. Myocyte nuclei (black arrowhead) also showed pERK expression. (B) In contrast, little pERK was detected in B10.BR allografts in response to F(ab’)2 DSA. (C) Endothelial expression of pERK was somewhat less extensive when NK cells were depleted during the time of DSA administration. (D) Isografts in B6.RAG1−/− recipients given HB13 showed little or no increase in pERK expression. Immunhistochemical stains with anti-pERK; original magnification 400×.
Table 3.
Effects of intact and F(ab’)2 DSA and NK depletion on pERK expression
| pERK Expression (% of cells) Endothelium |
||||||||
|---|---|---|---|---|---|---|---|---|
| Group | Donor | Recipient | Treatment | N | Arterial | Capillary | Myocyte | p-Value |
| 1 | B10.BR | B6.RAG1−/− | αH-2Kk(IgG2a) | 5 | 63 ±34 | 44±15 | 44 ±27 | versus group 3 p < 0.01 (all categories) |
| 2 | B10.BR | B6.RAG1−/− | αH-2Kk(IgG2a) + αNK1.1 | 5 | 34±18 | 25±29 | 17 ± 20 | versus group 1 p > 0.05 (all categories) |
| 3 | B10.BR | B6.RAG1−/− | αH-2Kk(IgG2a) F(ab’)2 | 5 | 7±8 | 2±4 | 2 ± 2 | versus group 4 p > 0.05 (all categories) |
| 4 | B6.RAG1−/− | B6.RAG1−/− | αH-2Kk(IgG2a) | 2 | 5±2 | 3±3 | 21 ±9 | |
Discussion
This study shows that NK cells play a necessary role for the development of antibody-mediated chronic rejection, whether or not the antibodies activate complement. NK cells were identified in the CAV lesions and depletion of NK cells prevented CAV. NK-cell depletion significantly attenuated the frequency and severity of antibody-mediated chronic rejection mediated by three different DSA of two different DSA isotypes, whether or not complement was functional. Removal of the Fc portion of the DSA eliminated its activity. These results argue that antibody alone cannot mediate the lesions and that macrophages, if they contribute, are insufficient without the participation of NK cells.
Microvascular complement deposition in allografts, particularly the C4d fragment of C4, has been a useful marker of acute and chronic antibody-mediated rejection (28). Ample studies support the necessary role of complement fixation in acute antibody-mediated rejection in humans and experimental animals as shown, for example, by the inhibition achieved with anti-C5 (11). However, complement fixation has not been proved for chronic rejection and we have shown that florid CAV can be promoted by noncomplement fixing antibodies and in complement deficient recipients (C3−/−; Ref. 7). In humans, endothelial gene transcript increases can be detected in a subset of patients with DSA and no detectable C4d deposition (29), arguing for a pathway less dependent on complement fixation. These studies identify a complement independent pathway that requires NK cells and, at least in mice, is the dominant pathway even in the presence of an intact complement system and complement fixing DSA.
NK cells, key players in innate immunity, have been recognized as participants in rejection and acceptance of transplanted organs (5,6). NK cells can affect target cells through natural cytotoxicity or antibody dependent cellular cytotoxicity (ADCC). Natural cytotoxicity occurs by a balance of stimulatory and inhibitory receptors that recognize self-antigens expressed by the target cells. This mechanism is relevant to the “hybrid resistance” of F1 recipients to parental bone marrow transplants and the CAV that develops in parent to F1 heart allografts. In contrast, ADCC is triggered by interaction of Fcγ RIII on NK cells with immunoglobulin bound to antigen on target cells, leading to NK activation (25,26). NK cells, by their Fc receptors, are necessary for hyperacute xenograft rejection mediated by noncomplement fixing anti-Gal antibodies (12). Depletion of NK cells with anti-asialo-GM1 prolongs survival of mouse to rat cardiac xenografts, arguing for their participation in acute antibody-mediated rejection (30).
Reed and colleagues demonstrated direct effects of MHC antibody on endothelial cells, not requiring complement or leukocytes (31). Stimulation of cultured human endothelial cells with w6/32, a mAb against class I HLA, increased endothelial cell proliferation, an effect also elicited by F(ab’)2 fragments (32). Among the changes noted was increased phosphorylation of ERK (pERK), indicative of increased ERK activity (33). Activation of the ERK pathway promotes cellular proliferation (34,35) and is evident in response to ischemia (36). Our data show a correlation with an upregulation of pERK in myocardial endothelial cells with DSA in vivo and inability of F(ab’)2 to mediate CAV, corroborating the previous work by Reed and colleagues. However, we found that little or no detectable upregulation of pERK occurred after administration of F(ab’)2 fragments of DSA. These results contrast with those of Jin et al. who reported that F(ab’)2 and intact DSA cause a similar increase in pERK in mouse allografts (37). The reason for the difference may be methodological due to differences in analysis (western blot vs. immunohistochemistry). Cardiac myocytes express pERK and this may confound interpretation of tissue extracts. Some of the the pERK expression, particularly that inmyocytes, may be a response to ischemia produced by the CAV (36). Depletion of NK cells did not fully prevent the pERK response to DSA, indicating that this response is less sensitive to NK cell levels, compatible with the ability of DSA alone to induce pERK in cultured endothelial cells (33). Proximal coronaries were not available for pERK staining due to lack of formalin-fixed tissue. However, we have observed increased pERK in the endothelium of affected arteries in samples of human allografts with chronic humoral rejection (Alessandrini et al., unpublished data). Although data from this manuscript and from Jin et al. (33) indicate that pERK is up-regulated in response to DSA, we have yet to show a direct relationship between pERK expression and the development of CAV.
Our studies do not rule out a role for macrophages that may augment the effects of NK cells and DSA. Studies by Lee and colleagues reported that IgG1 alloantibodies to major histocompatibility complex class I antigens can result in increased graft injury by stimulating endothelial cells to secrete IL-6 and MCP-1 and by activating mononuclear cells through their Fc receptors (13). These findings have been confirmed by others (38). However, in the present experiments, it is unlikely that macrophages are the primary responder because NK depletion prevented IgG2a DSA CAV induction and IgG2a binds to all major Fcγ receptors in the mouse, including the high affinity Fcγ RI on macrophages. We did note that γ-chain deficient mice given DSA do have some degree of CAV. The nature of this γ-chain independent pathway was not determined.
Recent studies suggest that NK cells participate in acute and chronic rejection of organ allografts in humans (39,40). NK-cell transcripts and intravascular accumulation of NK cells (CD56+) have been detected in human kidney allografts with DSA and chronic humoral rejection, even in the absence of complement deposition (41). NK cells also are increased in the blood and in grafts during chronic rejection of lung allografts (42). Blood NK cells are activated in patients with chronic rejection and the number of CD16(+) NK cells increased in the grafted lung during the progression of chronic rejection (30).
In summary, we have identified a mechanism for antibody-mediated chronic rejection in the mouse that is NK-cell dependent and independent of complement fixation, although it does require the Fc portion of the antibody. This is the principal pathway by which DSA mediates CAV in the mouse, even in the presence of complement fixing antibodies. The NK-DSA pathway may also be relevant to chronic antibody-mediated rejection in the human, which can occur with little or no complement fixation. Further search for evidence of this pathway in humans is warranted.
Acknowledgments
This work was supported in part by the Roche Organ Transplant Research Foundation (RBC), NIH grant RO1 HL071932 (JCM) and by a Basic Science Grant from the American Society of Transplantation (TH).
Glossary
Abbreviations
- CAV
chronic allograft vasculopathy
- DSA
donor specific antibody
- ERK
extracellular signal-regulated kinase
- Fc
crystallizable fragment (of immunoglobulin molecule)
- NK
natural killer
- PBS
phosphate-buffered saline
Footnotes
Disclosure
The authors of this manuscript have no conflicts to disclose as described by the American Journal of Transplantation.
References
- 1.Mehra MR. Contemporary concepts in prevention and treatment of cardiac allograft vasculopathy. Am J Transplant. 2006;6:1248–1256. doi: 10.1111/j.1600-6143.2006.01314.x. [DOI] [PubMed] [Google Scholar]
- 2.Taylor DO, Edwards LB, Boucek MM, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-third official adult heart transplantation report-2006. J Heart Lung Transplant. 2006;25:869–879. doi: 10.1016/j.healun.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Uehara S, Chase CM, Colvin RB, Madsen JC, Russell PS. T-cell depletion eliminates the development of cardiac allograft vasculopathy in mice rendered tolerant by the induction of mixed chimerism. Transplant Proc. 2006;38:3169–3171. doi: 10.1016/j.transproceed.2006.10.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uehara S, Chase CM, Cornell LD, Madsen JC, Russell PS, Colvin RB. Chronic cardiac transplant arteriopathy in mice: Relationship of alloantibody, C4d deposition and neointimal fibrosis. Am J Transplant. 2007;7:57–65. doi: 10.1111/j.1600-6143.2006.01599.x. [DOI] [PubMed] [Google Scholar]
- 5.Graham JA, Wilkinson RA, Hirohashi T, et al. Viral infection induces de novo lesions of coronary allograft vasculopathy through a natural killer cell-dependent pathway. Am J Transplant. 2009;9:2479–2484. doi: 10.1111/j.1600-6143.2009.02801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uehara S, Chase CM, Kitchens WH, et al. NK cells can trigger allograft vasculopathy: The role of hybrid resistance in solid organ allografts. J Immunol. 2005;175:3424–3430. doi: 10.4049/jimmunol.175.5.3424. [DOI] [PubMed] [Google Scholar]
- 7.Hirohashi T, Uehara S, Chase CM, et al. Complement independent antibody-mediated endarteritis and transplant arteriopathy in mice. Am J Transplant. 2010;10:510–517. doi: 10.1111/j.1600-6143.2009.02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell PS, Chase CM, Winn HJ, Colvin RB. Coronary atherosclerosis in transplanted mouse hearts. II. Importance of humoral immunity. J Immunol. 1994;152:5135–5141. [PubMed] [Google Scholar]
- 9.Galvani S, Auge N, Calise D, et al. HLA class I antibodies provoke graft arteriosclerosis in human arteries transplanted into SCID/beige mice. Am J Transplant. 2009;9:2607–2614. doi: 10.1111/j.1600-6143.2009.02804.x. [DOI] [PubMed] [Google Scholar]
- 10.Rahimi S, Qian Z, Layton J, Fox-Talbot K, Baldwin WM, III, Wasowska BA. Non-complement- and complement-activating antibodies synergize to cause rejection of cardiac allografts. Am J Transplant. 2004;4:326–334. doi: 10.1111/j.1600-6143.2004.00334.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Jiang J, Liu W, et al. Prevention of acute vascular rejection by a functionally blocking anti-C5 monoclonal antibody combined with cyclosporine. Transplantation. 2005;79:1121–1127. doi: 10.1097/01.tp.0000161218.58276.9a. [DOI] [PubMed] [Google Scholar]
- 12.Yin D, Zeng H, Ma L, et al. Cutting Edge: NK cells mediate IgG1-dependent hyperacute rejection of xenografts. J Immunol. 2004;172:7235–7238. doi: 10.4049/jimmunol.172.12.7235. [DOI] [PubMed] [Google Scholar]
- 13.Lee CY, Lotfi-Emran S, Erdinc M, et al. The involvement of FcR mechanisms in antibody-mediated rejection. Transplantation. 2007;84:1324–1334. doi: 10.1097/01.tp.0000287457.54761.53. [DOI] [PubMed] [Google Scholar]
- 14.Trotta R, Col JD, Yu J, et al. TGF-beta utilizes SMAD3 to inhibit CD16-mediated IFN-gamma production and antibody-dependent cellular cytotoxicity in human NK cells. J Immunol. 2008;181:3784–3792. doi: 10.4049/jimmunol.181.6.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roda JM, Parihar R, Lehman A, Mani A, Tridandapani S, Carson WE., III Interleukin-21 enhances NK cell activation in response to antibody-coated targets. J Immunol. 2006;177:120–129. doi: 10.4049/jimmunol.177.1.120. [DOI] [PubMed] [Google Scholar]
- 16.Bian H, Harris PE, Reed EF. Ligation of HLA class I molecules on smooth muscle cells with anti-HLA antibodies induces tyrosine phosphorylation, fibroblast growth factor receptor expression and cell proliferation. Int Immunol. 1998;10:1315–1323. doi: 10.1093/intimm/10.9.1315. [DOI] [PubMed] [Google Scholar]
- 17.Bian H, Reed EF. Alloantibody-mediated class I signal transduction in endothelial cells and smooth muscle cells: Enhancement by IFN-gamma and TNF-alpha. J Immunol. 1999;163:1010–1018. [PubMed] [Google Scholar]
- 18.Jin YP, Fishbein MC, Said JW, et al. Anti-HLA class I antibody-mediated activation of the PI3K/Akt signaling pathway and induction of Bcl-2 and Bcl-xL expression in endothelial cells. Hum Immunol. 2004;65:291–302. doi: 10.1016/j.humimm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Jin YP, Jindra PT, Gong KW, Lepin EJ, Reed EF. Anti-HLA class I antibodies activate endothelial cells and promote chronic rejection. Transplantation. 2005;79:S19–S21. doi: 10.1097/01.tp.0000153293.39132.44. [DOI] [PubMed] [Google Scholar]
- 20.Jin YP, Singh RP, Du ZY, Rajasekaran AK, Rozengurt E, Reed EF. Ligation of HLA class I molecules on endothelial cells induces phosphorylation of Src, paxillin, and focal adhesion kinase in an actin-dependent manner. J Immunol. 2002;168:5415–5423. doi: 10.4049/jimmunol.168.11.5415. [DOI] [PubMed] [Google Scholar]
- 21.Narayanan K, Jaramillo A, Phelan DL, Mohanakumar T. Pre-exposure to sub-saturating concentrations of HLA class I antibodies confers resistance to endothelial cells against antibody complement-mediated lysis by regulating Bad through the phosphatidylinositol 3-kinase/Akt pathway. Eur J Immunol. 2004;34:2303–2312. doi: 10.1002/eji.200324843. [DOI] [PubMed] [Google Scholar]
- 22.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Russell PS, Chase CM, Sykes M, Ito H, Shaffer J, Colvin RB. Tolerance, mixed chimerism, and chronic transplant arteriopathy. J Immunol. 2001;167:5731–5740. doi: 10.4049/jimmunol.167.10.5731. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong AT, Strauch AR, Starling RC, Sedmak DD, Orosz CG. Morphometric analysis of neointimal formation in murine cardiac allografts. Transplantation. 1997;63:941–947. doi: 10.1097/00007890-199704150-00006. [DOI] [PubMed] [Google Scholar]
- 25.Arase N, Arase H, Hirano S, Yokosuka T, Sakurai D, Saito T. IgE-mediated activation of NK cells through FcgRIII. J Immunol. 2003;170:3054–3058. doi: 10.4049/jimmunol.170.6.3054. [DOI] [PubMed] [Google Scholar]
- 26.Arase N, Arase H, Park SY, Ohno H, Ra C, Saito T. Association with FcRgamma is essential for activation signal through NKR-P1 (CD161) in natural killer (NK) cells and NK1.1 +T cells. J Exp Med. 1997;186:1957–1963. doi: 10.1084/jem.186.12.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vosshenrich CA, Ranson T, Samson SI, et al. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005;174:1213–1221. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- 28.Colvin RB. Antibody-mediated renal allograft rejection: Diagnosis and pathogenesis. J Am Soc Nephrol. 2007;18:1046–1056. doi: 10.1681/ASN.2007010073. [DOI] [PubMed] [Google Scholar]
- 29.Sis B, Halloran PF. Endothelial transcripts uncover a previously unknown phenotype: C4d–negative antibody-mediated rejection. Curr Opin Organ Transplant. 2010;15:42–48. doi: 10.1097/MOT.0b013e3283352a50. [DOI] [PubMed] [Google Scholar]
- 30.Chen D, Weber M, Lechler R, Dorling A. NK-cell-dependent acute xenograft rejection in the mouse heart-to-rat model. Xenotrans-plantation. 2006;13:408–414. doi: 10.1111/j.1399-3089.2006.00327.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Reed EF. Effect of antibodies on endothelium. Am J Transplant. 2009;9:2459–2465. doi: 10.1111/j.1600-6143.2009.02819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris PE, Bian H, Reed EF. Induction of high affinity fibroblast growth factor receptor expression and proliferation in human endothelial cells by anti-HLA antibodies: A possible mechanism for transplant atherosclerosis. J Immunol. 1997;159:5697–5704. [PubMed] [Google Scholar]
- 33.Jindra PT, Jin YP, Jacamo R, Rozengurt E, Reed EF. MHC class I and integrin ligation induce ERK activation via an mTORC2-dependent pathway. Biochem Biophys Res Commun. 2008;369:781–787. doi: 10.1016/j.bbrc.2008.02.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chadee DN, Kyriakis JM. MLK3 is required for mitogen activation of B-Raf, ERK and cell proliferation. Nat Cell Biol. 2004;6:770–776. doi: 10.1038/ncb1152. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Q, Heinke J, Vargas A, et al. ERK signaling is a central regulator for BMP-4 dependent capillary sprouting. Cardiovasc Res. 2007;76:390–399. doi: 10.1016/j.cardiores.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Andreucci M, Michael A, Kramers C, et al. Renal ischemia/reperfusion and ATP depletion/repletion in LLC-PK(1) cells result in phosphorylation of FKHR and FKHRL1. Kidney Int. 2003;64:1189–1198. doi: 10.1046/j.1523-1755.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- 37.Jindra PT, Hsueh A, Hong L, et al. Anti-MHC class I antibody activation of proliferation and survival signaling in murine cardiac allografts. J Immunol. 2008;180:2214–2224. doi: 10.4049/jimmunol.180.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reyes-Vargas E, Pavlov IY, Martins TB, Schwartz JJ, Hill HR, Delgado JC. Binding of anti-HLA class I antibody to endothelial cells produce an inflammatory cytokine secretory pattern. J Clin Lab Anal. 2009;23:157–160. doi: 10.1002/jcla.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gill RG. NK cells: Elusive participants in transplantation immunity and tolerance. Curr Opin Immunol. 2010;22:649–654. doi: 10.1016/j.coi.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beilke JN, Gill RG. Frontiers in nephrology: The varied faces of natural killer cells in transplantation-contributions to both allograft immunity and tolerance. J Am Soc Nephrol. 2007;18:2262–2267. doi: 10.1681/ASN.2007040423. [DOI] [PubMed] [Google Scholar]
- 41.Hidalgo LG, Sis B, Sellares J, et al. NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: Evidence for NK cell involvement in antibody-mediated rejection. Am J Transplant. 2010;10:1812–1822. doi: 10.1111/j.1600-6143.2010.03201.x. [DOI] [PubMed] [Google Scholar]
- 42.Fildes JE, Yonan N, Tunstall K, et al. Natural killer cells in peripheral blood and lung tissue are associated with chronic rejection after lung transplantation. J Heart Lung Transplant. 2008;27:203–207. doi: 10.1016/j.healun.2007.11.571. [DOI] [PubMed] [Google Scholar]