Abstract
An alanine scan was performed on the novel kappa opioid receptor (KOR) peptide ligand CJ-15,208 to determine which residues contribute to the potent in vivo agonist activity observed for the parent peptide. These cyclic tetrapeptides were synthesized by a combination of solid phase peptide synthesis of the linear precursors, followed by cyclization in solution. Like the parent peptide, each of the analogs exhibited agonist activity and KOR antagonist activity in an antinociceptive assay in vivo. Unlike the parent peptide, the agonist activity of the potent analogs was mediated predominantly if not exclusively by mu opioid receptors (MOR). Thus analogs 2 and 4, in which one of the phenylalanine residues was replaced by alanine, exhibited both potent MOR agonist activity and KOR antagonist activity in vivo. These peptides represent novel lead compounds for the development of peptide-based opioid analgesics.
Keywords: analgesics, kappa opioid receptor antagonists, peptides, structure-activity relationships
Introduction
Recent reports suggest that kappa opioid receptor (KOR) antagonists could have potential therapeutic application in the treatment of mood disorders and drug abuse.[1] Pretreatment with the nonpeptide KOR-selective antagonists nor-binaltorphimine (nor-BNI) or JDTic reduce immobility in the forced swim assay similar to antidepressants,[2] and reduced behavioral measures of anxiety in rats.[3] Pretreatment with these antagonists also prevented stress-induced reinstatement of extinguished cocaineseeking behavior.[2c, 4] Likewise, heroin-dependent patients treated with a “functional KOR antagonist” (buprenorphine plus naltrexone to block mu opioid receptors (MOR)) for 12 weeks showed significantly improved drug abstinence compared to patients treated only with naltrexone.[5]
These nonpeptide antagonists demonstrate notably prolonged durations of activity,[1, 6] antagonizing KOR for weeks after a single dose.[7] This unusual pharmacological profile can complicate their use as pharmacological tools and could conceivably slow their development for clinical use, sparking interest in shorter acting KOR-selective antagonists.
We have a long-standing interest in peptide ligands for KOR, particularly those that demonstrate KOR-selective antagonism. A number of analogs of the endogenous opioid peptide dynorphin A have demonstrated KOR antagonism (for example, see ref. [8]). Modifications to linear peptides can reduce proteolytic cleavage, so that the peptide’s activity is preserved after systemic administration. This was demonstrated for the KOR-selective peptide antagonist zyklophin ([N-benzylTyr1,cyclo(D-Asp5,Dap8)]dynorphin A-(1-11)NH2) developed in our laboratory,[8e] which exhibits KOR-selective antagonist activity following systemic (subcutaneous) administration,[9] and prevents stressinduced reinstatement of extinguished cocaine conditioned place preference after subcutaneous administration.[9]
The cyclic tetrapeptide CJ-15,208 was reported to preferentially bind to KOR and antagonize the activity of a KOR agonist in the rabbit vas deferens smooth muscle preparation,[10] but the stereochemistry of the Trp residue in this natural product was not determined. We therefore undertook the synthesis of both tryptophan isomers of this cyclic tetrapeptide,[11] and found that the optical rotation of the L-Trp isomer 1 was consistent with that reported for the natural product (Figure 1a). While the L-Trp peptide did not exhibit any agonist activity at either KOR or MOR in vitro,[12] it unexpectedly exhibited robust agonist activity in vivo in the 55°C warm water tail withdrawal antinociceptive assay in addition to KOR-selective antagonist activity.[12a]
Figure 1.


Structures of a) the cyclic tetrapeptide CJ-15,208, 1, and b) alanine analogs 2-5. The residues are numbered 1-4, arbitrarily starting with the Phe C-terminal to the Trp residue.
Therefore we undertook structure-activity relationship (SAR) studies of CJ-15,208, first performing an alanine scan (Figure 1b) of the peptide to determine which amino acid side chains were important for interaction with opioid receptors and the pharmacological activity observed in vivo. Here we report the results of these initial studies, including both basic in vitro and in vivo characterization of these analogs of CJ-15,208.
Results and Discussion
Synthesis
The cyclic tetrapeptides were synthesized by a combination of solid phase synthesis of the linear tetrapeptide precursors, followed by cyclization in solution (the synthesis of cyclo[Ala-DPro-Phe-Trp], 2, is shown in Scheme 1), using the optimized procedure described for CJ-15,208.[11] The cyclizations were performed by slow addition of the linear peptide precursor to the coupling reagent HATU and DIEA in DMF; under these conditions the formation of the dimeric cyclic octapeptide is minimal.[11] The peptides were purified by reversed phase HPLC.
Scheme 1.

Synthesis of peptide 2.
In vitro pharmacological characterization
The peptides were evaluated for opioid receptor affinity in radioligand binding assays using cloned opioid receptors.[13] Substitution of alanine had a large effect on affinities for KOR with generally less effect on affinities for MOR (Table 1). Unexpectedly, substitution of Phe1 (see Figure 1 for notation) increased both KOR and MOR affinities by 4.4- and 19-fold, respectively. In contrast, substitution of the other three residues in the cyclic tetrapeptide with alanine decreased KOR affinity from 3- to 44-fold (Table 1), with the largest decrease occurring when the Trp residue was replaced by Ala, followed by substitution of Phe3 (see Figure 1). These results suggested that Trp and Phe3 are important for KOR affinity. These substitutions, however, did not decrease MOR affinity, but in one case (replacement of D-Pro by NMe-D-Ala in 3) increased MOR affinity 4.4-fold, resulting in these three analogs exhibiting negligible selectivity for KOR over MOR. All of the peptides exhibited very low affinity for delta opioid receptors (DOR) in the binding assays.
Table 1.
Opioid receptor affinities of alanine analogs of CJ-15,208.
| Ki (nM ± SEM) | Selectivity | |||
|---|---|---|---|---|
| Peptide | KOR | MOR | DOR | KOR/MOR/DOR |
| 2 | 8.03 ± 1.67 | 32.1 ± 3.9 | 8680 ±1270 | 1/4.0/1080 |
| 3 | 113 ± 23 | 140 ± 9 | 1370 ± 70 | 1/1.2/12 |
| 4 | 663 ± 220 | 533 ± 28 | >10000 | 1.2/1/>15 |
| 5 | 1550 ± 290 | 687 ± 81 | >10000 | 2.3/1/>14 |
| 1 [a] | 35.4 ± 3.6 | 619 ± 87 | 4150 ±3020 | 1/17.5/117 |
From reference [12a]
The ligand-stimulated GTPγS binding assay was used to assess the efficacy and potency of the cyclic tetrapeptides. None of the peptides exhibited appreciable stimulation of GTPγS binding via either KOR or MOR, consistent with the lack of agonist activity of the parent peptide 1 in this assay.[12] The Ala analog 2 was a reasonably potent antagonist of both dynorphin A-(1-13)NH2 at KOR (KB= 2. 6 ± 0 . 8 nM) and [D-Ala2,NMePhe4,glycol]enkephalin (DAMGO) at MOR (KB= 7.3 ± 1.6 nM), consistent with its affinities for KOR and MOR. Notably, it was 25-fold more potent as a KOR antagonist than the parent peptide 1 (KB = 65.2 ± 01.6 nM), while retaining MOR antagonist potency similar to 1 (KB = 10.2 ± 1.7 nM).
In vivo pharmacological characterization
The opioid activity of the cyclic tetrapeptides was determined in vivo using C57Bl/6J mice in the 55 °C warm water tail withdrawal assay following intracerebroventricular (i.c.v.) administration. This initial evaluation was done following central administration to measure the inherent pharmacological activity of the analogs in vivo without the complications associated with distribution (i.e. blood-brain barrier penetration) that could affect activity following systemic administration. Like CJ-15,208, each of the alanine analogs exhibited antinociceptive activity in vivo albeit with varying potencies (Figure 2). Analogs 2 and 3 exhibited similar antinociceptive potencies to CJ-15,208 in this assay with ED50 (and 95% confidence interval) values = 1.49 (0.39-7.41) and 2.43 (0.71-8.85) nmol for 2 and 3, respectively, vs. 1.74 (0.62-4.82) nmol for CJ-15,208. Interestingly, analog 4 (ED50= 0.10 (0.03-0.35) nmol) is 17-fold more potent than the parent peptide. Peptide 5, in which the Trp residue was substituted by Ala, proved to be the least potent analog with an ED50 value of 6.97 (1.02-47.4) nmol, 4-fold lower than the parent peptide.
Figure 2.
The antinociceptive activity of the cyclic tetrapeptides was assessed in vivo following i.c.v. administration in the 55°C warm-water tail-withdrawal assay in C57Bl/6J mice. All points represent antinociception at peak response, which was 20 min (for 4), 30 min (for peptides 1, 2 and 5) or 40 min (peptide 3). All points represent average % antinociception ± SEM from 7-8 mice. Data for 1 from reference [12a].
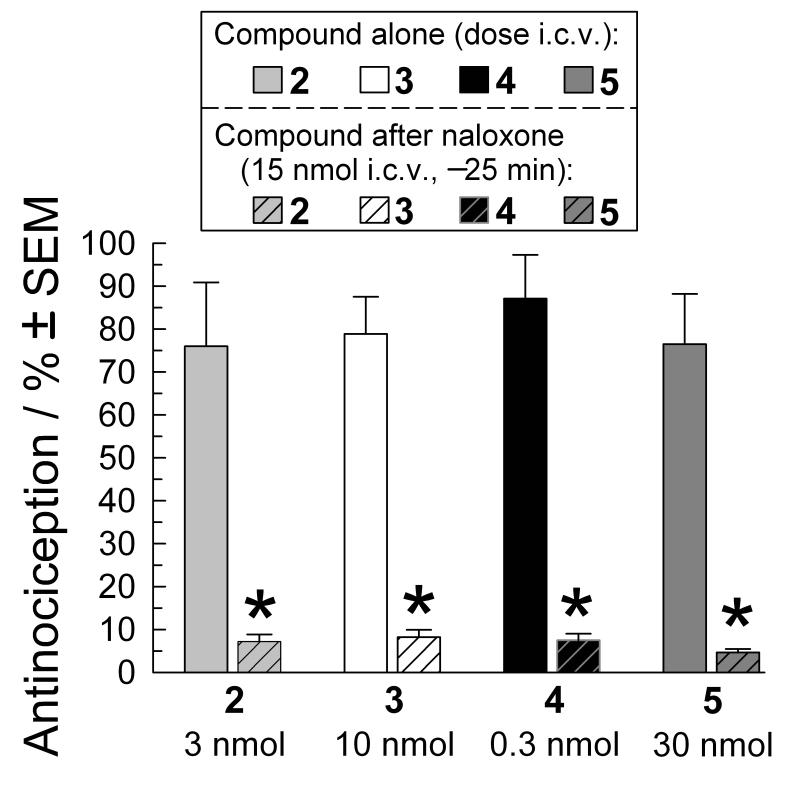
While consistent with the in vivo activity of the parent peptide CJ-15,208, the antinociceptive activity of these analogs was surprising, given their lack of agonist activity in the GTPγS assay in vitro and the relatively low opioid receptor affinities of analogs 3-5. Therefore we evaluated whether the antinociceptive activity was mediated through opioid receptors. In our initial testing, the antinociceptive activity of each peptide was completely blocked by pretreatment with the nonselective opioid antagonist naloxone (Figure 3), verifying opioid receptor involvement. We subsequently examined which opioid receptors were involved in the antinociceptive activity by pretreating test subjects with antagonists selective for MOR, KOR and DOR. The antinociceptive activity of each of the alanine analogs 2-5, administered at a dose producing 50-80% antinociception, was almost completely blocked by pretreatment with the MOR-selective irreversible antagonist β-funaltrexamine (β-FNA, Figure 4). Of interest, pretreatment with the KOR-selective antagonist nor-BNI produced differing effects, significantly antagonizing the antinociceptive activity of only peptide 5, with no effect on 3 or 4 (Figure 4). Pretreatment with nor-BNI reduced the antinociceptive activity of analog 2 by >30%, but the difference in the presence vs. absence of nor-BNI did not reach statistical significance. The DOR-selective antagonist naltrindole significantly reduced the antinociceptive activity of only peptide 5. Together, these results suggest that the antinociception induced by peptides 2-4 was mediated almost exclusively by MOR, although it is possible that KOR contributes to the antinociceptive activity of 2. In contrast all three receptors appear to contribute to the antinociceptive activity of 5. These results are in contrast to those for 1 where the antinociceptive activity appears to be predominantly mediated by KOR, with a lesser contribution by MOR.[12a]
Figure 3.
Cyclic tetrapeptide induced antinociception is opioid-receptor mediated. Peak antinociceptive activity of peptides 2 (3 nmol), 3 (10 nmol), 4 (0.3 nmol) and 5 (30 nmol) was determined in the 55°C warm-water tail-withdrawal assay after i.c.v. administration to C57Bl/6J mice (open bars). Naloxone pretreatment (15 nmol i.c.v., striped bars) 25 min prior to peptide administration significantly antagonized the effect of each cyclic tetrapeptide. Tail-withdrawal latencies were measured 30 minutes after injection of the cyclic tetrapeptide. Data represents average % antinociception ± SEM from 7-8 mice. *=significantly different from response of matching administered compound alone, p<0.05, One-way ANOVA followed by Tukey’s HSD post hoc test.
Figure 4.
Opioid-receptor-selective agonism by the cyclic tetrapeptides. The antinociceptive activity of peptides 2 (3 nmol), 3 (3 nmol), 4 (0.1 nmol) and 5 (30 nmol) was determined in the 55°C warm-water tail-withdrawal assay after i.c.v. administration to C57Bl/6J mice (solid bars). Antinociception was also assessed 24 h after administration in mice pretreated with β-FNA (5 mg/kg, s.c.; diagonally striped bars) or nor-BNI (10 mg/kg, i.p., wave-filled bars). Additional mice were pretreated with naltrindole (20 mg/kg, i.p., –15 min; hatched bars) before administration of one of the cyclic tetrapeptides. Tail-withdrawal latencies were measured in the mouse 55°C warm-water tail-withdrawal test 30 minutes after injection of the cyclic tetrapeptides 2, 4 and 5, or 40 min after peptide 3. Data represents average % antinociception ± S.E.M. from 8-16 mice. * = significantly different from response of matching administered compound alone, p<0.05, One-way ANOVA followed by Tukey’s HSD post hoc test.
We next examined the peptides for antagonist activity following dissipation of the agonist activity. For the parent peptide 1 significant agonist activity was detected for up to 100 min after administration of the highest dose (10 nmol), while for the analogs 2-5 significant agonist activity was detected for 70-80 min. To ensure that there was no residual agonist activity, the peptides were evaluated for antagonist activity 3 h after pretreatment. All of the analogs dose-dependently antagonized the antinociceptive effects of the KOR-selective agonist U50,488 (Figure 5). Peptides 2 and 4 appeared to be somewhat more potent than the parent peptide 1, whereas peptides 3 and 5 were less potent as KOR antagonists than 1. Importantly, the duration of the KOR antagonist activity for each of the peptides was relatively brief (less than 18 hours, Figure 6), substantially shorter than the duration of activity of the parent peptide 1 which exhibits significant KOR antagonist activity for at least 24 hours.[12a] The reason for the shorter duration of antagonist activity of the analogs compared to the parent peptide is unclear, but could be due to differences in hydrophobicity, with the more hydrophobic parent peptide being retained longer in the tissue.
Figure 5.
Dose-dependent antagonism of U50,488-induced antinociception by tested cyclic tetrapeptides. The antinociceptive effects of U50,488 (10 mg/kg, i.p.; thatched bar) were determined 40 minutes after administration in mice pretreated 3 h with peptides 1 (diamonds), 2 (circles), 3 (inverted triangles), 4 (filled triangles) and 5 (squares) in the 55°C warm-water tail-withdrawal assay after i.c.v. administration. Data represents average % antinociception ± S.E.M. from 8 mice. * = significantly different from response of U50,488 p<0.05, One-way ANOVA followed by Tukey’s HSD post hoc test. Data for 1 from reference [12a].
Figure 6.
Duration of cyclic tetrapeptide-mediated antagonism of U50,488-induced antinociception in the mouse 55°C warm-water tail-withdrawal test. Antinociception of U50,488 (10 mg/kg, i.p.; thatched bar) was determined in mice pretreated 3, 6, 18 or 24 h with peptides 2 (circles), 3 (inverted triangles), 4 (filled triangles) and 5 (squares), and for peptide 1 (diamonds) at 8, 18 and 24 h.[12a] Tail-withdrawal latencies were determined 40 minutes after agonist administration. Data represents average % antinociception ± S.E.M. from 8 mice/point. * = significantly different from response of U50,488 p<0.05, One-way ANOVA followed by Tukey’s HSD post hoc test.
The selectivity of the antagonist activity was next evaluated by examining the ability of the peptides to antagonize the antinociception produced by the MOR preferring agonist morphine or the DOR selective agonist SNC-80 (Figure 7). None of the peptides antagonized morphine. While peptide 2 did not significantly antagonize SNC-80, surprisingly peptides 3 and 5 did antagonize this agonist (the decrease in the antinociception of SNC-80 by pretreatment with 4 was not significant). This DOR antagonist activity was unexpected given the low affinity of these cyclic tetrapeptides for DOR.
Figure 7.
Receptor selectivity of the antagonism by the cyclic tetrapeptides in the mouse 55°C warm-water tail-withdrawal test. Antinociceptive activity of morphine (10 mg/kg, i.p., left set of bars) was not reduced by a 3 h pretreatment of the mice with the cyclic tetrapeptides 2 (10 nmol, striped black bar), 3 (1 nmol, striped white bar), 4 (1 nmol, striped gray bar) or 5 (10 nmol, striped dark gray bar). However, the antinociceptive effect of U50,488 (10 mg/kg, i.p., center set of bars) was significantly antagonized by pretreatment of the mice with any of the cyclic tetrapeptides. (For this set of tests peptide 2 was administered at 3 nmol, i.c.v.). In contrast, the antinociceptive effect of SNC-80 (100 nmol, i.c.v., right set of bars) was significantly prevented only by pretreatment with peptides 3 and 5. Tail-withdrawal latencies were measured in the mouse 55°C warm-water tail-withdrawal test 40 minutes after injection of the known selective agonists. Data represents average % antinociception ± S.E.M. from 8-16 mice. * = significantly different from response of matching administered agonist alone, p<0.05, One-way ANOVA followed by Tukey’s HSD post hoc test.
Conclusions
The alanine analogs of CJ-15,208 exhibited in vivo pharmacological profiles that were unexpected based on their opioid receptor affinities and lack of agonist activity in the GTPͣS assay in vitro. All of the analogs exhibited antinociceptive activity in the 55°C warm water tail withdrawal assay, with analog 4 exhibiting particularly potent agonist activity. This antinociceptive activity involves opioid receptors, as it is blocked by the nonselective opioid antagonist naloxone. Further examination with selective antagonists suggests that the antinociceptive activity of the more potent analogs 2-4 is predominantly if not entirely mediated through MOR activation, which contrasts with the parent peptide 1[12a] where the antinociception is predominantly mediated through activity at KOR with a smaller contribution by MOR. These alanine analogs also exhibited antagonist activity at KOR after dissipation of the agonist activity, which especially in the case of the potent antagonist 4 was unexpected given its low KOR affinity. The DOR antagonist activity of at least two of the analogs was also unexpected, given the very low DOR affinities of these compounds. Clearly, these analogs have different opioid activity profiles in vivo from what was expected from the in vitro assays and also from the results for the parent peptide 1.
The in vivo data suggests that there are different structural requirements for agonist vs. antagonist activity mediated by KOR and for the activation of KOR vs. MOR. All three of the aromatic residues appear to contribute to agonist activity mediated by KOR. Interestingly, only the antinociceptive activity of the lower potency agonist 5, in which the Trp residue was replaced by Ala, was significantly antagonized by nor-BNI. All of the peptides, however, antagonized KOR in vivo, although peptide 5 also exhibited relatively low potency as an antagonist. All of the analogs exhibited agonist activity that was mediated predominantly by MOR, suggesting that only two of the aromatic residues are sufficient for activation of MOR receptors. The analogs in which one of the Phe residues was replaced with alanine exhibited high potency both as MOR agonists and as KOR antagonists in vivo. These analogs are undergoing additional evaluation in vivo as lead compounds for potential development as analgesics.
While these peptides produce antinociception and antagonist activity that is clearly mediated through opioid receptors, the marked differences between the activity profiles in the in vitro vs. in vivo assays suggest that these compounds produce their opioid activity through more complex mechanisms than typical opioid receptor ligands. Notably, similar differences have been found between in vitro and in vivo opioid activity for other novel peptide-based antinociceptive compounds that are structurally distinct from these peptides.[14] Involvement of additional mechanisms in analgesic activity has been reported for other opioid peptides. For example, the indirect activation of opioid receptors by release of endogenous opioid peptides has been reported for several opioid peptides,[15] most notably for the potent and selective MOR peptides Dmt-DALDA (Dmt-D-Arg-Phe-Lys-NH2, Dmt = 2′,6′-dimethyltyrosine) and endomrophin-2. A non-opioid mechanism (inhibition of norepinephrine uptake) was also reported to contribute to the antinociceptive effects of Dmt-DALDA.[16] Additional studies are being conducted to explore possible mechanisms for the observed antinociceptive activity of these cyclic tetrapeptides.
In conclusion, these unusual ligands represent valuable compounds for further study and as novel lead compounds, especially analogs 2 and 4, for the development of peptide-based opioid analgesics. These studies are ongoing in our laboratories.
Experimental Section
Materials
Reagents for peptide synthesis were obtained from the following sources: Fmoc (fluorenylmethoxycarbonyl)-protected amino acids (Novabiochem (EMD), San Diego, CA), 2-chlorotrityl chloride resin (1.4 mmol/g, Novabiochem), coupling reagents HATU (2-(1H-7-azabenzotriazol-1-yl)-1,1,3,3-tetramethyl uronium hexafluorophosphate, Novabiochem), PyBOP (benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate, Novabiochem) and HOBt (1-hydroxybenzotriazole, Fluka, Milwaukee, WI), DIEA (N,N-diisopropylethylamine, Fluka), TFA (trifluoroacetic acid, Pierce, Rockford, IL), and HPLC-grade solvents (Fisher Scientific, Pittsburg, PA). The other solvents and routine chemicals were obtained from Fisher Scientific.
HPLC analyses and purifications were performed on Vydac 218TP C18 reversed phase columns (Grace Davison, 4.6 × 50 mm, 5 μ, and 22 × 250 mm, 10 μ, respectively).
Peptide synthesis:[11]
Solid phase synthesis of linear peptide precursors
The linear peptide precursors were synthesized using Fmoc-protected amino acids on a 2-chlorotrityl chloride resin and a custom made manual peptide synthesizer (CHOIR)[17] constructed in house. Following swelling of the resin in CH2Cl2 (2 × 10 min), the C-terminal Fmoc-protected amino acid (2 equiv) and (DIEA, 5 equiv) in CH2Cl2/N,N-dimethylformamide (DMF) (4:1, 5 mL per 0.5 g resin) were added to the resin, and the reaction gently agitated with N2 gas for 6 h. Additional CH2Cl2 was added every 30 min to maintain the solvent volume, and additional DIEA (5 equiv) was added to the reaction every 2 h. The resin was washed with CH2Cl2/DMF (1/1, 5x), and quantitative Fmoc analysis[18] then used to determine loading efficiency. A capping step was then performed using 15% MeOH and 5% DIEA in CH2Cl2 (2 × 10 min), and the resin washed with CH2Cl2/DMF (1:1, 5x). The Fmoc group was then removed with 20% piperidine in DMF (2 × 20 min), and the resin washed with CH2Cl2/DMF (1:1, 5x) and CH2Cl2 (5x).
Fmoc-protected amino acids (4 equiv) were coupled to the resin using PyBOP (4 equiv), HOBt (4 equiv) and DIEA (8 equiv) in CH2Cl2/DMF (1:1) for 2-4 h. The resin was washed after the coupling reactions with CH2Cl2/DMF (1:1, 5x) and CH2Cl2 (5x). The reactions were monitored to determine completion using the Kaiser test for primary amines or the chloranil test for the secondary amine of Pro. The Fmoc group was then removed as described above, and the deprotection/coupling cycle repeated to assemble the linear tetrapeptides. Finally, the resin was washed with CH2Cl2/DMF (1:1, 5x), CH2Cl2 (10x), iPrOH (2x), hexane (2x), CH2Cl2 (2x), MeOH (2x), and finally CH2Cl2 (2x).
The peptides were cleaved from the resin using 1% TFA in CH2Cl2.. Following swelling the resin in CH2Cl2 the TFA solution was mixed with the resin (5 mL × 2 min × 10), and the cleavage solution drained into a round bottom flask. This procedure was repeated until of the cleavage solution was collected in the round bottom flask. Following the cleavage, the resin was washed with CH2Cl2 (2x) and MeOH (2x). The combined solutions were evaporated to give the crude linear tetrapeptides which were used in the cyclizations without purification.
Cyclization reaction and final deprotection
The linear peptides were cyclized as follows: The crude linear peptide (0.5 equiv) in DMF (5-10 mL) was added dropwise at a rate of 1.6 mL/h (using a KD Scientific single infusion syringe pump and a 10 mL syringe) to a solution of HATU (0.75 equiv, 1 mM) and DIEA (8 equiv) in DMF over 6 h. After 6 h a second portion of HATU (0.75 equiv) was added to the reaction in one portion, and a second portion of linear peptide (0.5 mmol) in DMF (5-10 mL) was added dropwise at a rate of 1.6 mL/h as described above. The reaction was then allowed to stir for an additional 12-24 h. Following removal of the solvent under reduced pressure, the residue was dissolved in EtOAc/Et2O (4:1) or CH2Cl2 and the solution washed with 1N citric acid (2x), saturated bicarbonate (2x) and brine (2x). The organic layer was separated, dried (Na2SO4), and the solvent was removed under reduced pressure to give the crude cyclic peptide. This workup was not performed for peptide 5 because of its water solubility; instead following removal of the DMF the crude peptide was dissolved in water and lyophilized.
The Boc (t-butyloxycarbonyl) group on the indole group of Trp in the cyclic precursors of 2-4 was then removed by treating a solution of the cyclic peptide in CH2Cl2 (1 mL) with 50% TFA in CH2Cl2 (2 mL) for 30 min. The solution was then evaporated and the peptide triturated with aq 10% AcOH and the peptide then dried by lyophilization.
Purification and characterization
The cyclic peptides were purified by reversed phase HPLC (30-70% aq MeOH over 40 min, except for peptide 5 where the gradient was 20-60% aq MeOH over 40 min). The cyclic peptides were characterized by reversed phase HPLC and electrospray ionization mass spectrometry (see Supporting Information).
In vitro pharmacological evaluation
Radioligand binding assays
Opioid receptor affinities were determined in radioligand binding assay using membranes from Chinese hamster ovary (CHO) cells stably expressing KOR, MOR or DOR as previously described.[13] Incubations with isolated membrane protein were performed in triplicate with 12 different concentrations from 0.1 nM to 10 μM of the cyclic tetrapeptides for 90 min in 50 mM Tris, pH 7.4 at 22°C using [3H]diprenorphine, [3H]DAMGO and [3H]DPDPE as the radioligands for KOR, MOR and DOR, respectively. Nonspecific binding was determined in the presence of 10 μM unlabeled Dyn A-(1-13)NH2, DAMGO, and DPDPE for KOR, MOR and DOR, respectively. Reactions were terminated by rapid filtration over Whatman GF/B fiber filters using a Brandel M24-R cell harvester and the filters counted in 4 mL of Cytocint (ICN Radiochemicals) using a Beckman LS6800 scintillation counter. IC50 values were determined by nonlinear regression analysis to fit a logistic equation to the competition data using Prism software (GraphPad Software Co., La Jolla, California, USA). Ki values were calculated from the IC50values by the Cheng and Prusoff equation[19] using KD values of 0.45, 0.49, and 1.76 nM for [3H]diprenorphine, [3H]DAMGO, and [3H]DPDPE, respectively. The results presented are the mean ± SEM from at least three separate assays.
GTPγS assays
The binding of the GTP analog [35S]GTPγS to membranes was assayed following the method described by Siebenallar and Murray.[20] Binding was determined in a volume of 500 μL. The assay mixture contains 50 mM HEPES, pH 7.4, 1 mM EDTA, 5 mM magnesium acetate, 1 μM GDP, 1 rnM dithiothreitol, 100 mM NaCl, 1 mg bovine serum albumin per mL; and approximately 100,000 disintegrations per min (dpm) [35S]GTPγS (0.1 to 0.2 nM). Approximately 10 μg of KOR or MOR expressing CHO cell membrane protein was used per tube. Following 90 min incubation at 22°C, the assay was terminated by filtration under vacuum on a Brandel (Gaithersburg, MD) model M-48R cell harvester using Schleicher and Schuell Inc. (Keene, NH) number 32 glass fiber filters. The filters were rinsed with 4 × 4-mL washes of ice-cold 50 mM Tris HCl, pH 7.4 5 mM MgCl, at 5°C, to remove unbound [35S]GTPγS. Filter disks were then placed into counting vials to which 8 mL of Biocount scintillation fluid (Research Products International Corp., Mount Prospect, IL) was added. Filter-bound radioactivity was determined by liquid scintillation spectrometry (Beckman Instruments, Fullerton, CA,) following overnight extraction at room temperature. The amount of radioligand bound was <10% of the total added in all experiments. Specific binding is defined as total binding minus that occurring in the presence of 3 μM unlabeled GTPγS. Nonspecific binding was approximately 1% of the total binding at 0.1 nM [35S]GTPγS.
To evaluate the peptides for agonist activity the membranes were incubated with ten different concentrations of peptide (0.01 nM −1 μM). The antagonist activity of the peptides was determined by measuring the EC50 of an agonist (dynorphin A-(1-13)NH2 for KOR and DAMGO for MOR) in the absence or presence of four different concentrations (10 nM −3 μM) of the peptide. The pA2 was determined by Schild analysis,[21] and the results are reported as KB. values.
In vivo pharmacological evaluation
Animals
317 adult male C57Bl/6J mice weighing 20-25 grams were obtained from Jackson Labs (Bar Harbor, ME, USA), and were housed and cared for in accordance with the 2002 National Institute of Health Guide for the Care and Use of Laboratory Animals and as approved by the TPIMS Institutional Animal Care Committee, operating under the OLAW approval number A4618-01. All mice were group housed, four to a cage, in self-standing plastic cages within the animal care facility. The colony room was illuminated on a 12-h light-dark cycle, with the lights on at 7 am. Food pellets and distilled water were available ad libitum. Note that C57Bl/6J mice were selected for this study because of their established responses to thermal noxious stimuli and antinociceptive testing.[22] All compounds other than the peptides were obtained from Sigma (St. Louis, Missouri, USA).
Intracerebroventricular administration technique
Intracerebroventricular (i.c.v.) injections were made directly into the lateral ventricle according to the modified method of Haley and McCormick.[23] The volume of all i.c.v. injections was 5 μL, using a 10-μL Hamilton microliter syringe. The mouse was lightly anesthetized with isoflurane, an incision was made in the scalp, and the injection was made 2 mm lateral and 2 mm caudal to bregma at a depth of 3 mm.
Antinociceptive testing
The 55°C warm-water tail-withdrawal assay was performed in C57Bl/6J mice as previously described.[24] Briefly, warm (55°C) water in a 2-L heated water bath was used as the thermal nociceptive stimulus, with the latency of the mouse to withdraw its tail from the water taken as the endpoint. After determining baseline tail-withdrawal latencies, mice were administered a graded dose of compound though the i.c.v. route. Intracerebroventricular injections (5 μL, using a 10 μL Hamilton syringe) were performed as described above;[24] the cyclic tetrapeptides were administered in 50% DMSO/50% sterile saline (0.9%). To determine agonist activity, the tail-withdrawal latency was determined every 10 min following administration of a cyclic tetrapeptide for 3 h, or until latencies returned to baseline values.
A cut-off time of 15 s was used in this study; if the mouse failed to display a tail-withdrawal response during that time, the tail was removed from the water and the animal was assigned a maximal antinociceptive score of 100%. At each time point, antinociception was calculated according to the following formula: % antinociception = 100 × (test latency – control latency)/(15 – control latency).
To determine the opioid receptor selectivity of the agonist activity of peptides 2-5, mice were pretreated with a single dose of β-FNA (5 mgkg−1, s.c.) or nor-BNI (10 mgkg−1, i.p.) 23.3 h in advance of administration of a graded dose of a cyclic tetrapeptide compound (0.1-30 nmol i.c.v). Additional mice were pretreated prior to the administration of a cyclic tetrapeptide compound with the opioid receptor nonselective antagonist naloxone (15 nmol, i.c.v., −25 min), or naltrindole (20 mgkg−1, i.p., −15 min), with antinociceptive testing 40 min later. Reference agonists and antagonists were administered using sterile saline (0.9%) as the vehicle, except for SNC-80 which was dissolved in 35% DMSO/65% saline.
To determine antagonist activity, mice were pretreated with a cyclic tetrapeptide 150 min prior to the administration of the MOR-preferring agonist morphine (10 mgkg−1, i.p.), KOR-selective agonist U50,488 (10 mgkg−1, i.p.) or DOR-selective agonist SNC-80 (100 nmol, i.c.v.). Antinociception produced by these established agonists was then measured 30 min after administration. Additionally, to determine the duration of KOR antagonist activity, additional mice were pretreated for 7.3, 17.3, or 23.3 prior to administration of U50,488 as described above.
Statistical analysis
Radioligand binding results represent the mean ± SEM obtained from 3-5 independent experiments each performed in triplicate. IC50 values were calculated by least squares fit to a logarithm-probit analysis. The Ki values of unlabeled compounds were calculated from the equation Ki = IC50/(1+S), where S=(concentration of radioligand)/(KD of radioligand),[19] and reported as the mean ± SEM of at least three independent experiments. KB values from the GTPγS assay represent the mean ± SEM from 2-4 experiments.
All tail-withdrawal data points shown are the means of 7-16 mice, with SEM represented by error bars. Data for antinociception experiments were analyzed with ANOVA using the Prism 5.0 software package (GraphPad, La Jolla, California, USA). Analyses examined the main effect of baseline and post-treatment tail-withdrawal latencies to determine statistical significance for all tail-withdrawal data. Significant effects were further analyzed using Tukey’s HSD post-hoc testing. All data are presented as mean ± SEM, with significance set at P<0.05.
Supplementary Material
Acknowledgements
This research was supported by grants from the National Institute on Drug Abuse R01 DA018832 and R01 DA023924 and funds from the State of Florida.
Footnotes
Supporting information for this article is available on the WWW under http://www.chemmedchem.org or from the author.
References
- [1].Aldrich JV, McLaughlin JP. AAPS J. 2009;11:312–322. doi: 10.1208/s12248-009-9105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2] a).Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WCJ, Jones RM, Portoghese PS, Carlezon WAJ. J. Pharmacol. Exp. Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]; b) McLaughlin JP, Popovici M. Marton, Chavkin C. J. Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Beardsley PM, Howard JL, Shelton KL, Carroll FI. Psychopharmacology (Berl) 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- [3].Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr. J. Pharmacol. Exp. Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- [4].Redila VA, Chavkin C. Psychopharmacology (Berl) 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5] a).Rothman RB, Gorelick DA, Heishman SJ, Eichmiller PR, Hill BH, Norbeck J, Liberto JG. J. Substance Abuse Treat. 2000;18:277–281. doi: 10.1016/s0740-5472(99)00074-4. [DOI] [PubMed] [Google Scholar]; b) Gerra G, Fantoma A, Zaimovic A. J. Psychopharmacol. 2006;20:806–814. doi: 10.1177/0269881106060835. [DOI] [PubMed] [Google Scholar]
- [6].Metcalf MD, Coop A. AAPS J. 2005;7:E704–722. doi: 10.1208/aapsj070371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7] a).Horan P, Taylor J, Yamamura HI, Porreca F. J. Pharmacol. Exp. Ther. 1992;260:1237–1243. [PubMed] [Google Scholar]; b) Carroll FI, Harris LS, Aceto MD. Eur. J. Pharmacol. 2005;524:89–94. doi: 10.1016/j.ejphar.2005.09.013. [DOI] [PubMed] [Google Scholar]
- [8] a).Wan Q, Murray TF, Aldrich JV. J. Med. Chem. 1999;42:3011–3013. doi: 10.1021/jm9901071. [DOI] [PubMed] [Google Scholar]; b) Lu Y, Nguyen TM-D, Weltrowska G, Berezowska I, Lemieux C, Chung NN, Schiller PW. J. Med. Chem. 2001;44:3048–3053. doi: 10.1021/jm0101186. [DOI] [PubMed] [Google Scholar]; c) Bennett MA, Murray TF, Aldrich JV. J. Med. Chem. 2002;45:5617–5619. doi: 10.1021/jm025575g. [DOI] [PubMed] [Google Scholar]; d) Vig BS, Murray TF, Aldrich JV. J. Med. Chem. 2003;46:1279–1282. doi: 10.1021/jm0256023. [DOI] [PubMed] [Google Scholar]; e) Patkar KA, Yan X, Murray TF, Aldrich JV. J. Med. Chem. 2005;48:4500–4503. doi: 10.1021/jm050105i. [DOI] [PubMed] [Google Scholar]
- [9].Aldrich JV, Patkar KA, McLaughlin JP. Proc. Natl. Acad. Sci. U.S.A. 2009;106:18396–18401. doi: 10.1073/pnas.0910180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Saito T, Hirai H, Kim Y-J, Kojima Y, Matsunaga Y, Nishida H, Sakakibara T, Suga O, Sujaku T, Kojima N. J. Antibiot. 2002;55:847–854. doi: 10.7164/antibiotics.55.847. [DOI] [PubMed] [Google Scholar]
- [11] a).Kulkarni SS, Ross NC, McLaughlin JP, Aldrich JV. Adv. Exp. Med. Biol. 2009;611:269–270. doi: 10.1007/978-0-387-73657-0_121. [DOI] [PubMed] [Google Scholar]; b) Ross NC, Kulkarni SS, McLaughlin JP, Aldrich JV. Tetrahedron Lett. 2010;51:5020–5023. doi: 10.1016/j.tetlet.2010.07.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12] a).Ross NC, Reilley KJ, Murray TF, Aldrich JV, McLaughlin JP. Br. J. Pharmacol. doi: 10.1111/j.1476-5381.2011.01544.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dolle RE, Michaut M, Teipel B. Martinez, Seida PR, Ajello CW, Muller AL, DeHaven RN, Carroll PJ. Bioorg. Med. Chem. Lett. 2009;19:3647–3650. doi: 10.1016/j.bmcl.2009.04.105. [DOI] [PubMed] [Google Scholar]
- [13].Arttamangkul S, Ishmael JE, Murray TF, Grandy DK, DeLander GE, Kieffer BL, Aldrich JV. J. Med. Chem. 1997;40:1211–1218. doi: 10.1021/jm960753p. [DOI] [PubMed] [Google Scholar]
- [14].Reilley KJ, Giulianotti M, Dooley CT, Nefzi A, McLaughlin JP, Houghten RA. AAPS J. 2010;12:318–329. doi: 10.1208/s12248-010-9191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15] a).Ohsawa M, Mizoguchi H, Narita M, Nagase H, Kampine JP, Tseng LF. J. Pharmacol. Exp. Ther. 2001;298:592–597. [PubMed] [Google Scholar]; b) Ohsawa M, Shiraki M, Mizoguchi H, Narita M, Kawai K, Nagase H, Cheng EY, Tseng LF. Neurosci. Lett. 2001;316:1–4. doi: 10.1016/s0304-3940(01)02334-5. [DOI] [PubMed] [Google Scholar]; c) Sakurada S, Hayashi T, Yuhki M, Orito T, Zadina JE, Kastin AJ, Fujimura T, Murayama K, Sakurada C, Sakurada T, Narita M, Suzuki T, Tan-no K, Tseng LF. Eur. J. Pharmacol. 2001;427:203–210. doi: 10.1016/s0014-2999(01)01238-9. [DOI] [PubMed] [Google Scholar]; d) Szeto HH, Soong Y, Wu D, Qian X, Zhao GM. J. Pharmacol. Exp. Ther. 2003;305:696–702. doi: 10.1124/jpet.102.048561. [DOI] [PubMed] [Google Scholar]; e) Mizoguchi H, Bagetta G, Sakurada T, Sakurada S. Peptides. 2011;32:421–427. doi: 10.1016/j.peptides.2010.11.013. [DOI] [PubMed] [Google Scholar]
- [16].Shimoyama M, Shimoyama N, Zhao GM, Schiller PW, Szeto HH. J. Pharmacol. Exp. Ther. 2001;297:364–371. [PubMed] [Google Scholar]
- [17].Vig BS, Aldrich JV. Aldrichimica Acta. 2004;37:2. [Google Scholar]
- [18].Chan WC, White PD. Fmoc Solid Phase Peptide Synthesis: A Practical Approach. Oxford University Press Inc.; New York: 2000. pp. 41–76. [Google Scholar]
- [19].Cheng YC, Prusoff WH. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- [20].Siebenaller JF, Murray TF. Biol. Bull.-US. 1999;197:388–394. doi: 10.2307/1542793. [DOI] [PubMed] [Google Scholar]
- [21].Schild HO. Br. J. Pharmacol. 1947;2:189–206. doi: 10.1111/j.1476-5381.1947.tb00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22] a).Mogil JS, Kest B, Sadowski B, Belknap JK. J. Pharmacol. Exp. Ther. 1996;276:532–544. [PubMed] [Google Scholar]; b) Kest B, Hopkins E, Palmese CA, Adler M, Mogil JS. Pharmacol. Biochem. Behav. 2002;73:821–828. doi: 10.1016/s0091-3057(02)00908-5. [DOI] [PubMed] [Google Scholar]
- [23].Haley TJ, McCormick WG. Br J Pharmacol Chemother. 1957;12:12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].McLaughlin JP, Hill KP, Jiang Q, Sebastian A, Archer S, Bidlack JM. J. Pharmacol. Exp. Ther. 1999;289:304–311. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.