Abstract
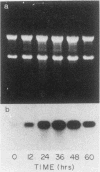
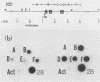
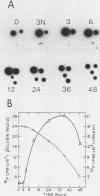
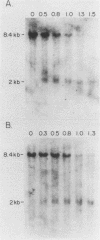
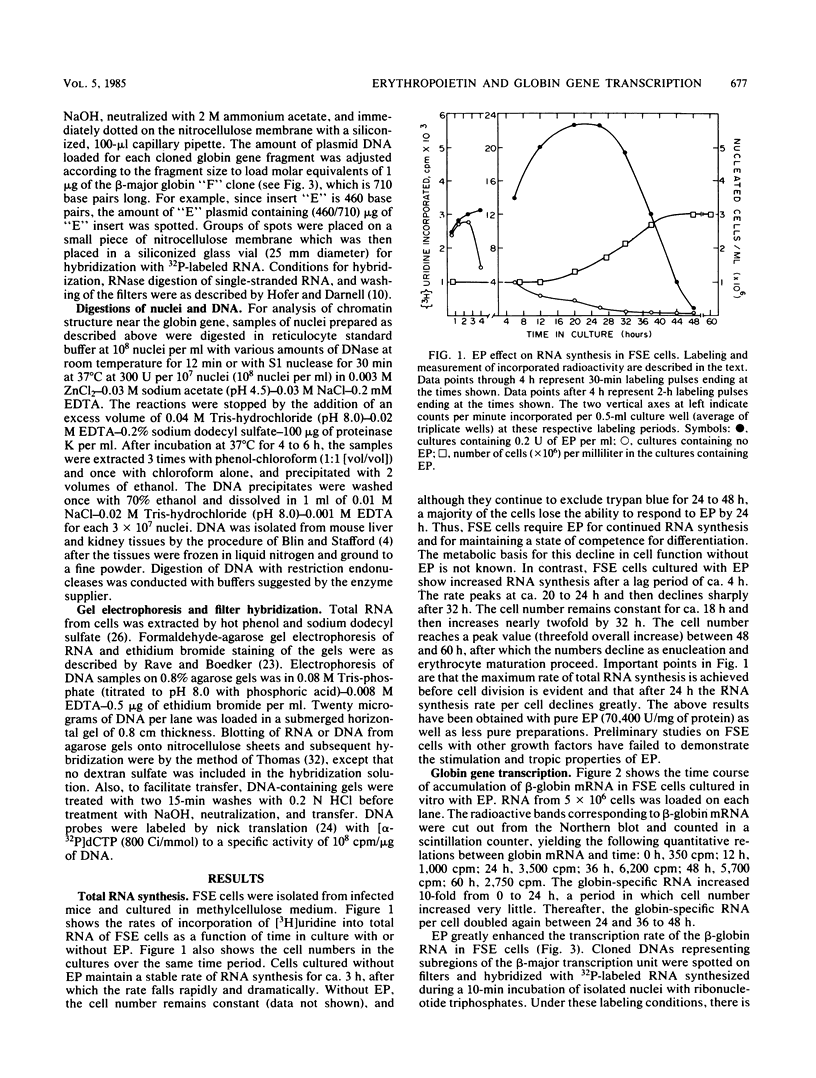
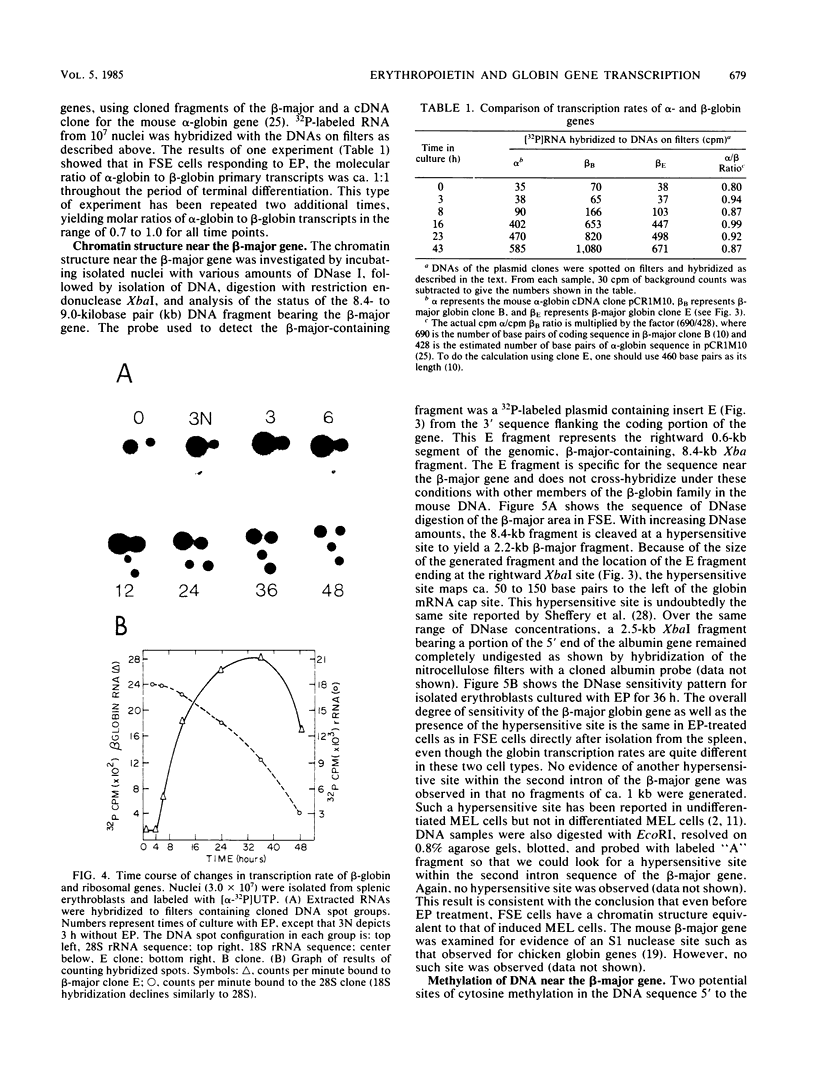
Splenic erythroblasts of mice infected with the anemia-inducing strain of Friend virus can be isolated in large numbers with less than 5% contamination with other cell types. In short-term culture, the isolated cells will initiate globin synthesis and undergo other aspects of terminal differentiation only if erythropoietin (EP) is added to the medium. An early effect of the hormone on these cells is stimulation of total RNA synthesis. EP also causes initiation of transcription of the beta-globin genes after a lag period of 4 to 6 h. By 6 h, the transcription rate of beta-globin RNA is enhanced threefold, and by 12 h, it is nearly maximal at ca. 20 times the level of control cells which received no EP. Transcription rates of alpha and beta-globin genes are approximately equal to each other throughout the period of terminal differentiation. In the splenic erythroblasts, the chromatin structure in the vicinity of the beta-major globin gene was analyzed with two nucleases during these transcription rate changes. No S1 nuclease-hypersensitive site is detectable near the gene. The beta-major gene is quite sensitive to DNase I in comparison with the albumin gene; however, the level of sensitivity is the same before EP addition as it is during maximal gene transcription after EP addition. Also, a hypersensitive site near the 5' cap site of the beta-major gene is quantitatively equivalent both before and after EP addition. Analysis of cytosine methylation at two sites upstream from the gene showed no changes upon induction of beta-globin gene transcription by EP. Thus, the initiation of beta-globin transcription by EP appears to be at some step after chromatin structural alteration such as synthesis, release, or activation of a specific transcription initiation factor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELRAD A. A., STEEVES R. A. ASSAY FOR FRIEND LEUKEMIA VIRUS: RAPID QUANTITATIVE METHOD BASED ON ENUMERATION OF MACROSCOPIC SPLEEN FOCI IN MICE. Virology. 1964 Nov;24:513–518. doi: 10.1016/0042-6822(64)90199-0. [DOI] [PubMed] [Google Scholar]
- Balcarek J. M., McMorris F. A. DNase I hypersensitive sites of globin genes of uninduced Friend erythroleukemia cells and changes during induction with dimethyl sulfoxide. J Biol Chem. 1983 Sep 10;258(17):10622–10628. [PubMed] [Google Scholar]
- Bazill G. W., Haynes M., Garland J., Dexter T. M. Characterization and partial purification of a haemopoietic cell growth factor in WEHI-3 cell conditioned medium. Biochem J. 1983 Mar 15;210(3):747–759. doi: 10.1042/bj2100747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondurant M., Koury M., Krantz S. B., Blevins T., Duncan D. T. Isolation of erythropoietin-sensitive cells from Friend virus-infected marrow cultures: characteristics of the erythropoietin response. Blood. 1983 Apr;61(4):751–758. [PubMed] [Google Scholar]
- Burgess A. W., Metcalf D., Russell S. H., Nicola N. A. Granulocyte/macrophage-, megakaryocyte-, eosinophil- and erythroid-colony-stimulating factors produced by mouse spleen cells. Biochem J. 1980 Feb 1;185(2):301–314. doi: 10.1042/bj1850301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill P., Kabat D. Unexpectedly large size of globin messenger ribonucleic acid. Proc Natl Acad Sci U S A. 1971 Jan;68(1):72–75. doi: 10.1073/pnas.68.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M., Goldwasser E. On the mechanism of erythropoietin-induced differentiation. V. Characterization of the ribonucleic acid formed as a result of erythropoietin action. Biochemistry. 1969 May;8(5):1795–1805. doi: 10.1021/bi00833a003. [DOI] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Activation of globin genes during chicken development. Cell. 1981 May;24(2):393–401. doi: 10.1016/0092-8674(81)90329-9. [DOI] [PubMed] [Google Scholar]
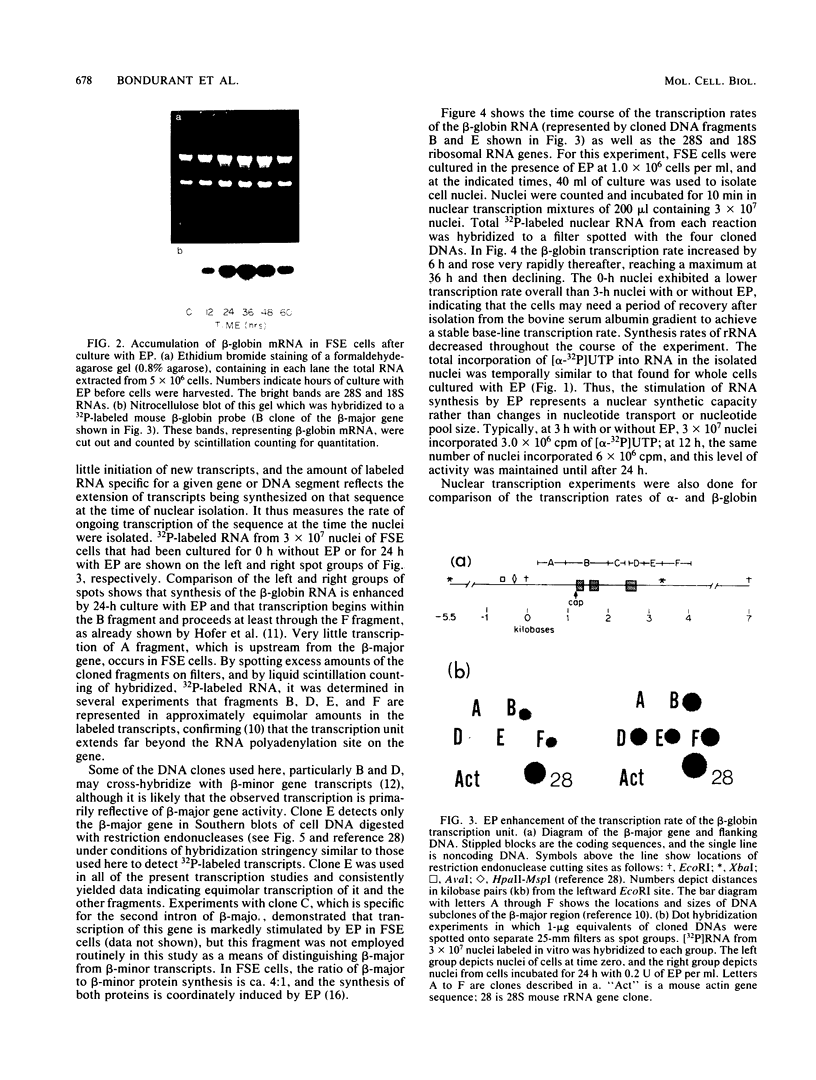
- Hofer E., Darnell J. E., Jr The primary transcription unit of the mouse beta-major globin gene. Cell. 1981 Feb;23(2):585–593. doi: 10.1016/0092-8674(81)90154-9. [DOI] [PubMed] [Google Scholar]
- Hofer E., Hofer-Warbinek R., Darnell J. E., Jr Globin RNA transcription: a possible termination site and demonstration of transcriptional control correlated with altered chromatin structure. Cell. 1982 Jul;29(3):887–893. doi: 10.1016/0092-8674(82)90450-0. [DOI] [PubMed] [Google Scholar]
- Jahn C. L., Hutchison C. A., 3rd, Phillips S. J., Weaver S., Haigwood N. L., Voliva C. F., Edgell M. H. DNA sequence organization of the beta-globin complex in the BALB/c mouse. Cell. 1980 Aug;21(1):159–168. doi: 10.1016/0092-8674(80)90123-3. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene M. A., Corces V., Lowenhaupt K., Elgin S. C. DNase I hypersensitive sites in Drosophila chromatin occur at the 5' ends of regions of transcription. Proc Natl Acad Sci U S A. 1981 Jan;78(1):143–146. doi: 10.1073/pnas.78.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman L., Peters S., Woodward-Jack J., Myers J. Alterations in the metabolism of transfer RNA during erythroid differentiation of the Friend erythroleukemia cell. Exp Cell Res. 1980 Oct;129(2):415–424. doi: 10.1016/0014-4827(80)90510-8. [DOI] [PubMed] [Google Scholar]
- Koury M. J., Bondurant M. C., Duncan D. T., Krantz S. B., Hankins W. D. Specific differentiation events induced by erythropoietin in cells infected in vitro with the anemia strain of Friend virus. Proc Natl Acad Sci U S A. 1982 Jan;79(2):635–639. doi: 10.1073/pnas.79.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koury M. J., Sawyer S. T., Bondurant M. C. Splenic erythroblasts in anemia-inducing Friend disease: a source of cells for studies of erythropoietin-mediated differentiation. J Cell Physiol. 1984 Dec;121(3):526–532. doi: 10.1002/jcp.1041210311. [DOI] [PubMed] [Google Scholar]
- Kuncio G. S., Goldstein L. Small nuclear RNAs in cellular growth and differentiation. I: metabolic alterations seen in Friend erythroleukemic cells. J Cell Physiol. 1981 Nov;109(2):235–241. doi: 10.1002/jcp.1041090206. [DOI] [PubMed] [Google Scholar]
- Larsen A., Weintraub H. An altered DNA conformation detected by S1 nuclease occurs at specific regions in active chick globin chromatin. Cell. 1982 Jun;29(2):609–622. doi: 10.1016/0092-8674(82)90177-5. [DOI] [PubMed] [Google Scholar]
- Maniatis G. M., Rifkind R. A., Bank A., Marks P. A. Early stimulation of RNA synthesis by erythropoietin in cultures of erythroid precursor cells. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3189–3194. doi: 10.1073/pnas.70.11.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudel U., Salmon J., Fibach E., Terada M., Rifkind R., Marks P. A., Bank A. Accumulation of alpha- and beta-globin messenger RNAs in mouse erythroleukemia cells. Cell. 1977 Oct;12(2):463–469. doi: 10.1016/0092-8674(77)90122-2. [DOI] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rougeon F., Mach B. Cloning and amplification of alpha and beta mouse globin gene sequences synthesised in vitro. Gene. 1977 May;1(3-4):229–239. doi: 10.1016/0378-1119(77)90047-6. [DOI] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- Shaul Y., Ginzburg I., Aviv H. Preferential transcription and nuclear transport of globin gene sequences, as control steps leading to final differentiation of murine erythroleukemic cells. Eur J Biochem. 1982 Nov 15;128(2-3):637–642. doi: 10.1111/j.1432-1033.1982.tb07011.x. [DOI] [PubMed] [Google Scholar]
- Sheffery M., Rifkind R. A., Marks P. A. Murine erythroleukemia cell differentiation: DNase I hypersensitivity and DNA methylation near the globin genes. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1180–1184. doi: 10.1073/pnas.79.4.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherton C. C., Kabat D. Changes in RNA and protein metabolism preceding onset of hemoglobin synthesis in cultured Friend leukemia cells. Dev Biol. 1976 Jan;48(1):118–131. doi: 10.1016/0012-1606(76)90051-8. [DOI] [PubMed] [Google Scholar]
- Soeiro R., Darnell J. E. Competition hybridization by "pre-saturation" of HeLa cell DNA. J Mol Biol. 1969 Sep 28;44(3):551–562. doi: 10.1016/0022-2836(69)90379-9. [DOI] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triadou P., Lelong J. C., Gros F., Crepin M. Tissue-specific formation of transcription-initiation complexes at the 5' end of the mouse beta major globin gene. Eur J Biochem. 1983 Sep 1;135(1):163–169. doi: 10.1111/j.1432-1033.1983.tb07632.x. [DOI] [PubMed] [Google Scholar]
- Weber J., Jelinek W., Darnell J. E., Jr The definition of a large viral transcription unit late in Ad2 infection of HeLa cells: mapping of nascent RNA molecules labeled in isolated nuclei. Cell. 1977 Apr;10(4):611–616. doi: 10.1016/0092-8674(77)90093-9. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Beug H., Groudine M., Graf T. Temperature-sensitive changes in the structure of globin chromatin in lines of red cell precursors transformed by ts-AEV. Cell. 1982 Apr;28(4):931–940. doi: 10.1016/0092-8674(82)90072-1. [DOI] [PubMed] [Google Scholar]