Abstract
Background
Treatments for obsessive-compulsive disorder (OCD) usually lead to incomplete symptom relief and take a long-time to reach full effect. Convergent evidence suggests that glutamate abnormalities contribute to the pathogenesis of OCD. Ketamine is a potent noncompetitive antagonist of the N-methyl-D-aspartate glutamate receptor. Trials have reported rapid antidepressant effects after low-dose ketamine infusion.
Methods
We conducted an open-label trial of ketamine (0.5mg/kg IV over 40 minutes) in 10 subjects with treatment-refractory OCD. Response was defined as a greater than 35% improvement in OCD symptoms and greater than a 50% improvement in depression symptoms from baseline at any time between 1–3 days following infusion.
Results
None of 10 subjects experienced a response in OCD symptoms in the first 3 days following ketamine. Four of 7 patients with comorbid depression experienced an antidepressant response to ketamine in the first 3 days following infusion. Both OCD and depression symptoms demonstrated a statistically significant improvement in the first 3 days following infusion compared to baseline, but the OCD response was <12%. The percentage reduction in depressive symptoms in the first 3 days following ketamine infusion was significantly greater than the reduction in OCD symptoms.
Discussion
Ketamine effects on OCD symptoms, in contrast to depressive symptoms, did not appear to persist or progress after the acute effects of ketamine had dissipated.
Trial Registration
Ketamine Infusion for Obsessive-Compulsive Disorder, http://clinicaltrials.gov/ct2/show/NCT01349231, NCT01349231.
Keywords: obsessive-compulsive disorder, major depressive disorder, ketamine, clinical trial, glutamate, pharmacological therapy
Introduction
Roughly one-third of patients with obsessive-compulsive disorder (OCD) fail to experience significant clinical benefit from first-line interventions such as cognitive behavioral therapy (CBT) or pharmacotherapy with selective serotonin reuptake inhibitors (SSRIs) (1). Antipsychotic augmentation is the only pharmacological strategy for treatment-refractory OCD with demonstrated efficacy in multiple double-blind trials (2); but antipsychotic augmentation only benefits around 1 in 3 of these patients (2). When they do respond, OCD patients typically experience the full treatment benefits of first-line interventions only after a time-lag of two to three months. SSRIs take longer to work and are often required in higher doses to treat OCD symptoms compared to depressive symptoms (3, 4). Failure of symptom relief and delay of symptom relief from first-line treatments are sources of substantial morbidity and decreased quality of life in OCD patients.
Converging lines of evidence from neuroimaging, genetic and pharmacological studies support the importance of glutamate abnormalities in the pathogenesis of OCD (5). Higher glutamate levels have been demonstrated in the cerebrospinal fluid of treatment-naïve OCD patients compared to healthy controls (6). In magnetic resonance spectroscopy studies, altered concentrations of glutamate and related compounds have been demonstrated in the caudate nucleus and anterior cingulate cortex of treatment-naïve children with OCD compared to normal controls (7, 8). These levels have been shown to normalize with successful pharmacological treatment (7). In genetic studies, single nucleotide polymorphisms within the glutamate transporter gene SLC1A1 have been associated with the diagnosis of OCD (9–13). Open-label, pharmacological treatment studies have suggested that glutamate modulating agents such as riluzole (14–16), N-acetylcysteine (17) and memantine (18) may hold promise for the treatment of OCD.
Ketamine is a moderately potent non-competitive antagonist of the N-methyl-d-aspartate (NMDA) receptor, a major type of glutamate receptor in the brain. Ketamine can also have effects on other neurotransmitter systems, including the opioid and muscarinic systems, especially at anesthetic doses. A double-blind, saline-controlled study demonstrated that a single dose of ketamine (0.5 mg/kg, intravenously) had rapid antidepressant effects in depressed patients (19). In these subjects ketamine infusion produced mild psychotomimetic symptoms and euphoria that dissipated within 120 minutes, while the antidepressant effects of ketamine infusion emerged over the first 180 minutes and persisted over 72 hours (19). Fifty percent of depressed patients receiving ketamine had a remission of their depressive symptoms at Day 3 compared to 12.5% in the placebo infusion group (19). The rapid onset of persistent antidepressant effects following a single infusion of ketamine has been replicated in several controlled trials (20–22).
Between one-third and two-thirds of OCD patients suffer from comorbid depression (23, 24). A recently published case reported a temporary antiobsessional effect of intravenuous ketamine infusion in a woman with treatment-refractory OCD (25). We conducted an open label trial of ketamine for treatment-refractory OCD in order to investigate its possible efficacy and to determine the time-course of any anti-obsessional effects.
METHODS
Patient Selection
Subjects were recruited through the Yale OCD Research Clinic. The Yale OCD Research Clinic is a tertiary referral clinic with a research mission that recruits subjects through referrals from community providers and the International OCD Foundation, word-of-mouth, and internet and newspaper advertising.
Screening assessment for eligibility included a detailed psychiatric and medical evaluation performed by a licensed psychiatrist (MHB) and a Structured Clinical Interview for DSM-IV and multiple ratings of psychiatric symptomatology (described in the Study Assessment section) by an experienced research nurse (SW). Subjects had severe, treatment-refractory OCD, as defined by a number of eligibility criteria. (1) Subjects were male or female (post-menopausal, surgically sterile, or negative pregnancy test at screening and agreement to utilize an established birth control, including complete abstinence, during the testing period) between the age of 18 and 65 yrs. (2) All subjects had a DSM IV-TR diagnosis of obsessive-compulsive disorder by structured clinical interview (SCID), with a Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) score >24 (26, 27). (3) OCD was treatment-refractory, as defined by a Y-BOCS>24 despite two SSRI trials of adequate dose and duration and having been offered prior CBT treatment. (4)Subjects were required to have had stable doses of all psychiatric medications for the month prior to treatment and have had no changes of dosage of SSRIs or clomipramine for at least 2 months prior to study enrollment. Most subjects received their treatment elsewhere; doses and duration of previous medication trials were verified through consultation with treating psychiatrists and review of past medical and pharmacy records. (5) Subjects were medically and neurologically healthy on the basis of physical examination, medical laboratory assessment, EKG, and medical history. Individuals with stable medical conditions or taking medications without major CNS effects (e.g., oral hypoglycemics) were eligible if their medications had not been adjusted in the month prior to entry. (6) Subjects could have no lifetime history of substance dependence diagnosis by SCID (excluding tobacco) and had to have a urine toxicology screen negative for drug of abuse (28). (7) All subjects were required to provide written informed consent according to the Yale HIC guidelines. Subjects were compensated $500 for full participation in this trial. This trial received approval from the Yale Human Investigations Committee and was registered on clinicaltrials.gov (NCT01349231).
Study Intervention
Subjects were hospitalized at the Connecticut Mental Health Center Clinical Neuroscience Research Unit for 1 week prior to and 1 week following ketamine infusion in order to maintain a consistent environment in which to assess OCD symptoms. Following an overnight fast, ketamine 0.5 mg/kg was administered via a continuous constant rate IV infusion over a 40-minute period at the Yale Center for Clinical Investigation facilities within Yale-New Haven Hospital. The ketamine infusion procedures were modeled after previous ketamine infusion studies in depressed patients (19–21). A research nurse and a physician were present at all times during the procedure and for at least 1 hour following infusion. Heart rate, blood pressure and oxygen saturation were continuously monitored during and for an hour following the ketamine infusion.
Study Assessments
Structured clinical ratings were performed at screening/baseline, 1, 2, 3 hours and 1, 2, 3, 5 and 7 days following ketamine infusion. Clinical ratings were conducted to assess OCD and depression severity as well potential side-effects of ketamine throughout the trial. The ratings included: 1) Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) (26, 27); 2) Hamilton Depression Rating Scale-17 (HDRS-17) (29, 30); 3) Clinician-Administered Dissociative States Scale (CADSS) (31); and 4) the Clinical Global Impression (CGI) scale (32). Rating scales were limited in number to minimize the duration of assessments, as there was a short interval of time between assessments in the first 3 hours following ketamine infusion. The HDRS and Y-BOCS ratings were slightly modified to account for the compressed temporal context of the assessments during the assessments performed 1, 2 and 3 hours following infusion. Items 1 (time occupied by obsessive thoughts) and 6 (time occupied by compulsions) from the Y-BOCS were modified such that the frequency of these events was emphasized rather than both event the actual time-spent. For instance, for Item 1 we asked the question “How often do these obsessions occur?” rather than both, “How much time is occupied by obsessive thoughts? And How often do these obsessions occur?” For the HDRS-17 ratings 1,2 and 3 hours following infusion the answers to the insomnia related items were carried forward from the previous night. Subjects were allowed to undergo changes in psychiatric medications starting 7 days following ketamine infusion within the study protocol. We additionally conducted symptom ratings at 10, 14, 21 and 28 days after ketamine-infusion to monitor safety. We do not to report results from days 10–28 in this study because (1) half of subjects enrolled in this trial underwent other medication changes 1–2 weeks following infusion, (2) 3 of 10 subjects left the inpatient unit at 1 week following infusion and (3) ratings of symptom severity did not change appreciably in any of the subjects after day 7.
Statistical Analysis
The primary goal of this trial was to determine the character and duration of OCD response to ketamine in order to provide information for a potential, larger, saline-controlled trial of ketamine in the treatment of OCD. We decided a priori that if 3 or more subjects with OCD exhibited an OCD treatment response at any point 1–7 days following infusion, this would provide sufficient evidence to pursue a larger trial. A treatment response was defined as a 35% or greater reduction in Y-BOCS severity from baseline, as is convention for OCD. Assuming that the treatment response to ketamine observed in OCD was similar to that observed in depression (conservatively 50% of subjects), there would be a 98% probability of having at least 3 of 10 subjects have an OCD response to ketamine infusion.
We used Wilcoxon Rank Sum Tests to compare (1) average severity of OCD symptoms (Y-BOCS total score) for the first 3 days following infusion to baseline, (2) average severity of depressive symptoms for the first 3 days following infusion to baseline and (3) cumulative percent reduction (area under the curve) in OCD symptoms compared to percent reduction in depressive symptoms in the first 3 days following infusion. Averages of rating on the first 3 days following infusion were used in order to reduce the number of hypotheses tested, given that we did not known the exact time course of potential ketamine response in OCD. Ratings at 1 and 2 hours after infusion were not included in the primary analysis in order to minimize the influence of the acute psychotomimetic effects of ketamine on ratings immediately following the infusion. When average symptom severity scores were significantly different from baseline, individual Wilcoxon Rank Sum Tests were conducted at individual time-points, relative to baseline. Hypothesis testing was not corrected for multiple comparisons.
The same procedure was used for comparing the percent reduction in OCD and depression symptoms. We used a Pearson correlation to assess the degree of correlation between reduction in OCD and depressive symptoms. Only subjects with significant current depressive symptoms (HDRS-17≥16) were included in the analysis of the effects of ketamine on depression. We used the Fisher’s Exact Test to compare the proportion of OCD treatment responders to depression treatment responders. All statistical analyses were performed in IBM SPSS Statistics 19 for Windows (Chicago, Illinois).
RESULTS
Subjects
10 subjects with treatment-refractory OCD were enrolled in this trial. Subjects had severe OCD symptoms (YBOCS average: 32.9 +/− 1.9 , range: 31–36) despite multiple previous SRI trials of adequate dose and duration (SRI trials: 4.1 +/− 1.2, range: 2–5). Every enrolled subject had additionally undergone previous cognitive behavioral therapy for OCD and antipsychotic augmentation (antipsychotic trials: 2.7 +/− 1.6, range: 1–6). Average age at ketamine infusion was 41.7 +/− 13.5 years (range: 18–64). Duration of OCD symptoms prior to ketamine infusion was 24.6 +/− 17.4 years (range: 4–54). Three subjects were medication-free at the time of ketamine infusion. The other 7 subjects were on SRIs (6 at maximal tolerated dose). Four of these patients were also taking antipsychotic medications and 1 subject was taking glutamate-modulating agents (both n-acetylcysteine and riluzole). Further details of the individual subjects enrolled in the trial are depicted in Table 1.
TABLE 1.
Characteristics of Included Subjects
| Past Trials | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Gender | Duration of Illness |
OCD Symptom Dimensions | MDD | Other Psychiatric Diagnoses | Current Medications | SRI | Antipsychotics | Glutamatergic Agents |
| 30 | Male | 5 | cleaning | Past | Social Phobia | citalopram 80mg, risperidone 2mg, clonazepam 2mg | 2 | 1 | 0 |
| 64 | Female | 54 | forbidden thoughts, cleaning, hoarding | Past | PTSD | fluvoxamine 300mg, riluzole 100mg, NAC 3600mg, aripiprazole 1mg | 3 | 1 | 2 |
| 44 | Male | 24 | Symmetry (repeating), hoarding, cleaning | Current | Social Phobia | zoloft 200mg, clonazepam 0.5mg | 5 | 6 | 0 |
| 36 | Male | 20 | forbidden thoughts | Current | NONE | clomipramine 250mg, olanzapine 15mg | 3 | 2 | 2 |
| 48 | Male | 31 | cleaning, hoarding | Current | NONE | fluvoxamine 300mg, trazodone 300mg | 5 | 3 | 0 |
| 23 | Male | 4 | cleaning | Current | Social Phobia, Eating Disorder NOS | NONE | 3 | 2 | 0 |
| 47 | Female | 34 | cleaning, forbidden thoughts | Current | Trichotillomania | NONE | 5 | 3 | 0 |
| 40 | Male | 22 | symmetry (repeating), cleaning | Current | Past tic disorder | fluoxetme 80mg, clomipramme 150mg, seroquet 25, clonazepam 2mg | 5 | 5 | 0 |
| 18 | Female | 6 | cleaning | Current | Eating Disorder NOS, skin picking | NONE | 5 | 2 | 0 |
| 23 | Female | 10 | forbidden thoughts | Past | Trichotillomania, PTSD | escitalopram 20mg, clomipramine 50 mg | 5 | 2 | 0 |
Effect of Ketamine on Obsessive-Compulsive Disorder
There was a significant acute, but transient, improvement in OCD symptoms 1–3 hours following ketamine infusion, which largely dissipated by the next day. Y-BOCS improvement was slight (peaking at 11%) but statistically significant over days 1–3 following ketamine infusion (Wilcoxon Rank Sum Test: T=−2.81, N=10, p=0.005). None of the 10 subjects exhibited an OCD response to ketamine infusion, defined a priori as a 35% improvement in Y-BOCS at any point between 1–3 days following the infusion. The effects of ketamine on the obsession and compulsion subscale scores of the Y-BOCS followed the same pattern and were not significantly different from one another (Figure S1 in the Supplement).
Effect of Ketamine on Depressive Symptoms
Four of the 7 OCD subjects with significant comorbid depressive symptoms experienced a depression response to ketamine within the first 3 days following infusion. Average HDRS-17 scores in the first 3 days following ketamine infusion were also significantly reduced compared to baseline (Wilcoxon Rank Sum Test: T=2.20, N=7, p=0.03). One of the three subjects who were not on other medications at the time of infusion experienced a response to ketamine in terms of their depressive symptoms.
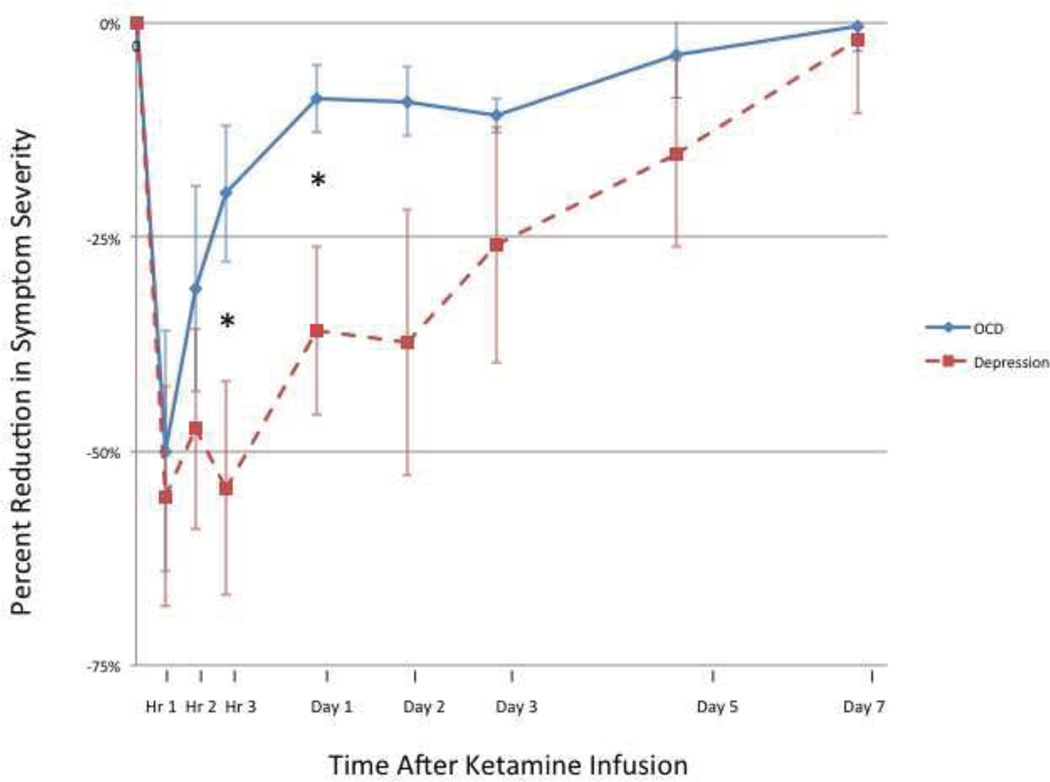
Comparative Effects of Ketamine on OCD and Depression
Figure 1 compares the reduction in OCD and depression symptom severity in the first 7 days following ketamine infusion. The percent reduction of depressive symptoms in response to ketamine was significantly greater than the reduction in OCD symptoms in the first 3 days following infusion (Wilcoxon Rank Sum Test: T=−2.0, N=7, p=0.043). There was a significant correlation between the percent reduction in OCD and depressive symptoms in the first 3 days following infusion among the 7 OCD subjects with comorbid depression (Pearson r=0.82, p=0.02; Figure S2 in the Supplement).
Figure 1. Effect of Ketamine on OCD vs. Depression Symptoms.
There was a significantly greater reduction in depressive compared to OCD symptoms in the first 3 days following ketamine infusion (Wilcoxon Rank Sum Test: T=−2.0, N=7, p=0.043). * indicates p<0.05.
Treatment Responders to Ketamine
None of the 10 subjects experienced an OCD response to ketamine, defined a priori as a 35% reduction in Y-BOCS at any time during days 1–3 following infusion. Additionally, no subject was “very much” or “much” improved on the CGI during the same time period (Figure S3 in the Supplement). Four of 7 subjects with significant comorbid depressive symptoms experienced a depression response, defined a priori as a 50% improvement in HAM-D17 at any point during the same time period. The difference in the proportion of responders in the two domains was statistically significant (Fisher’s exact test p<0.02).
Safety and Tolerability
The ketamine procedure was well tolerated and completed by all subjects. One subject experienced a transient increase in systolic blood pressure (<30% above baseline, maximum 160/80) which lasted 10 minutes. No subjects experienced tachycardia (heart rate greater than 100 beats per minute) during the infusion.
Half of subjects reported some dissociative symptoms during infusion, but CADSS scores remained low in the hour of infusion (mean=1.4, range: 0–5). Subjects reported gaps in memory (n=3), sensory distortions (i.e. perioral perasthesias, n=2), a feeling that time was moving in slow motion (n=2), disconnected feeling from reality (n=1). No subjects reported any dissociative symptoms beyond the 1 hour time point No subjects reported or exhibited paranoia or hallucinations during the infusion. Two of the 3 non-depressed subjects reported dysphoria, anxiety and passive suicidal ideation within the first two days following ketamine infusion.
DISCUSSION
In our trial, ketamine demonstrated an acute effect on both OCD and depressive symptoms. Ketamine effects on OCD symptoms (in contrast to depressive symptoms) did not appear to persist or progress after the acute effects of ketamine had dissipated. Despite the fact that our uncontrolled trial demonstrated a modest statistically improvement in the first 3 days following the acute effects of ketamine infusion, it provides strong data that single dose of ketamine does not have the same sustained/persistent potent effect on OCD symptoms that it does on depression. Several aspects of our data support this conclusion. (1) None of 10 subjects enrolled in this trial demonstrated an OCD response to ketamine. Furthermore, (2) no subject rated their OCD symptoms as “very much improved” or “much improved” on the CGI 1–7 days following ketamine infusion. (3) Although statistically significant, the improvement in OCD symptoms 1–7 days following infusion was quite modest (peaking at an 11% reduction on Day 2) compared to the greater than 60% reduction in OCD symptoms observed at the 1 hour time point when the acute psychomometic and dissociative effects of ketamine were still present.
By contrast, comorbid depressive symptoms in OCD symptoms demonstrated significant and a relatively prolonged response after ketamine infusion. 59% (4 of 7) subjects with comorbid depressive symptoms exhibited a depression response to ketamine infusion. This response rate is quite consistent with previous trials in depression that have reported response rates between 50–70% 24–72hrs following ketamine infusion (19–21).
The results of our uncontrolled trial differed from a previous case report that reported a rapid antiobsessional effect of ketamine that persisted for several days following infusion (25). Although we noticed a short-lived improvement in OCD symptoms following ketamine infusion, we did not observe a prolonged response in any subjects. We additionally did not observe a differential effect of ketamine on obsessions versus compulsions. Furthermore, ketamine effects on OCD symptoms (in contrast to depressive symptoms) did not appear to persist or progress after the acute effects of ketamine had dissipated.
Ketamine is not the only challenge paradigm that has been shown to differentially affect depressive and OCD symptom. Tryptophan depletion, which temporarily reduces the availability of whole brain serotonin, has been demonstrated to exacerbate depressive but not OCD symptoms in OCD subjects whose symptoms were responsive to SRI medications (33–35). Additionally, several FDA-approved medications to treat depression are not effective in the treatment of OCD. These pharmacological agents include bupropion (36), buspirone (37), trazodone (38), non-selective tricyclic antidepressants (39–41) and monoamine oxidase inhibitors (42, 43).
In light of this negative conclusion, it is important to note a couple significant limitations to this trial. The current trial was small, uncontrolled and unblinded. We made the decision to design a trial in this manner because the time course of OCD response to ketamine was unknown and establishing this time-course would be critical to properly designing a definitive controlled trial. Additionally, establishing a credible placebo control for ketamine research is problematic, given the predictable and common acute physiologic (increase in heart rate and blood pressure) and psychological (dissociative symptoms, euphoria, perioral paresthesias, nausea etc.) effects of ketamine. However, treatment benefits are usually exaggerated in uncontrolled, unblinded trials, and thus one would expect the treatment effects of ketamine to be exaggerated, not minimized, in an open label trial such as this.
Comparing percent symptom reduction across different scales in different disorders (Figure 3) is potentially problematic. This comparison assumes that the scales are linear, and that percentage reduction in symptoms across OCD and depression scales would be similar. That being said, the percent reduction of OCD and depression symptoms was nearly identical during the acute period after infusion and differed only in the follow-up period 3 hours-7 days following infusion. In addition, if there were an improvement in OCD symptoms that was not captured by the Y-BOCS due to nonlinearities or other limitations, one would expect it to be reflected in the CGI. No such CGI benefit was observed.
Our trial suggests that ketamine infusion following a protocol that has now become standard (0.5mg/kg infused over 40 minutes) may have acute anti-obsessional effects but does not have the same sustained beneficial effects on OCD symptoms that it has in depression. The acute benefit of ketamine on OCD symptoms needs to be confirmed in controlled trials and cannot be divorced from the psychomometic and dissociative effects of ketamine. Additionally, we cannot eliminate the possibility that other environmental effects caused acute changes in OCD symptoms, as the ketamine infusion took place in a different context (a medical hospital) than where other ratings were conducted (an inpatient psychiatric unit). If present, an acute effect of ketamine would suggest that immediate effects of ketamine on glutamate neurotransmission may be important in OCD. We would suggest that future controlled trials of ketamine in OCD should utilize an active (i.e. midazolam) rather than saline control because the immediate period following ketamine infusion may be of particular interest in OCD and therefore controlling for some of the nonspecific psychomometic and dissociative effects of ketamine will be particularly important. Rigorous trials of other promising glutamate modulating agents that can be given repeatedly such as riluzole, n-acetylcysteine and memantine remain important and needed (44). The lack of prolonged anti-OCD effects of ketamine suggest the delayed effects of ketamine on synaptic plasticity may not be as important in the pathogenesis of OCD (45). The lack of a prolonged anti-OCD effect of ketamine does not eliminate the possibility that prolonged or repeated dosing of ketamine may have a persistent effect on OCD symptoms. Higher doses of SSRI medications are more effective in the treatment of OCD (3). Additionally, ketamine may have a more prolonged effect in a less treatment-refractory OCD population (such as medication free, treatment-naïve or pediatric OCD subjects); however it would be difficult to justify the use of ketamine in these patient populations given the presence of other effective and benign treatments in these populations.
Supplementary Material
Acknowledgements
The authors acknowledge the NARSAD (MHB), the APIRE/Eli Lilly Psychiatric Research Fellowship (MHB), National Institutes of Health grants K23MH091240 (MHB) and K08MH081190 (CP), the AACAP/ Eli Lilly Pilot Research Award (MHB), the Trichotillomania Learning Center (MHB), UL1 RR024139 (MHB), from the National Center for Research Resources, a component of the National Institutes of Health, and NIH roadmap for Medical Research (MHB), and the State of Connecticut, Department of Mental Health and Addiction Services for its support of the Ribicoff Research Facilities (MHB, CP, GS).
Disclosures:
Dr. Bloch has received funding from the National Institutes of Health the APIRE/Eli Lilly Psychiatric Research Fellowship (MHB), the AACAP/ Eli Lilly Pilot Research Award, the Trichotillomania Learning Center, NARSAD, and UL1 RR024139 from the National Center for Research Resources, a component of the National Institutes of Health, and NIH roadmap for Medical Research.
Dr. Leckman has received research funding from the National Institutes of Health, Tourette Syndrome Association, Talecris Biotherapeutics and C8Sciences, Klingenstein Third Generation Foundation (medical student fellowship program). James F. Leckman has received book royalties from John Wiley and Sons, McGraw Hill, Oxford University Press.
Dr Krystal has served as a scientific consultant to the following companies (The Individual Consultant Agreements listed below are less than $10 000 per year): Aisling Capital, Astellas Pharma Global Development, AstraZeneca Pharmaceuticals, Biocortech, Brintnall & Nicolini, Easton Associates, Gilead Sciences, GlaxoSmithKline, Janssen Pharmaceuticals, Lundbeck Research USA, Medivation, Merz Pharmaceuticals, MK Medical Communications, F Hoffmann-La Roche, SK Holdings, Sunovion Pharmaceuticals, Takeda Industries, Teva Pharmaceutical Industries. He is on the Scientific Advisory Board for the following companies: Abbott Laboratories, Bristol-Myers Squibb, Eisai, Eli Lilly, Forest Laboratories, Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Naurex, Pfizer Pharmaceuticals, Shire Pharmaceuticals. He holds less than $150 in exercisable warrant options with Tetragenex Pharmaceuticals. He is on the Board of Directors: Coalition for Translational Research in Alcohol and Substance Use Disorders. He is President Elect: American College of Neuropsychopharmacology. He is the principal investigator of a multicenter study in which Janssen Research Foundation has provided drug and some support to the Department of Veterans Affairs. He is on the Editorial Board, Editor of Biological Psychiatry (Income Greater than $10 000). He has Patents and Inventions: 1) Seibyl JP, Krystal JH, Charney DS. Dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia. Patent number: 5 447 948, 5 September 1995; 2) he is a co-inventor with Dr Gerard Sanacora on a filed patent application by Yale University related to targeting the glutamatergic system for the treatment of neuropsychiatric disorders (PCTWO06108055A1); (3) Intranasal Administration of Ketamine to Treat Depression (pending).
Dr. Bhagwagar is a full time employee and stock holder at Bristol Myers Squibb He is funded in part by a NARSAD Young Investigator Award (Dr Bhagwagar), the National Institutes of Health (grant no. K23-MH077914), and a Clinical and Translational Science Award (grant no. UL1 RR024139) from the National Center for Research Resources to Yale University.
Dr. Sanacora has received consulting fees form AstraZeneca, Avanir, Bristol-Myers Squibb, Evotec, Eli Lilly & Co., Hoffman La-Roche, Johnson & Johnson, Novartis, and Novum Pharmaceuticals in the last 24 months. He has received additional grant support from AstraZeneca, Bristol-Myers Squibb, Hoffman La-Roche, Merck & Co., and Sunovion Inc. He has also received free study drug from Sanofi-Aventis.
Dr. Pittenger is a consultant for F. Hoffman La Roche Pharmaceuticals, Inc., and has received research support from Pfizer Pharmaceuticals, Inc.
Footnotes
All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Foa EB, Liebowitz MR, Kozak MJ, Davies S, Campeas R, Franklin ME, et al. Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 2005;162(1):151–161. doi: 10.1176/appi.ajp.162.1.151. [DOI] [PubMed] [Google Scholar]
- 2.Bloch MH, Landeros-Weisenberger A, Kelmendi B, Coric V, Bracken MB, Leckman JF. A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Mol Psychiatry. 2006;11(7):622–632. doi: 10.1038/sj.mp.4001823. [DOI] [PubMed] [Google Scholar]
- 3.Bloch MH, McGuire J, Landeros-Weisenberger A, Leckman JF, Pittenger C. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850–855. doi: 10.1038/mp.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bollini P, Pampallona S, Tibaldi G, Kupelnick B, Munizza C. Effectiveness of antidepressants. Meta-analysis of dose-effect relationships in randomised clinical trials. Br J Psychiatry. 1999;174:297–303. doi: 10.1192/bjp.174.4.297. [DOI] [PubMed] [Google Scholar]
- 5.Pittenger C, Bloch MH, Williams K. Glutamate abnormalities in obsessive compulsive disorder: Neurobiology, pathophysiology, and treatment. Pharmacol Ther. 2011;132(3):314–332. doi: 10.1016/j.pharmthera.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakrabarty K, Bhattacharyya S, Christopher R, Khanna S. Glutamatergic dysfunction in OCD. Neuropsychopharmacology. 2005;30(9):1735–1740. doi: 10.1038/sj.npp.1300733. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg DR, MacMaster FP, Keshavan MS, Fitzgerald KD, Stewart CM, Moore GJ. Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. J Am Acad Child Adolesc Psychiatry. 2000;39(9):1096–1103. doi: 10.1097/00004583-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg DR, Mirza Y, Russell A, Tang J, Smith JM, Banerjee SP, et al. Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. J Am Acad Child Adolesc Psychiatry. 2004;43(9):1146–1153. doi: 10.1097/01.chi.0000132812.44664.2d. [DOI] [PubMed] [Google Scholar]
- 9.Arnold PD, Sicard T, Burroughs E, Richter MA, Kennedy JL. Glutamate transporter gene SLC1A1 associated with obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63(7):769–776. doi: 10.1001/archpsyc.63.7.769. [DOI] [PubMed] [Google Scholar]
- 10.Dickel DE, Veenstra-VanderWeele J, Cox NJ, Wu X, Fischer DJ, Van Etten-Lee M, et al. Association testing of the positional and functional candidate gene SLC1A1/EAAC1 in early-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63(7):778–785. doi: 10.1001/archpsyc.63.7.778. [DOI] [PubMed] [Google Scholar]
- 11.Samuels J, Wang Y, Riddle MA, Greenberg BD, Fyer AJ, McCracken JT, et al. Comprehensive family-based association study of the glutamate transporter gene SLC1A1 in obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(4):472–477. doi: 10.1002/ajmg.b.31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shugart YY, Wang Y, Samuels JF, Grados MA, Greenberg BD, Knowles JA, et al. A family-based association study of the glutamate transporter gene SLC1A1 in obsessivecompulsive disorder in 378 families. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(6):886–892. doi: 10.1002/ajmg.b.30914. [DOI] [PubMed] [Google Scholar]
- 13.Stewart SE, Fagerness JA, Platko J, Smoller JW, Scharf JM, Illmann C, et al. Association of the SLC1A1 glutamate transporter gene and obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(8):1027–1033. doi: 10.1002/ajmg.b.30533. [DOI] [PubMed] [Google Scholar]
- 14.Coric V, Taskiran S, Pittenger C, Wasylink S, Mathalon DH, Valentine G, et al. Riluzole augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial. Biol Psychiatry. 2005;58(5):424–428. doi: 10.1016/j.biopsych.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 15.Grant P, Lougee L, M H, Swedo S. An open-label trial of riluzole, a glutamate antagonist, in children with treatment-refractory obsessive-compulsive disorder. Journal of Child and Adolescent Psychopharmacology. doi: 10.1089/cap.2007.0021. in press. [DOI] [PubMed] [Google Scholar]
- 16.Grant P, Lougee L, Hirschtritt M, Swedo SE. An open-label trial of riluzole, a glutamate antagonist, in children with treatment-resistant obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. 2007;17(6):761–767. doi: 10.1089/cap.2007.0021. [DOI] [PubMed] [Google Scholar]
- 17.Lafleur DL, Pittenger C, Kelmendi B, Gardner T, Wasylink S, Malison RT, et al. N-acetylcysteine augmentation in serotonin reuptake inhibitor refractory obsessive-compulsive disorder. Psychopharmacology (Berl) 2006;184(2):254–256. doi: 10.1007/s00213-005-0246-6. [DOI] [PubMed] [Google Scholar]
- 18.Stewart SE, Jenike EA, Hezel DM, Stack DE, Dodman NH, Shuster L, et al. A single-blinded case-control study of memantine in severe obsessive-compulsive disorder. J Clin Psychopharmacol. 2010;30(1):34–39. doi: 10.1097/JCP.0b013e3181c856de. [DOI] [PubMed] [Google Scholar]
- 19.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 20.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 21.Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, et al. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res. 2011;191(2):122–127. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pigott TA, L'Heureux F, Dubbert B, Bernstein S, Murphy DL. Obsessive compulsive disorder: comorbid conditions. The Journal of clinical psychiatry. 1994;55(Suppl):15–27. discussion 8–32. [PubMed] [Google Scholar]
- 24.Robins LN, Helzer JE, Weissman MM, Orvaschel H, Gruenberg E, Burke JD, Jr, et al. Lifetime prevalence of specific psychiatric disorders in three sites. Archives of general psychiatry. 1984;41(10):949–958. doi: 10.1001/archpsyc.1984.01790210031005. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez CI, Kegeles LS, Flood P, Simpson HB. Rapid resolution of obsessions after an infusion of intravenous ketamine in a patient with treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2011;72(4):567–569. doi: 10.4088/JCP.10l06653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, et al. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch Gen Psychiatry. 1989;46(11):1012–1016. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- 27.Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 28.First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2001. [Google Scholar]
- 29.Hamilton M. Rating depressive patients. J Clin Psychiatry. 1980;41(12 Pt 2):21–24. [PubMed] [Google Scholar]
- 30.Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology. 1967(6):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 31.Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998;11(1):125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- 32.Guy W. ECDEU assessment manual for psychopharmacology. Rockville, MD: National Institute of Mental Health; 1976. [Google Scholar]
- 33.Smeraldi E, Diaferia G, Erzegovesi S, Lucca A, Bellodi L, Moja EA. Tryptophan depletion in obsessive-compulsive patients. Biol Psychiatry. 1996;40(5):398–402. doi: 10.1016/0006-3223(95)00393-2. [DOI] [PubMed] [Google Scholar]
- 34.Barr LC, Goodman WK, McDougle CJ, Delgado PL, Heninger GR, Charney DS, et al. Tryptophan depletion in patients with obsessive-compulsive disorder who respond to serotonin reuptake inhibitors. Arch Gen Psychiatry. 1994;51(4):309–317. doi: 10.1001/archpsyc.1994.03950040053007. [DOI] [PubMed] [Google Scholar]
- 35.Berney A, Sookman D, Leyton M, Young SN, Benkelfat C. Lack of effects on core obsessive-compulsive symptoms of tryptophan depletion during symptom provocation in remitted obsessive-compulsive disorder patients. Biol Psychiatry. 2006;59(9):853–857. doi: 10.1016/j.biopsych.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 36.Vulink NC, Denys D, Westenberg HG. Bupropion for patients with obsessive-compulsive disorder: an open-label, fixed-dose study. J Clin Psychiatry. 2005;66(2):228–230. doi: 10.4088/jcp.v66n0211. [DOI] [PubMed] [Google Scholar]
- 37.McDougle CJ, Goodman WK, Leckman JF, Holzer JC, Barr LC, McCance-Katz E, et al. Limited therapeutic effect of addition of buspirone in fluvoxamine-refractory obsessive-compulsive disorder. Am J Psychiatry. 1993;150(4):647–649. doi: 10.1176/ajp.150.4.647. [DOI] [PubMed] [Google Scholar]
- 38.Pigott TA, L'Heureux F, Rubenstein CS, Bernstein SE, Hill JL, Murphy DL. A double-blind, placebo controlled study of trazodone in patients with obsessive-compulsive disorder. J Clin Psychopharmacol. 1992;12(3):156–162. [PubMed] [Google Scholar]
- 39.Foa EB, Kozak MJ, Steketee GS, McCarthy PR. Treatment of depressive and obsessive-compulsive symptoms in OCD by imipramine and behaviour therapy. Br J Clin Psychol. 1992;31(Pt 3):279–292. doi: 10.1111/j.2044-8260.1992.tb00995.x. [DOI] [PubMed] [Google Scholar]
- 40.Leonard HL, Swedo SE, Rapoport JL, Koby EV, Lenane MC, Cheslow DL, et al. Treatment of obsessive-compulsive disorder with clomipramine and desipramine in children and adolescents. A double-blind crossover comparison. Arch Gen Psychiatry. 1989;46(12):1088–1092. doi: 10.1001/archpsyc.1989.01810120030006. [DOI] [PubMed] [Google Scholar]
- 41.Foa EB, Steketee G, Kozak MJ, Dugger D. Effects of imipramine on depression and obsessive-compulsive symptoms. Psychiatry Res. 1987;21(2):123–136. doi: 10.1016/0165-1781(87)90070-9. [DOI] [PubMed] [Google Scholar]
- 42.Insel TR, Murphy DL, Cohen RM, Alterman I, Kilts C, Linnoila M. Obsessive-compulsive disorder. A double-blind trial of clomipramine and clorgyline. Arch Gen Psychiatry. 1983;40(6):605–612. doi: 10.1001/archpsyc.1983.04390010015002. [DOI] [PubMed] [Google Scholar]
- 43.Jenike MA, Baer L, Minichiello WE, Rauch SL, Buttolph ML. Placebo-controlled trial of fluoxetine and phenelzine for obsessive-compulsive disorder. Am J Psychiatry. 1997;154(9):1261–1264. doi: 10.1176/ajp.154.9.1261. [DOI] [PubMed] [Google Scholar]
- 44.Pittenger C, Bloch MH, Williams K. Glutamate abnormalities in obsessive compulsive disorder: neurobiology, pathophysiology, and treatment. Pharmacology & therapeutics. 2011;132(3):314–332. doi: 10.1016/j.pharmthera.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duman RS, Li N, Liu RJ, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62(1):35–41. doi: 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.