Abstract
The kinematic strategy encoded in motor cortical areas for classic straight-line reaching is remarkably simple and consistent across subjects, despite the complicated musculoskeletal dynamics that are involved. As tasks become more challenging, however, different conscious strategies may be utilized to improve perceived behavioral performance. We discovered that additional spatial information appeared both in single neurons and in the population code of monkey dorsal premotor cortex when obstacles impeded direct reach paths. The neural correlate of movement planning varied between subjects in a manner consistent with the use of different strategies to optimize task completion. These distinct planning strategies were manifested in the timing and strength of the information contained in the neural population code.
Humans and other primates are adept at reaching for visually-identified targets, an important element of the behavioral repertoire. The neural mechanisms supporting this have long been an active area of investigation. For reaching movements, the output of the motor system is a sequence of muscle activations which guide the hand appropriately through space to achieve the goal of the reach. Before movement initiation, sensory information and cognitive processes interact to form an initial movement plan. During this preparatory period, neural activity relating to the upcoming reach can be seen in a network of frontal and parietal cortical areas, including the premotor (PM) and primary motor (M1) cortices (1–4). Instructed-delay reaching tasks, an experimental paradigm in which a monkey is shown a target but must withhold movement until a ‘go’ cue is given, allow this preparatory neural activity to be probed. Electrophysiological (5–7), imaging (8, 9), and transcranial magnetic stimulation (10) studies highlight dorsal premotor cortex (PMd) as a critical area for planning reaching movements. However, the precise relationship between neural activity in PMd and reaching behavior remains unresolved. Many PMd neurons show a sensitivity of firing rate to direction, be it the direction of arm movement (1, 6, 11, 12), an effector in visual space (13), a target location (14), visuospatial attention (15, 16), or other parameters. Unfortunately, virtually all of these factors correlate with each other under normal circumstances - the hand movement and visual movement are correlated, the target direction and movement direction are correlated, and so forth. Neural activity related to any of these parameters will thus also correlate with the others. The widely-used center-out task (17), in which a subject makes hand movements in all directions from a central starting point, is particularly susceptible to this limitation. To interrogate directional tuning properties in more detail, tasks designed to decorrelate certain aspects of the behavior, such as arm movement from visual cursor movement (13, 18) or position from velocity (19), are commonly employed. We dissociated target direction from initial movement direction, allowing us to independently examine the effects of target and planned movement directions on preparatory neural activity.
To investigate the importance of target selection and motor planning in PMd and provide a framework for relating our findings to classic center-out based studies of motor cortical function, we adopted the following experimental approach: 1) train rhesus macaques to perform two reaching tasks, a standard center-out and a complex obstacle-avoidance paradigm; 2) record single-unit activity from PMd while the monkeys perform both tasks in a blocked design; 3) quantify the relationship between the firing of individual neurons and various parameters by fitting cosine tuning curves; 4) construct population decoding models from these tuning properties; 5) evaluate the performance of the models at predicting behavior by decoding the recorded neural responses.
The behavioral tasks consist of arm movements in free space within a virtual reality simulator, with hand position mapped onto a frontoparallel plane (20) (Fig. 1 and fig. S1). Both tasks involve a set of instructed delay periods with information revealed sequentially prior to a ‘go’ cue. The basic center-out task consists of direct reaching movements in eight directions from five different starting locations. Since the hand is held at different locations and the target is unknown during delay 1, the preferred position gradient for each neuron can be determined without interference from the formation of a movement plan. In the second delay period the target is revealed; during this time neuronal preferred direction can be determined analogous to prior studies. The obstacle-avoidance task begins with the same delay sequence but with a single, central starting position. During a third delay period, an obstacle is revealed which may or may not require the monkey to make an indirect hand trajectory to reach the target successfully. In both tasks, after a random length final delay, the sphere representing the start position is extinguished, indicating that the reach can begin. Successful movements (those that acquire the target while avoiding the obstacle, if necessary) are rewarded following a target hold period. Two monkeys performed the reaching tasks while acute intracortical recordings were taken from contralateral PMd (20). A total of 723 single units were recorded (monkey G, 343; monkey H, 380).
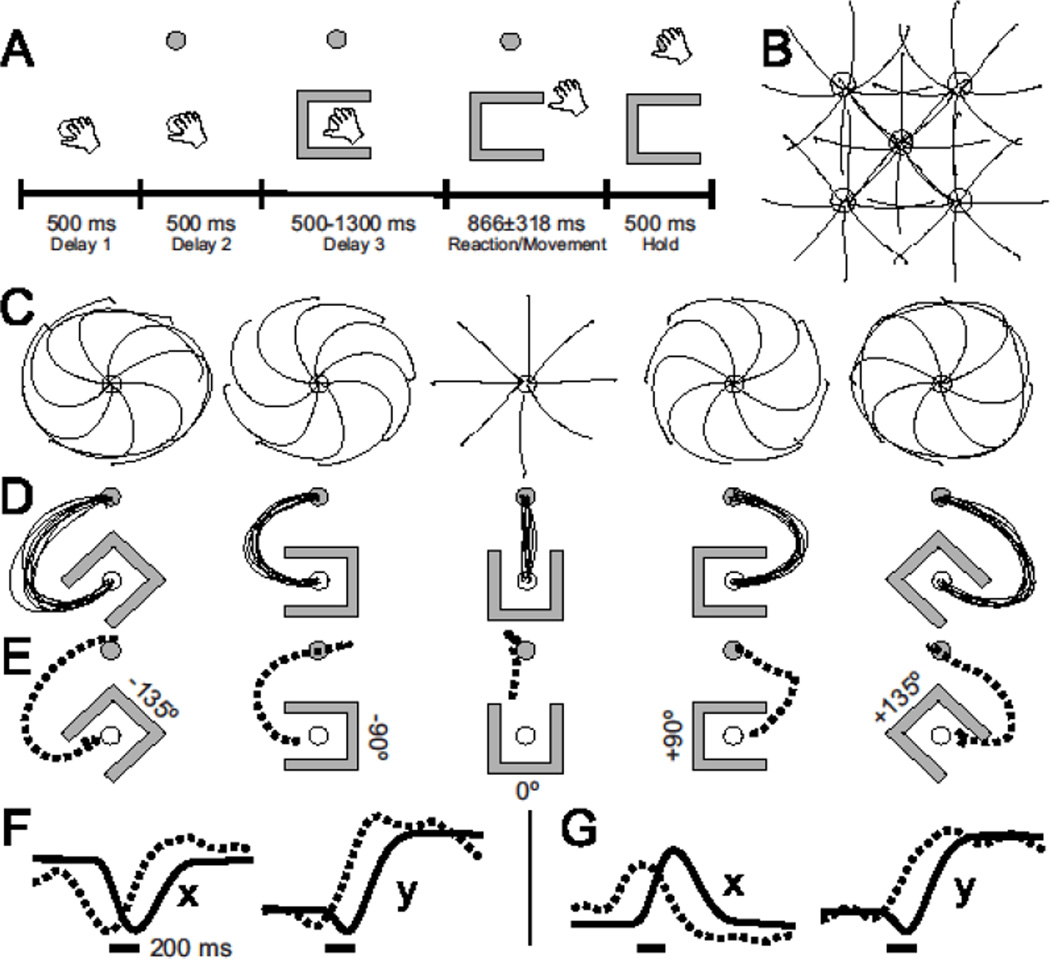
Fig. 1.
Kinematics and neural decoding of hand trajectory (monkey G). A) Single trial timeline for the obstacle-avoidance task. B) Mean hand trajectories for the 40 unique center-target combinations in the center-out task. C) Mean hand trajectories for the 40 unique target-obstacle combinations. For clarity the trajectories have been separated into panels according to orientation of the obstacle relative to the target direction. D) The trajectories from C, rotated such that the target direction is always straight up. E) Neural prediction of hand path for the conditions in D, decoded with the position-PV during the movement epoch. F) x- and y-components of hand position (solid line) and neural prediction (dashed line) for the −135° condition (D-E, left). Scale bar: 200ms. G) Same as F, for +135° condition (D-E, right).
Population vector (PV) analysis (17) was used to investigate the spatial and temporal aspects of the neuronal population representation of planned and executed movements. Position and velocity encoding of the hand are represented at the single neuron level within motor cortex (19). To evaluate contributions of these parameters in PMd, we calculated the preferred movement direction and position gradient of each neuron from delay-period activity in the center-out task. These tuning properties were then used to construct two PV decoders, one for position and another for velocity. The PV models were applied to the obstacle-avoidance data to decode time-resolved estimates of instantaneous firing rates during the planning and movement epochs (20). Taking advantage of the rotational symmetry of the task, we collapsed all trials (Fig. 1C and fig. S2) down to the 5 relative orientations of target and obstacle (Fig. 1D-E), effectively increasing the repetitions of each movement. Figure 1E shows the neural prediction of hand path for each relative orientation, as decoded by the positional PV from movement-epoch activity. The neural population response preceded the actual hand movement by approximately 200 ms (Fig. 1F-G). The velocity PV decoded from movement time activity yielded poor reconstructions (Fig. S3) suggesting that PMd activity better represents hand position versus hand velocity during movement.
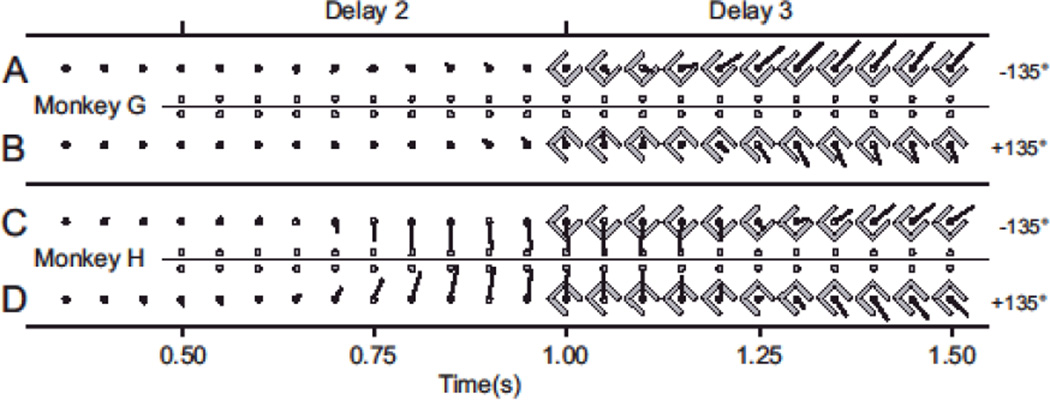
During the preparatory period, the velocity-based PV predicts the initial direction of movement required to escape the obstacle. The temporal evolution of the neural activity suggests that the two monkeys likely adopted different strategies on the task. Figure 2 illustrates the behavior of the velocity-based PV for two trial conditions for each animal, as it changes over the course of the delay periods. The lengths and directions of the PV for all trial types are shown in Fig. 3. In monkey G, the length of the PV remained insignificant until the final delay period, and pointed in the direction of the upcoming hand movement soon after lengthening. The PV for monkey H, in contrast, achieved significant length and pointed toward the target during the second delay, when only the target was shown. When the obstacle was revealed, the vector changed to the new direction. In both monkeys, the velocity-based decoder (built from target-tuning properties in the center-out task) ultimately predicted the movement direction rather than the target direction, suggesting that directionally-tuned planning activity during a simple reaching task primarily represents the planned initial hand direction rather than the final spatial goal of the reach.
Fig. 2.
Decoding delay-period neural activity with the velocity-based PV. A-B) Monkey G, +/− 135° trials. The PV remains insignificant until the obstacle is shown. C-D) Same as A-B, for monkey H. The PV initially reflects the target direction before switching to the initial hand direction.
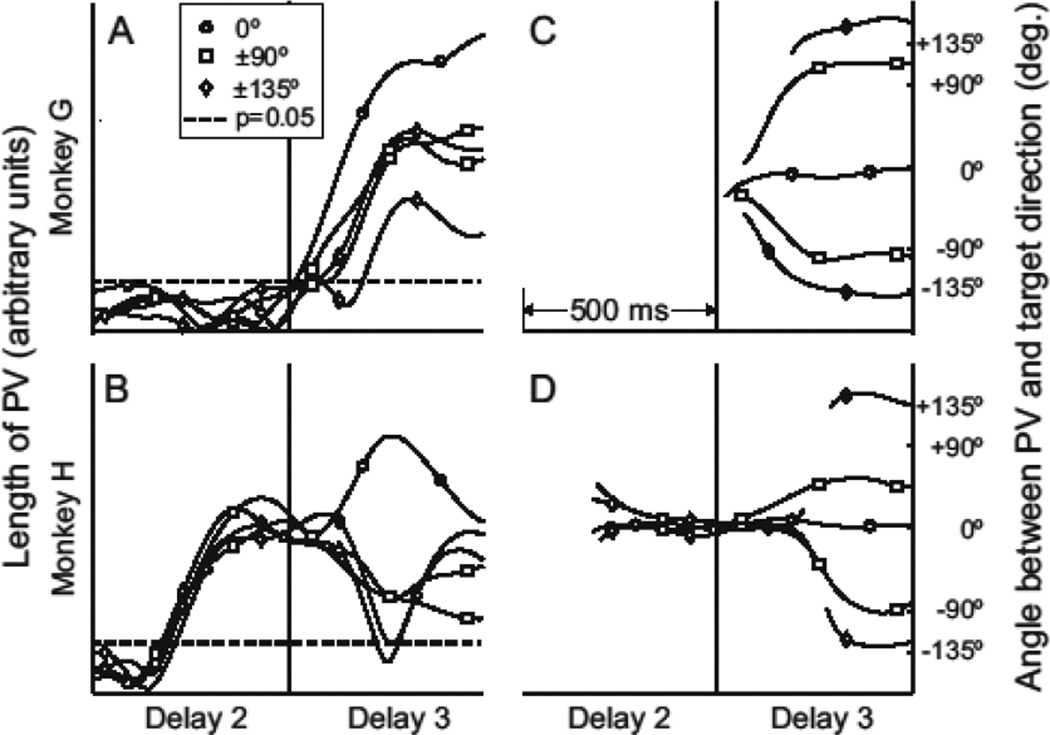
Fig. 3.
Planning strategies seen in the velocity-based PV response. A-B) PV length for each monkey during delay 2 and 3 for the 5 relative orientations of target and obstacle. Monkey H lengthens the PV immediately after target onset while monkey G does not. C-D) Angular difference between PV and center-target vector. Lines are hidden where the length of the PV is insignificant.
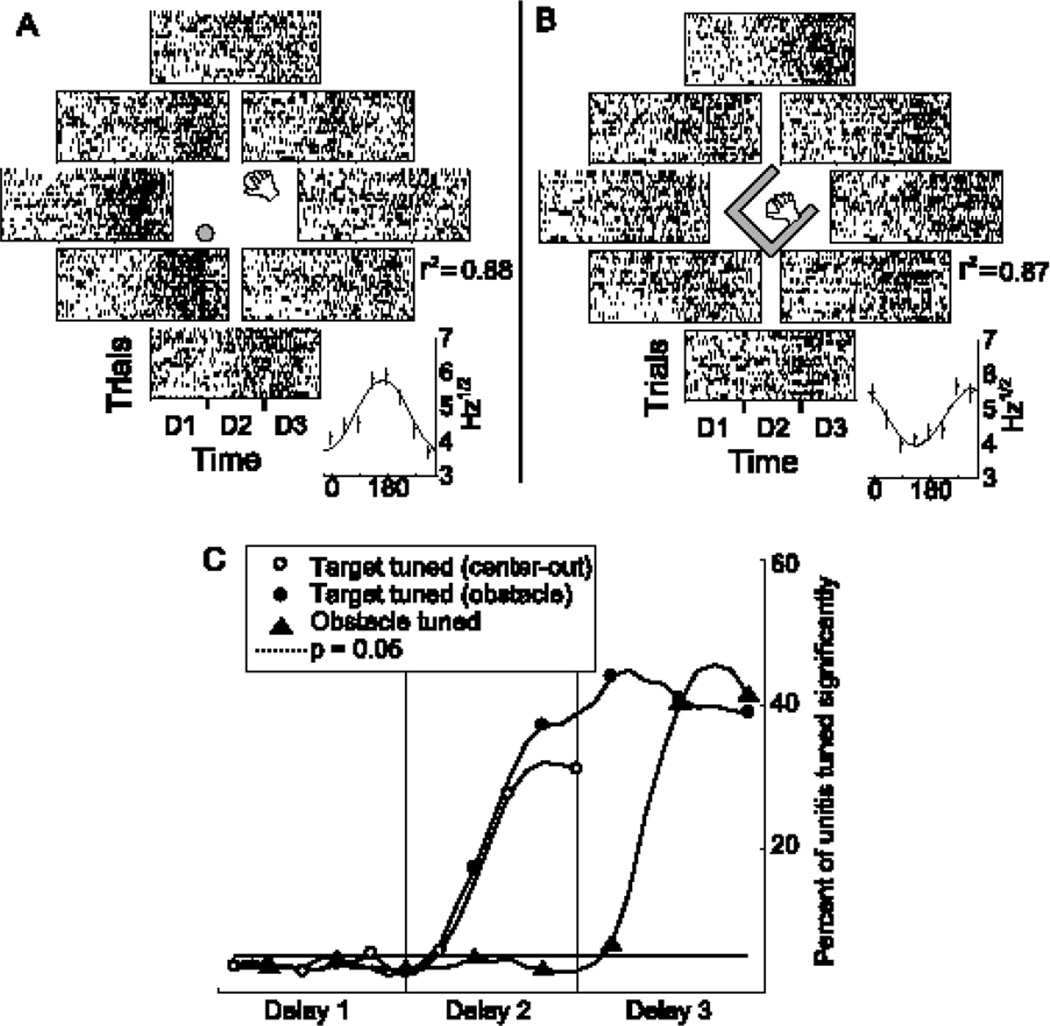
We next asked whether, in a more complex situation, a representation of the target might also be present in PMd. The obstacle-avoidance task fully decorrelates the target and initial movement directions, allowing us to perform regression analyses of the two parameters independently. Individual neurons can significantly tune to both target and movement directions simultaneously (Fig. 4). This suggests that single cells in PMd participate in the population coding of a high-dimensional space which includes both the initial segment and ultimate goal of a movement. Some of the variance left unexplained by a particular tuning model can be accounted for by tuning to additional parameters, while some is truly noise (either intrinsic or due to sampling effects). Of the entire sample of recorded units, some tuned significantly to target direction, others to movement direction, some to both and some to neither. An insignificant proportion of units showed tuning before the relevant information was revealed, confirming that the directional parameters were successfully separated and were not anticipated by the monkey before the visual cues were given. Upon display of the target or obstacle, the directional information began to influence neural responses, with over 60% of all units tuning significantly to at least one parameter during the final delay. The diverse single neuron tuning properties in this study are reminiscent of the heterogeneity of neural responses reported previously in the spatial (21) and temporal (22) domains. It has been hypothesized that this heterogeneity allows the neural population to act as a basis set for encoding multiple movement parameters (3, 22). Our results support this view: the myriad ways that multiple spatial and temporal parameters are combined in the firing of single neurons leads to complex rate codes that can nonetheless be decoded in the context of the whole population. The combination of position, velocity and goal encoding is particularly relevant to computational models of reach planning that utilize current state and target information to generate desired movement vectors (23, 24).
Fig. 4.
A single neuron simultaneously tuning to target and obstacle directions during the delay period. A) Spike rasters during delay periods 1–3 for a single neuron. Each row is a single trial, and the panel in which a row is located indicates the target direction for that trial. Inset: cosine fit to target direction during delay 3. This neuron prefers targets down and left. B) The data shown in A have been reorganized according to the direction of the obstacle opening for each trial. Inset: cosine fit to obstacle direction during delay 3. The same neuron that prefers targets down and left (from A) prefers obstacle openings pointing up and to the right. Two independent directional parameters are encoded simultaneously via noisy cosine tuning. C) Percentage of all recorded neurons significantly tuned (p < 0.05 for cosine fit) as a function of time. Tuning to each independent parameter occurs only after the information regarding that parameter is revealed to the monkeys.
The single neuron regression analyses suggest that target direction, in addition to initial movement direction, is present in the population code in PMd, at least in some circumstances. We tested this using PV models built from preferred target directions during the final delay of the obstacle-avoidance task, when the animals were aware of both the target and the necessary hand path (25). The results of this analysis confirm that by using a population model built from target tuning properties measured in the appropriate setting, target direction can be decoded independently of initial hand direction during the planning period (Fig. S4, S5). This target representation appeared in the population code only when indirect movements were being planned: during delay period 3 of trials where an obstacle-avoiding, curved trajectory was needed, the PV lengthened and pointed toward the target. In contrast, when the obstacle did not interfere with a direct movement to the target, the PV was significantly shorter (Fig. S5). Target encoding was also absent during delay 2, before the obstacle was displayed. During that period, the velocity-based PV analysis indicated that monkey G was not yet planning a movement, and monkey H was preparing a direct reach (Figs. 2, 3). As neither monkey was planning an indirect trajectory at that time, the lack of a significant target-PV response is expected. A third result consistent with these findings is that the velocity-based decoder - built by regressing against target direction in the center-out task - predicts initial movement direction, not target direction, when applied to indirect movements. This suggests that the goal representation was significantly weaker than the movement representation during the center-out task. In this framework, target encoding would be expected to be minimal during that task, since all movements are unimpeded during center-out reaching. The finding that ultimate goal direction is strongly represented in PMd for indirect relative to direct reaches suggests a potential role in shaping the reach beyond the initial segment of movement, similar to sequential reaching tasks (26, 27), but this hypothesis was not explicitly tested.
The population analyses reveal that the time course of preparatory neural activity in premotor cortex is subject to top-down modulation suggestive of distinct cognitive strategies. It appears that monkey G waited until all information was known before generating a movement plan, while monkey H planned to move directly to the target until the obstacle instructed him otherwise. In monkey H, the early planner, the direction decoding vector initially lengthened in the direction of the target. Once the obstacle was revealed, the population vector shortened and rotated to the required hand movement direction before lengthening again. In contrast, the PV from monkey G only achieved significant length during the final delay period, when the task was fully specified. As the vectors lengthened, they quickly stabilized in the direction of the initial movement required by the obstacle. This occurred sooner in monkey G than in monkey H (Fig. 3 C, D).
Although the monkeys were trained similarly and performed identical tasks, the behavioral (Fig. S6) and neural lines of evidence indicate that the two animals utilized different approaches when planning obstacle-avoidance reaches. Why might one monkey have adopted a strategy requiring a change of plan? One explanation has to do with the task structure. On of the trials the obstacle did not impede a straight movement (0° trials), and on another of the trials the ‘go’ cue was given instead of an obstacle appearing (catch trials). Thus, ⅓ of the time an initial movement plan would in fact be valid, and on half of those trials it would be useful immediately. We tested for behavioral correlates of planning by examining the angular deviation of the take-off angle (hand direction relative to target direction at movement initiation) on 0° trials, when both monkeys’ PVs were significant, versus catch trials, when only monkey H had a significant PV. For 0° trials, the difference in accuracy of the two monkeys was not statistically significant. On catch trials however, monkey H, had a substantial accuracy advantage (Fig. S6C).
The work presented here makes a number of points about the role of PMd in the movement planning and execution process. First, information about multiple independent spatial parameters is often embedded in the firing rate of a single neuron (e.g. Fig. 4A-B). The precise combination of parameters relevant to each cell is highly variable, leading to heterogeneity in the responses of individual neurons. Despite these spatially and temporally complex responses, a simple linear decoding scheme can meaningfully extract lower-dimensional information from the population as a whole. Second, the timing and strength of spatial information in the population code suggests that PMd activity is modulated both by task demands and by the particular planning strategy being used. The directional tuning observed in classic studies of center-out reaching is predictive of the initial hand movement direction, not the target direction, when those parameters are not separated. The time course with which population activity resolved to a significant directional prediction was consistent with two distinct approaches to the task, in which a tradeoff between planning speed and reach accuracy could be seen. A target representation, distinct from the initial movement representation, was also seen in the neural population. The strength of this representation was reduced for direct relative to indirect reaches, which suggests that relevant information can be selectively encoded as it is needed for the task. Finally, using position tuning properties in a population decoder provided a high-fidelity prediction of the hand trajectory during movement, consistent with prior reports of position coding in premotor areas (28, 29). Although the velocity-based decoder strongly predicted the initial hand direction prior to movement initiation, it did not predict the hand velocity particularly well during execution of the movement. These findings contrast with population decoding in M1, which shows good prediction of velocity and relatively worse performance when estimating position (19).
Supplementary Material
Acknowledgements
We are grateful for the support of the NIH Grant R01NS057813, NIH F30 NS071690-01, and NSF IGERT 0548890
Footnotes
Supplementary Materials:
Materials and Methods
Figures S1–S6
References (30–32)
References and Notes
- 1.Wise SP. The primate premotor cortex: past, present, and preparatory. Annu. Rev. Neurosci. 1985;8:1–19. doi: 10.1146/annurev.ne.08.030185.000245. [DOI] [PubMed] [Google Scholar]
- 2.Georgopoulos AP, Crutcher MD, Schwartz AB. Cognitive spatial-motor processes. 3. Motor cortical prediction of movement direction during an instructed delay period. Exp. Brain Res. 1989;75:183–194. doi: 10.1007/BF00248541. [DOI] [PubMed] [Google Scholar]
- 3.Kalaska JF, Crammond DJ. Cerebral cortical mechanisms of reaching movements. Science. 1992;255:1517–1523. doi: 10.1126/science.1549781. [DOI] [PubMed] [Google Scholar]
- 4.Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu. Rev. Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- 5.Crammond DJ, Kalaska JF. Prior information in motor and premotor cortex: activity during the delay period and effect on pre-movement activity. J Neurophysiol. 2000;84:986–1005. doi: 10.1152/jn.2000.84.2.986. [DOI] [PubMed] [Google Scholar]
- 6.Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron. 2005;45:801–814. doi: 10.1016/j.neuron.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Churchland MM, Shenoy KV. Delay of movement caused by disruption of cortical preparatory activity. J. Neurophysiol. 2007;97:348–359. doi: 10.1152/jn.00808.2006. [DOI] [PubMed] [Google Scholar]
- 8.Simon SR, et al. Spatial attention and memory versus motor preparation: premotor cortex involvement as revealed by fMRI. J. Neurophysiol. 2002;88:2047–2057. doi: 10.1152/jn.2002.88.4.2047. [DOI] [PubMed] [Google Scholar]
- 9.Kurata K, Tsuji T, Naraki S, Seino M, Abe Y. Activation of the dorsal premotor cortex and pre-supplementary motor area of humans during an auditory conditional motor task. J. Neurophysiol. 2000;84:1667–1672. doi: 10.1152/jn.2000.84.3.1667. [DOI] [PubMed] [Google Scholar]
- 10.Busan P, et al. Effect of transcranial magnetic stimulation (TMS) on parietal and premotor cortex during planning of reaching movements. PLoS ONE. 2009;4 doi: 10.1371/journal.pone.0004621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinrich M, Wise SP. The premotor cortex of the monkey. J. Neurosci. 1982;2:1329–1345. doi: 10.1523/JNEUROSCI.02-09-01329.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moran DW, Schwartz AB. Motor cortical representation of speed and direction during reaching. J. Neurophysiol. 1999;82:2676–92. doi: 10.1152/jn.1999.82.5.2676. [DOI] [PubMed] [Google Scholar]
- 13.Ochiai T, Mushiake H, Tanji J. Effects of image motion in the dorsal premotor cortex during planning of an arm movement. J. Neurophysiol. 2002;88:2167–2171. doi: 10.1152/jn.2002.88.4.2167. [DOI] [PubMed] [Google Scholar]
- 14.Shen L, Alexander GE. Preferential representation of instructed target location versus limb trajectory in dorsal premotor area. J. Neurophysiol. 1997;77:1195–1212. doi: 10.1152/jn.1997.77.3.1195. [DOI] [PubMed] [Google Scholar]
- 15.di Pellegrino G, Wise SP. Effects of attention on visuomotor activity in the premotor and prefrontal cortex of a primate. Somatosens. Mot. Res. 1993;10:245–262. doi: 10.3109/08990229309028835. [DOI] [PubMed] [Google Scholar]
- 16.Lebedev MA, Wise SP. Tuning for the orientation of spatial attention in dorsal premotor cortex. Eur. J. Neurosci. 2001;13:1002–1008. doi: 10.1046/j.0953-816x.2001.01457.x. [DOI] [PubMed] [Google Scholar]
- 17.Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science. 1986;233:1416–1419. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz AB, Moran DW, Reina GA. Differential representation of perception and action in the frontal cortex. Science. 2004;303:380–383. doi: 10.1126/science.1087788. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Chan SS, Heldman DA, Moran DW. Motor cortical representation of position and velocity during reaching. J. Neurophysiol. 2007;97:4258–4270. doi: 10.1152/jn.01180.2006. [DOI] [PubMed] [Google Scholar]
- 20.Materials and methods are available as supporting material on Science online [Google Scholar]
- 21.Hocherman S, Wise SP. Effects of hand movement path on motor cortical activity in awake, behaving rhesus monkeys. Exp. Brain Res. 1991;83:285–302. doi: 10.1007/BF00231153. [DOI] [PubMed] [Google Scholar]
- 22.Churchland MM, Shenoy KV. Temporal complexity and heterogeneity of singleneuron activity in premotor and motor cortex. J. Neurophysiol. 2007;97:4235–4257. doi: 10.1152/jn.00095.2007. [DOI] [PubMed] [Google Scholar]
- 23.Shadmehr R, Wise SP. The computational neurobiology of reaching and pointing. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- 24.Schaal S, Mohajerian P, Ijspeert A. Dynamics systems vs. optimal control - a unifying view. Prog. Brain Res. 2007;165:425–445. doi: 10.1016/S0079-6123(06)65027-9. [DOI] [PubMed] [Google Scholar]
- 25.A leave-one-out cross-validation was utilized for this decoder, as these models were built and tested on the same task. (20) [Google Scholar]
- 26.Catalan MJ, Honda M, Weeks RA, Cohen LG, Hallett M. The functional neuroanatomy of simple and complex sequential finger movements: a PET study. Brain. 1998;121(Pt 2):253–264. doi: 10.1093/brain/121.2.253. [DOI] [PubMed] [Google Scholar]
- 27.Baldauf D. Chunking movements into sequence: the visual pre-selection of subsequent goals. Neuropsychologia. 2011;49:1383–1387. doi: 10.1016/j.neuropsychologia.2011.01.041. [DOI] [PubMed] [Google Scholar]
- 28.Fogassi L, et al. Space coding by premotor cortex. Exp. Brain Res. 1992;89:686–690. doi: 10.1007/BF00229894. [DOI] [PubMed] [Google Scholar]
- 29.Pesaran B, Nelson MJ, Andersen RA. Dorsal premotor neurons encode the relative position of the hand, eye, and goal during reach planning. Neuron. 2006;51:125–134. doi: 10.1016/j.neuron.2006.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quian Quiroga R, Nadasdy Z, Ben-Shaul Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput. 2004;16:1661–1687. doi: 10.1162/089976604774201631. [DOI] [PubMed] [Google Scholar]
- 31.Reina GA, Moran DW, Schwartz AB. On the relationship between joint angular velocity and motor cortical discharge during reaching. J. Neurophysiol. 2001;85:2576–2589. doi: 10.1152/jn.2001.85.6.2576. [DOI] [PubMed] [Google Scholar]
- 32.Berens P. CircStat: a MATLAB toolbox for circular statistics. J. Statistical Software. 2009;31:1–21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.