Abstract
Background:
Surgical hemostasis is achieved using adjunctive hemostats when conventional methods fail.
Objective:
This study compares the effectiveness of two adjunctive gelatin-thrombin hemostats.
Hypothesis:
To determine effectiveness, hemostats were compared in vivo, in vitro, and using scanning electron microscopy (SEM).
Methods:
In vivo, a heparinized porcine liver abrasion model was used to compare hemostatic success, degree of bleeding, and blood loss at 2, 5, and 10 minutes post-treatment. In vitro, thrombin in the supernatant of each hemostat and Red Blood Cells (RBC'S) in the supernatant of clots formed by each was compared.
Results:
Ultrastructure of one gelatin was smooth and the other stellate. In vivo, smooth gelatin provided superior hemostatic success at 5 (85% vs. 60%; OR: 5.3; 95% CI: 1.66 to 17.9) and 10 mins (72.5% vs. 47.5%; OR: 5.0; 95% CI: 1.55 to 16.1). Smooth gelatin had a statistically different degree of bleeding at 5 (0.58 ± 0.87 [Mean ± SD] vs. 1.03 ± 1.12; OR: 3.36; 95% CI: 1.34 to 8.41) and 10 mins (1.13 ± 1.14 vs. 1.65 ± 1.05; OR: 3.87; 95% CI: 1.62 to 9.21). Mean blood loss was less with smooth gelatin at 2 (0.07 ± 0.19 vs. 0.13 ± 0.63 ml/min), 5 (0.04 ± 0.13 vs. 0.23 ± 0.45 ml/min), and 10 mins (0.09 ± 0.24 vs. 0.21 ± 0.32 ml/min). In vitro, supernatant of smooth gelatin had significantly less thrombin (6.81 vs. 10.9 IU/ml, p = .001), and significantly less RBC's than stellate gelatin (0.07 vs. 0.09 × 106/ul, p = .0085).
Conclusion:
Smooth gelatin has an increased ability to retain thrombin and RBC's in vitro which may explain why it provides superior hemostatic effectiveness, superior control of bleeding, and greater reduced blood loss in vivo.
Keywords: gelatin, thrombin, hemostat, adjunctive hemostats, hemostatic efficacy, bleeding model, floseal, surgiflo
INTRODUCTION
Bleeding is an expected complication to all surgical procedures. If untreated, bleeding can lead to dehiscence, infection, or hematoma resulting in surgical failure; or lead to hemarthrosis, hemothorax, or hemopericardium increasing secondary morbidity and mortality. Unlike adhesives and sealants, hemostats are intraoperative devices that treat bleeding to increase the likelihood of successful surgical outcomes [1–3].
Topical hemostatic agents include plant- or marine-derived material (i.e., cellulose, polysaccharides, chitosan), nonhuman-derived proteins (i.e., gelatin, collagen), human-derived proteins (i.e., thrombin, fibrinogen), and/or recombinant proteins (i.e., thrombin, aprotinin). Gelatin and topical thrombin are most commonly used, alone or in combination. Gelatin induces hemostasis by platelet activation and mechanical tamponade, while thrombin cleaves fibrinogen to form fibrin [4]. Milled gelatin can be prepared with thrombin as a flowable hemostatic agent. Flowable hemostatic agents offer unique advantages over nonflowable hemostats, such as conforming to wound geometries, filling deep lesions, and having the ability to remove excess material with irrigation. Flowable hemostatic agents are demonstrated to be more effective than conventional methods in multiple surgical specialties in randomized clinical trials [5–7].
The efficacy of commercially available thrombin derived from different species and recombinant sources have been proven to be similar [8, 9]. The use of human- or recombinant-derived thrombin is, however, favored because antibodies against bovine thrombin may form and attack human Factor V resulting in Factor V deficiency [7]. In contrast to thrombin, the efficacy of commercially available gelatin derived from different species has not been investigated though the physiochemical properties of gelatin vary based on the source and process of extraction [10].
This study compared two combination flowable hemostats, a bovine gelatin and a porcine gelatin hemostat combined with human thrombin, using scanning electron microscopy (SEM), and in vivo and in vitro test systems. SEM images were used to compare the ultrastructure of the gelatin size and surface variations. An in vivo heparinized porcine liver abrasion model was used to compare in vivo hemostatic success, degree of bleeding, and continued blood loss after treatment. An in vitro test system was used to compare thrombin in the supernatant of each hemostat alone, and used to compare the thrombin and the number of Red Blood Cells (RBC's) in the supernatant of each hemostat mixed with blood as a metric of clot formation.
MATERIALS AND METHODS
Hemostatic Agents
FLOSEAL VH S/D [Hemostatic Matrix] (Baxter Healthcare Corporation, Deerfield, Illinois, USA) and SURGIFLO [Hemostatic Matrix] (Ethicon Inc., Somerville, New Jersey, USA) were compared in this study. FLOSEAL, containing lyophilized bovine gelatin, was prepared with human thrombin provided in the hemostatic kit. SURGIFLO, containing partially reconstituted porcine gelatin was prepared with EVITHROM [Topical Human Thrombin] (Ethicon Inc., Somerville, New Jersey, USA). All products were stored and prepared according to their respective Instructions for Use [12–14]. Gelatin products were not prepared using the same thrombin, because the thrombin from one manufacturer may have affected the reconstitution of the other gelatin. Collagen procurement, treatment, and other manufacturing processes cannot be controlled for in this study.
FLOSEAL thrombin was reconstituted with calcium chloride (40 μmol/ml) for a thrombin concentration of approximately 500 IU/ml. FLOSEAL gelatin from a 10 ml kit was then hydrated with 8 ml of the thrombin solution yielding approximately 10 ml of final product with approximately 400 IU/ml of thrombin. EVITHROM was thawed and had a thrombin concentration of approximately 1,000 IU/ml. SURGIFLO gelatin was mixed with 4 ml of thrombin solution yielding approximately 10 ml of final product with approximately 400 IU/ml of thrombin. Both gelatins had the same final thrombin concentration of approximately 400 IU/ml. One lot of each product was used in the SEM and in vivo comparisons, while three lots of each product were used in the in vitro comparison.
METHODS
SEM
Samples of each hemostat were dehydrated in a graded ethanol series to 100% ethanol. They were then placed into graded solutions of ethanol and hexamethyldisilazane (HMDS) followed by fresh 100% HMDS and allowed to air dry. The specimens were then mounted onto aluminum SEM supports with carbon tape and coated with palladium for conductivity using a Denton Desk IV Sputter/Etch Unit (Denton Vacuum, LLC, Moorestown, New Jersey, USA). Samples were then morphologically examined using a Jeol JSM-7600F Scanning Electron Microscope (Jeol USA, Inc., Peabody, Massachusetts, USA) and representative images were taken.
In vivo Comparison
All animal activities were performed according to the Animal Welfare Act and The Guide for the Care and Use of Laboratory Animals in an AAALAC accredited institution. The study protocol was approved by the Institutional Animal Care and Use Committee prior to starting the work. Five female pigs with a mean weight of 52.6 kg, ranging 49.2–56.2 kg, were premedicated with midazolam (0.3 mg/kg, IM) and mask-induced with isoflurane in a 2:1 nitrous oxide-to-oxygen carrier. After intubation, pigs were ventilated and maintained under anesthesia using isoflurane. Warmed lactated Ringer's solution was given intravenously at a continuous rate infusion throughout the study.
A heparinized porcine liver abrasion model was used to compare the two treatments. This model is a refinement of the liver square model [15]. In this refined model, a series of two liver abrasions are created using a hand-drill (Dremel Stylus Model 1100-01, Robert Bosch Tool Corporation, Mt. Prospect, Illinois, USA) fixed with medium grade sandpaper (3M, St. Paul, Minnesota, USA). The refined model reduces variability by using standardized 1 cm diameter, 3–4 mm deep lesions. The liver abrasion model allows a high number of lesions per animal reducing the overall number of animals used such that a total of 80 lesions were performed in a total of 5 animals.
The treatments were compared at 2, 5, and 10 min after application by a single observer, blinded to treatment. The observer assessed the degree to which each lesion bleed after treatment using a defined, graded scale of 0 to 5; where 0 is no bleeding and 5 is severe bleeding (Figure 1) [15]. The degree of bleeding is an ordinal metric of hemostasis. While, hemostatic success is a binary metric of hemostasis based on the degree of bleeding, in which hemostatic success was predefined as “No Bleeding” or as an “Ooze.”
FIGURE 1 .
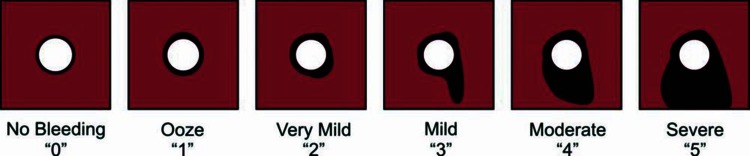
Graded scale used to assess the degree of bleeding from each lesion, where “no bleeding” and “an ooze” are hemostatic success. Each box depicts a treated hepatic abrasion with different amounts of blood loss.
If hemostatic success was not achieved at 2, 5, or 10 min after treatment, then the rate of blood loss was quantified using dry preweighted gauze to collect continual blood loss for 1 min. The initial weight of the gauze was subtracted from the final weight. The g/min were converted to ml/min using a conversion of 1 g equaling 1 ml [16]. The rate of blood loss was recorded as zero if hemostatic success was achieved because no to minimal blood loss could be quantified.
A research technician randomized the hemostatic agents within a lesion series using a random number sequence. The same research technician prepared the agents in unmarked syringes and presented each to a single surgeon who applied the hemostats in the assigned random order at the time of use to avoid test item confusion. The surgeon was blinded to the randomization and hemostatic agent being applied. The two lesions in each series were treated at approximately the same time to avoid difference in coagulation that may result from treating each independently.
Hemostatic agents were applied to each lesion and approximated with saline-dampened gauze for 2 min. At 2 and 5 min after application, degree of bleeding was assessed and blood loss was measured without disturbing the product. Excess product on the two lesions was equally and simultaneously irrigated away using saline after the 5-min assessment and blood loss measurement. At 10 min after application, bleeding assessment and blood collection were performed again.
Differences in coagulation factors and clotting times of humans and pigs [17, 18] were overcome by heparinization. Heparinization also increased the sensitivity of the test system by increasing the strength of the bleeds. Each pig received a bolus dose of heparin to achieve an Activated Clotting Time (ACT) within the “safe zone” for cardiopulmonary bypass (i.e., 300 to 600 s) [19, 20]. The “safe zone” is an objective criteria used by perfusionists to determine the safety of using cardiopulmonary bypass. The model, therefore, is clinically relevant as it represents appropriate levels of heparinization mimicking either clinical practice or disease. The ACT was measured every 20 min using a clinical coagulation instrument (Hemochron Whole Blood Coagulation System, International Technidyne Corporation, Piscataway, New Jersey, USA).
In vitro Comparison
A thrombin chromogenic assay (DiaPharma Group, Inc., West Chester, Ohio, USA) was used to measure thrombin in the supernatant of each gelatin when prepared with thrombin. A 50 μl aliquot of gelatin and thrombin, not mixed with blood, was left undisturbed in a tissue culture plate stored in an incubator at 37°C in 5% CO2 for 30 min. Then, 2 mL of phosphate buffer solution was added and the sample was stored again in an incubator at 37°C in 5% CO2 for 30 min. Finally, the sample was centrifuged at 2,000 g for 10 min to separate the gelatin particles, and the supernatant was collected and analyzed.
A thrombin chromogenic assay (ibid.) and a clinical hematology instrument (ADVIA®2120 Hematology System, Siemens Corporation, New York, USA) were used to measure thrombin and RBC's in the supernatant of each gelatin when prepared with thrombin and after being mixed with blood, respectively. A 50 μl aliquot of gelatin and thrombin was mixed with 300 μl of blood then treated as above. Human Type O blood from three separate donors was used. Blood was not pooled.
Statistical Analysis
The in vivo sampling unit was the liver lesion with 40 lesions per group for a total of 80 lesions to detect a difference in rates of 75% versus 35%, with an α = 0.05 and a 90% power. The statistical analysis was performed on the observed degree of bleeding score and hemostatic success percent.
Logistic regression was used to evaluate the treatment effect at 2, 5, and 10 min post-treatment using SAS® (SAS Institute Inc., Cary, North Carolina, USA). A binomial model of success percent and a proportional odds model of observed bleeding score were used. Independent variables included treatment group, pig, liver lobe, and initial bleeding score at baseline. The odds ratios and 95% confidence intervals were computed at each time point post-treatment. The rate of blood loss in ml/min was summarized for comparison.
The in vitro sampling unit was the supernatant of each product with three samples per lot of product per blood donor (i.e., a total of 27 samples per product were compared). A mixed effect model was used to compare mean differences of thrombin and RBC's before and after being mixed with blood. Factors in the model included treatment group, random lot effect nested in treatment, random donor effect, treatment by donor interaction, and donor by lot interaction nested in treatment. Because of the small number of donors and lots in this study, compound symmetry assumption was used for the variance-covariance structure.
RESULTS
SEM
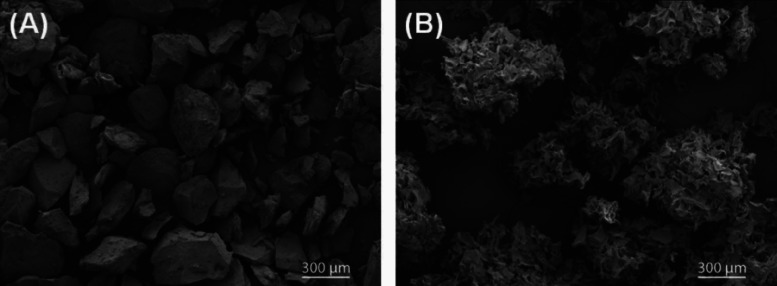
The ultrastructure of the two hemostats is drastically different (Figure 2). FLOSEAL gelatin is smooth discrete particles of round and angular shapes, while SURGIFLO gelatin is stellate coalescing particles of ribbon shape.
FIGURE 2 .
Scanning electron microscopic images of (A) bovine-derived gelatin (FLOSEAL) with a large-smooth appearance and (B) porcine-derived gelatin (SURGIFLO) with a small-stellate appearance.
In vivo Comparison
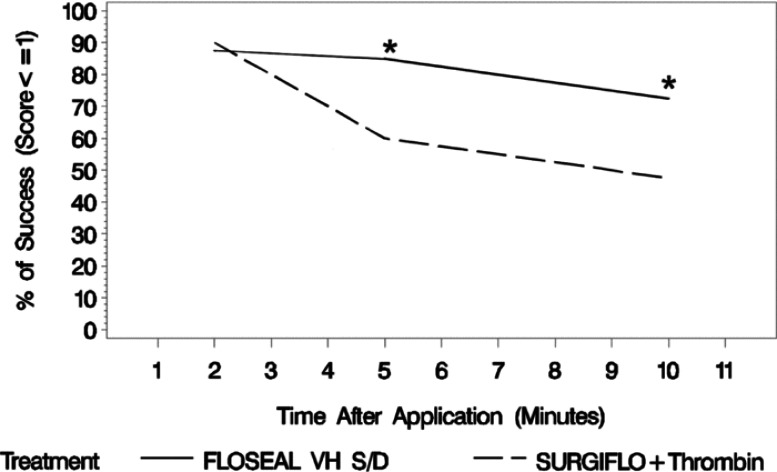
The hemostatic success at 2 min after application was similar between smooth gelatin (35 of 40 lesions, 87.5%) and stellate gelatin (36 of 40 lesions, 90.0%). The hemostatic success of the two agents, however, diverged overtime with smooth gelatin providing superior hemostatic success to stellate gelatin at 5 min (34 of 40, 85% vs. 24 of 40, 60%) and 10 min (29 of 40, 72.5% vs. 19 of 40, 47.5%) after application (Figure 3). The agents diverged greatest between 2 and 5 min after application and prior to irrigation of excess product. Thereafter, the performance was paralleled between the groups with stellate gelatin having the lower hemostatic success and greater rate of rebleeding over time. The odds ratio of binomial success demonstrates the superiority of smooth gelatin to stellate gelatin measured at 5 and 10 min after application (Table 1).
FIGURE 3 .
Hemostatic success at each time point post-treatment where FLOSEAL, a smooth gelatin, has a hemostatic success percent much greater than SURGIFLO, a stellate gelatin, over time (n = 40 per group per time point). Statistical significance is based on an odds ratio of binomial model of success, where FLOSEAL with thrombin is significantly different from SURIGFLO with thrombin at 5 and 10 min (*).
TABLE 1 .
Results of the multiple logistic regression for hemostatic effectiveness based on the odds ratio of binomial model of success percent, and for control of bleeding based on the odds ratio of proportional model of bleeding score. A 95% lower confidence limit greater than 1.0 indicates statistical significance and favors the numerator of the comparison. The greater the value above 1.0, the greater the significance
| Model | Minutes After application | Pig effect p-value | Lobe effect p-value | Baseline effect p-value | Comparison | Odds ratio | 95% lower confidence limit | 95% upper confidence limit |
|---|---|---|---|---|---|---|---|---|
| Hemostatic effectiveness | 2 | .8997 | .7138 | .0004 | FLOSEAL/SURGIFLO+Thrombin | 0.9249 | 0.1926 | 4.4405 |
| – | 5 | .0675 | .2691 | .0023 | FLOSEAL/SURGIFLO+Thrombin | 5.3247 | 1.5950 | 17.7763 |
| – | 10 | .0176 | .2100 | .0124 | FLOSEAL/SURGIFLO+Thrombin | 4.9878 | 1.5489 | 16.0616 |
| Control of bleeding | 2 | .5099 | .2629 | .0000 | FLOSEAL/SURGIFLO+Thrombin | 1.7315 | 0.4786 | 6.2642 |
| – | 5 | .0019 | .0131 | .0001 | FLOSEAL/SURGIFLO+Thrombin | 3.3570 | 1.3394 | 8.4138 |
| – | 10 | .0000 | .3001 | .0000 | FLOSEAL/SURGIFLO+Thrombin | 3.8653 | 1.6216 | 9.2135 |
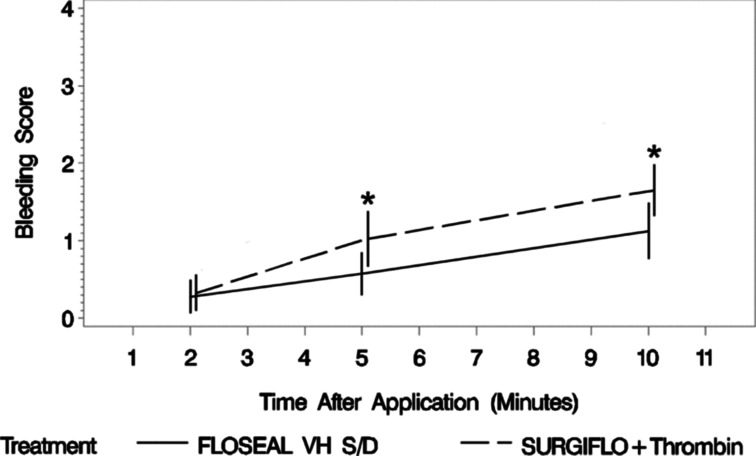
The mean degree of bleeding at 2 min after application and upon removing the dampened gauze was similar between smooth gelatin (0.275 ± 0.679 [Mean ± SD], N = 40) and stellate gelatin (0.325 ± 0.730, N = 40). The degree of bleeding of the two agents then diverged between 2 and 5 min after application with stellate gelatin yielding a significantly higher degree of bleeding (0.575 ± 0.874 vs. 1.025 ± 1.121, N = 40 per group). The difference between the agents remained similar between 5 and 10 min after application but favored smooth gelatin to stellate gelatin (Figure 4). The odds ratio of proportional odds demonstrates increasing superiority of smooth gelatin to stellate gelatin over time (Table 1).
FIGURE 4 .
Degree of bleeding at each time point post-treatment where FLOSEAL, a smooth gelatin, has the lowest bleeding scores over time (n = 40 per group). Statistical significance is based on an odds ratio of proportional model of bleeding score, where FLOSEAL is significantly different from SURGIFLO with thrombin at 5 and 10 min (*). The whiskers represent plus and minus two times the standard error.
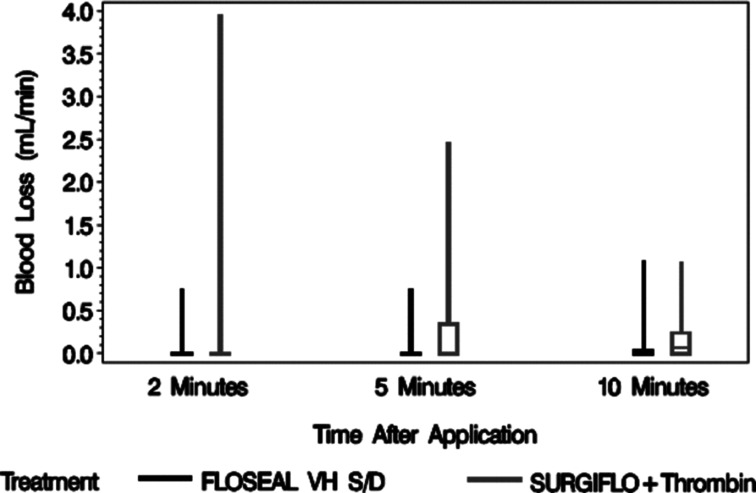
The rate of blood loss prior to treatment was not recorded. The rate of blood loss for smooth gelatin was 46% lower than stellate gelatin at 2 min (0.07 ± 0.19 ml/min vs. 0.13 ± 0.63 ml/min, N = 40 per group), 83% lower at 5 min (0.04 ± 0.13 ml/min vs. 0.23 ± 0.45 ml/min, N = 40 per group), and 57% lower at 10 min (0.09 ± 0.24 ml/min vs. 0.21 ± 0.32 ml/min, N = 40 per group) after application. The range of blood loss for stellate gelatin decreased over time, but increased on average overtime (Figure 5).
FIGURE 5 .
Box whisker plot for the rate of blood loss 2, 5, and 10 min after application. FLOSEAL, a smooth gelatin, consistently had the lowest amount of blood loss over time.
The mean baseline ACT in this model was 120.8 s (N = 5) with a median of 125.0 s and range of 111–128 s. The mean heparinized ACT was 498.2 s (N = 71) with a median of 407.0 s and a range of 227–1,286 s. The mean heparinized ACT was equivalent to 4.2× baseline with a median of 3.5× baseline.
In vitro Comparison
Smooth gelatin retained significantly more thrombin than stellate gelatin as measured in the supernatant of each before being mixed with blood (6.81 vs. 10.89 IU/ml, p = .0013) and after a clot was formed (0.613 vs. 1.289 IU/ml, p = .0003) (Table 2). The amount of thrombin in the supernatant of each reduced after being mixed with blood. Smooth gelatin retained significantly more RBC's than stellate gelatin as measured in the supernatant of each after a clot was formed (0.0685 vs. 0.0911 × 106/uL, p = .0085).
TABLE 2. .
Mixed Effect Model Results for Mean Difference of Thrombin, Thrombin with Blood, and Red Blood Cells (RBC's) where, statistically, FLOSEAL, containing smooth gelatin, had significantly lower nonretained thrombin and RBC's than SURGIFLO, containing stellate gelatin
| Metric | FLOSEAL VH S/D mean (n = 27) | SURGIFLO+Thrombin Mean (n = 27) | Mean difference | Lower 95% CI | Upper 95% CI | p-value of mean difference |
|---|---|---|---|---|---|---|
| Product thrombin (IU/ml) | 6.81 | 10.89 | −4.08 | −5.48 | −2.67 | 0.0013 |
| Product+Blood thrombin (IU/ml) | 0.613 | 1.289 | −0.676 | −0.841 | −0.511 | 0.0003 |
| RBC's (×106/ul) | 0.0685 | 0.0911 | −0.0226 | −0.0356 | −0.0096 | 0.0085 |
DISCUSSION
This paper investigated the differences between two gelatin-thrombin hemostats. The two hemostats demonstrated different in vivo performance, which is likely due to different ultrastructure. The reason for the different ultrastructures was not investigated and is of interest for future investigations. The extraction method can influence the isoionic point and viscosity of the gelatin, which may affect its ultrastructure [11]. However, the likely difference is that porcine-derived gelatin has a higher isoelectric point, lower kinemetic viscosity, and different amino acid composition (i.e., decreased alanine, glycine, isoleucine, hydroxyproline, and increased tyrosine) than bovine-derived gelatin [21]. The species differences are likely the cause for the different appearances (i.e. stellate gelatin being porcine-derived and smooth gelatin being bovine-derived) and subsequent performance differences.
Smooth gelatin has a superior in vivo performance to stellate gelatin over time. The difference may result from a greater ability to retain thrombin and ability for clot formation, which may be a result of the different gelatin shapes. The ability to retain a greater thrombin concentration in or on gelatin particles increases the amount of thrombin delivered to and maintained at the site of bleeding over time. While, the ability to trap RBC's indicates that a more effective hemostatic plug is formed. The greater thrombin concentration and more effective clot formation decreases the time to hemostasis, increases control of bleeding, and reduces blood loss.
The lesser ability of stellate gelatin to retain thrombin may explain its inferior performance in vivo. The volume of thrombin added to stellate can be increased; however, this will likely decrease its viscosity, causing more product to be washed away during continued bleeding. Alternatively, the concentration of thrombin added to stellate gelatin can be increased. But, the conversion of fibrinogen to fibrin is maximized at a one milligram-to-two unit ratio of fibrinogen to thrombin [22, 23]. And, when measured in vivo the natural occurring concentration of fibrinogen is 145–348 mg/dl and concentration of prothrombin is 270–330 U/ml for a ratio of 1 mg:122 IU [24, 25]. A thrombin concentration greater than 400 IU/ml is then unlikely to increase the efficacy of stellate gelatin. Both gelatin forms were prepared with the same thrombin concentration, approximately 400 IU/ml, in this study.
The strength of this study is that it used an in vivo model relevant to clinical practice, an in vitro model, and SEM to offer a possible explanation as to why differences are seen. The in vivo differences between the products are corroborated by the products’ indications: FLOSEAL, containing smooth gelatin, is indicated for all types of bleeding, including arterial spurting; SURGIFLO, containing stellate gelatin, is indicated for only venous, capillary, and arteriolar bleeding when prepared with either thrombin or saline [12, 13]. The clinical indications and efficacy differences carry clinically meaningful differences.
The efficacy of the gelatin products was compared in a retrospective case series of patients under-going a laparoscopic partial nephrectomy [26]. Clinical outcomes—not intraoperative performance—were measured in this study. This clinical comparison agrees with our data in that a lower amount of blood loss is seen with the smooth gelatin (25–650 ml) than with the stellate gelatin (50–1,500 ml) [26].
The limitation of our study is that it only compares the agents in one tissue type and in one lesion type, a limitation of all standardized bleeding models. This limitation, however, is addressed by comparing clinical data, which represents several different tissue types and lesion types. When clinical data is compared, the smooth gelatin had a greater percentage of patients reaching complete hemostasis at 3, 6, and 10 min after application (85%, 93%, 97%, respectively) than with the stellate gelatin (64%, 90%, 95%, respectively) [12, 13]. These clinical differences become more pronounced in patients with existing unfavorable clinical conditions (e.g., hypocoagulopathic conditions due to heparinization, antithrombotic therapies, antiplatelet therapies, hemodilution, thrombocytopenia, etc.), as similar to our in vivo data.
CONCLUSIONS
This study demonstrates that two flowable gelatin hemostats have different ultrastructures which may result in in vivo efficacy differences. It was demonstrated that smooth gelatin provides superior hemostatic efficacy, superior control of bleeding, and reduced blood loss over time relative to stellate gelatin using a heparinized porcine bleeding model. This study further demonstrated that the smooth gelatin retains significantly greater thrombin concentrations and RBC's than stellate gelatin, statistically. The in vitro findings may explain the in vivo differences between the two gelatins.
ACKNOWLEDGMENTS
The authors thank Jim DiOrio and Mary Ann Murphy for their imaging expertise and performing the SEM, and Huub Kreuwel for reviewing the manuscript. The authors also thank the technical and administrative staff at their respective institutions.
Declaration of Interest: Drs. Kevin M. Lewis, Holly D. Atlee, and Lawrence Lin; Mrs. Angela J. Mannone; and Mr. Joseph Dwyer are employees of Baxter Healthcare Corporation. Dr. Andreas Goppelt is an employee of Baxter Innovations GmbH. Dr. Heinz Redl is a consultant to Baxter Innovations GmbH.
REFERENCES
- [1].Spontnitz WD, Burks SG. Hemostats, sealants, and adhesives II: Update as well as how and when to use the components of the surgical toolbox. Clin Appl Thromb Hemost. 2010;16:497–514. doi: 10.1177/1076029610363589. [DOI] [PubMed] [Google Scholar]
- [2].Achneck HE, Sileshi B, Jamiolkowski RM, et al. A comprehensive review of topical hemostatic agents. Ann Surg. 2010;251:217–228. doi: 10.1097/SLA.0b013e3181c3bcca. [DOI] [PubMed] [Google Scholar]
- [3].Boucher BA, Traub O. Achieving hemostasis in the surgical field. Pharmacotherapy. 2009;29:S2–S7. doi: 10.1592/phco.29.pt2.2S. [DOI] [PubMed] [Google Scholar]
- [4].Oz MC, Cosgrove DM, 3rd, Badduke BR, et al. Controlled clinical trial of a novel hemostatic agent in cardiac surgery. Ann Thorac Surg. 2000;69:1376–1382. doi: 10.1016/s0003-4975(00)01194-2. [DOI] [PubMed] [Google Scholar]
- [5].Raga F, Sanz-Cortes M, Bonilla F, et al. Reducing blood loss at myomectomy with use of a gelatin-thrombin matrix hemostatic sealant. Fertil Steril. 2009;92:356–360. doi: 10.1016/j.fertnstert.2008.04.038. [DOI] [PubMed] [Google Scholar]
- [6].Nasso G, Piancone F, Bonifazi R, et al. Prospective, randomized clinical trial of the FloSeal matrix sealant in cardiac surgery. Ann Thorac Surg. 2009;88(5):1520–1526. doi: 10.1016/j.athoracsur.2009.07.014. [DOI] [PubMed] [Google Scholar]
- [7].Mozet C, Prettin C, Dietze M, et al. Use of floseal and effects on wound healing and pain in adults undergoing tonsillectomy: Randomised comparison versus electrocautery. Eur Arch Otorhinolaryngol. 2011 Dec 30 doi: 10.1007/s00405-011-1904-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [8].Chapman WC, Singla N, Genyk Y, et al. A phase 3, randomized, double-blind comparative study of the efficacy and safety of topical recombinant human thrombin and bovine thrombin in surgical hemostasis. J Am Coll Surgeons. 2007;205(2):256–265. doi: 10.1016/j.jamcollsurg.2007.03.020. [DOI] [PubMed] [Google Scholar]
- [9].Doria C, Fischer CP, Wood CG, et al. Phase 3, randomized, double-blind study of plasma-derived human thrombin versus bovine thrombin in achieving hemostasis in patients undergoing surgery. Curr Med Res Opin. 2008;24:785–794. doi: 10.1185/030079908X273426. [DOI] [PubMed] [Google Scholar]
- [10].Lawson JH, Lynn KA, Vanmatre RM, et al. Antihuman factor V antibodies after use of relatively pure bovine thrombin. Ann Thorac Surg. 2005;79:1037–1038. doi: 10.1016/j.athoracsur.2003.09.110. [DOI] [PubMed] [Google Scholar]
- [11].Djagny KB, Wang Z, Xu S. Gelatin: A valuable protein for food and pharmaceutical industries: Review. Crit Rev Food Sci Nutr. 2001;41:481–492. doi: 10.1080/20014091091904. [DOI] [PubMed] [Google Scholar]
- [12].FLOSEAL VH S. /D Instructions for Use. Deerfield, IL: Baxter Healthcare Corporation; 2005. [Google Scholar]
- [13].SURGIFLO Instructions for Use. Somerville, NJ: J & J Wound Management; 2008. [Google Scholar]
- [14].EVITHROM Prescribing Information. Somerville, NJ: J & J Wound Management; 2007. [Google Scholar]
- [15].Adams G, Manson J, Hasselblad V, et al. Acute in-vivo evaluation of bleeding with GelfoamTM plus saline and GelfoamTM Plus human thrombin using a liver square lesion model in swine. J Thromb Thrombolysis. 2009;28:1–5. doi: 10.1007/s11239-008-0249-3. [DOI] [PubMed] [Google Scholar]
- [16].Thornton JA. Estimation of blood loss during surgery. Ann R Coll Surg Engl. 1963;33:164–174. [PMC free article] [PubMed] [Google Scholar]
- [17].Lewis J. Pigs. In: Comparative Hemostasis in Vertebrates. Lewis J, editor. New York: Plenum Press; 1996. pp. 286–297. [Google Scholar]
- [18].Hahn N, Popov-Cenic S, Dorer A. Basic values of blood coagulation parameters in pigs (Sus scrofa domesticus) [German] Berl Munch Tierarztl Wochenschr. 1996;109:23–27. [PubMed] [Google Scholar]
- [19].Bull BS, Huse WM, Brauer FS, et al. Heparin therapy during extracorporeal circulation II: The use of a dose response curve to individualize heparin and protamine dosage. J Thorac Cardiovasc Surg. 1975;69:685–689. [PubMed] [Google Scholar]
- [20].Fitzgerald DJ, Patel A, Body SC, et al. The relationship between heparin level and activated clotting time in the adult cardiac surgery population. Perfusion. 2009;24:93–96. doi: 10.1177/0267659109106729. [DOI] [PubMed] [Google Scholar]
- [21].Eastoe JE. The amino acid composition of mammalian collagen and gelatin. Biochem J 1955. 61:589–600. doi: 10.1042/bj0610589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mosesson MW, DiOrio JP, Müller MF, et al. Studies on the ultrastructure of fibrin lacking fibrinopeptide B (β-Fibrin) Blood. 1987;69:1073–1081. [PubMed] [Google Scholar]
- [23].Weisel JW,, Nagaswami C. Computer modeling of fibrin polymerization kinetics correlated with electron microscope and turbidity observations: clot structure and assembly are kinetically controlled. Biophys J. 1992;63:111–128. doi: 10.1016/S0006-3495(92)81594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Oswald MW, Hunt HH, Lazarchick J. Normal range of plasma fibrinogen. Am J Med Technol. 1983;49:57–59. [PubMed] [Google Scholar]
- [25].Shapiro SS, Martinez J. Human prothrombin metabolism in normal man and in hypocoagulable subjects. J Clin Invest. 1969;48:1292–1298. doi: 10.1172/JCI106095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nogueira L, Katz D, Pinochet R, et al. Comparison of gelatin matrix-thrombin sealants used during laparoscopic partial nephrectomy. BJU Int. 2008;102:1670–1674. doi: 10.1111/j.1464-410X.2008.07926.x. [DOI] [PubMed] [Google Scholar]