Abstract
Purpose
To explore non-invasive, protein-based, membrane array technology as a means to evaluate the global immune and angiogenic profile of tear proteins in patients with active ocular cicatricial pemphigoid (OCP).
Methods
Forty-three proteins consisting of cytokines, angiogenic/growth factors, and immuno-inflammatory modulators were measured by membrane array in tear samples of four control patients and four OCP patients during active disease and after treatment.
Results
Signals for several distinct and consistent molecular entities were upregulated in all four active OCP tear samples relative to controls. In particular, IL-8 and MMP-9 were elevated during active disease and decreased following systemic immunomodulatory therapy.
Conclusions
Protein array analysis may provide a well-tolerated assay to monitor levels of inflammatory markers in the tears of OCP patients in response to therapy.
Keywords: cicatrizing conjunctivitis, ocular cicatricial pemphigoid, inflammation, protein array, tears
Ocular cicatricial pemphigoid (OCP) is characterized by chronic inflammation and subsequent conjunctival and corneal scarring. The clinical manifestations of inflammation in OCP are associated with the expression of various fibrogenic cytokines. Prior studies have examined mRNA and protein expression of individual cytokines in conjunctival biopsy specimens of patients with active disease.1 The goal of this study was to explore protein-based, membrane array technology as a means of evaluating the global immune and angiogenic profile of tear proteins collected via Schirmer strip from the eyes of patients with active OCP.
MATERIALS AND METHODS
Patients
This prospective study was approved by the Committee for Human Research at the University of California at San Francisco and followed the tenets of the Declaration of Helsinki. Details of the study were explained and informed consent was obtained. Two groups of patients were recruited prospectively:
Consecutive patients presenting to the Proctor Foundation with a new diagnosis of cicatrizing conjunctivitis or subepithelial scarring and without a history of systemic or topical immunosuppressive therapy within one month of presentation. Only patients with a positive biopsy showing linear junctional deposition of IgG and complement component C3 on direct immunofluorescence were included.
Control patients free of ocular disease as evaluated by history and biomicroscopy. Patients with a history of contact lens wear were excluded.
Tear Collection
Tears were collected by Schirmer strip (EagleVision, Memphis, TN) without anesthesia from both eyes of four OCP patients and four age-matched patients with negative ocular and systemic disease histories. Strips were left in place for 5 minutes then immediately stored at −80 °C until processing.
Membrane Array Analysis of Tears
Assays were carried out using components of the membrane array kit purchased from RayBiotech Inc., Norcross, GA. Protein extracted from strips combined from both eyes was applied to membrane arrays specific for 43 angiogenic modulators. Schirmer strips were extracted in 400 μl phosphate buffered saline (PBS) with vortexing at room temperature for one hour. Following centrifugation, extract supernatants were adjusted to 1 ml per 10mm of strip in blocking buffer. Each array contained duplicate dots of capture antibodies that were specific for each protein as well as positive and negative controls. The 20 proteins included on Array 1 and the 23 proteins included on Array 2 are summarized in Table 1. Membrane arrays were blocked in 2 ml of 5% biotin-free casein colloidal buffer (RDI, Flanders, NJ) in PBS at pH 7.4. All incubations were carried out with constant rocking at room temperature. After two hours, the blocking solution was discarded and 1 ml of strip extract was added to each membrane. After two hours, the membranes were washed six times for five minutes each with 2 ml of PBS and 0.05% Tween-20 followed by six washes of five minutes each in PBS without detergent. The membranes were then incubated with the supplied cocktail of biotinylated-secondary antibodies diluted to one-half of the recommended concentration in 1 ml of biotin-free casein colloidal buffer for 2 hours at room temperature. The washing sequence was repeated and the membranes were incubated with 1 ml of the supplied horseradish peroxidase (HRP)-conjugated streptavidin diluted 1:20,000 in the casein blocking solution. After one half-hour, the washing sequence was repeated. Matched sets of tear samples and control arrays were developed and imaged in tandem. Negative controls consisted of membranes developed in the absence of tears. Membranes were incubated with 1 ml of ECL Advance Western Blotting Detection Kit (Amersham Biosciences, Piscataway, NJ) and imaged using a Bio-Rad ChemiDoc XRS image station equipped with an enhanced sensitivity −45°C cooled-backed 12–bit CCD.2 Relative protein levels were quantified by densitometry and analyzed in R using the Statistical Analysis of Microarrays (SAM) package.3 Fold-changes were calculated for each protein as the mean post-treatment/pre-treatment value for each patient.
Table 1.
Proteins included in Arrays 1 and 2.
| Array 1 | Array 2 | ||
|---|---|---|---|
| Protein | Abbreviation | Protein | Abbreviation |
| Angiogenin | Angiogenin | Angiopoeitin-1 | Angiopoeitin-1 |
| Epidermal Growth Factor | EGF | Angiopoeitin-2 | Angiopoeitin-2 |
| Epithelial neutrophil-activating protein | 78ENA-78 | Angiostatin | Angiostatin |
| Basic Fibroblast Growth Factor | bFGF | Endostatin | Endostatin |
| Growth Related Oncogene | GRO | Granulocyte-colony Stimulating Factor | G-CSF |
| Interferon Gamma | IFN-γ | Granulocyte-macrophage Colony Stimulating Factor | GM-CSF |
| Insulin-like growth factor-1 | IGF-1 | I-309 | I-309 |
| Interleukin 6 | IL-6 | Interleukin 10 | IL-10 |
| Interleukin 8 | IL-8 | Interleukin 1 Alpha | IL-1α |
| Leptin | LEPTIN | Interleukin 1 Beta | IL-1β |
| Monocyte Chemoattractant Protein 1 | MCP-1 | Interleukin 2 | IL-2 |
| Platelet-derived Growth Factor BB | PDGF-BB | Interleukin 4 | IL-4 |
| Placenta Growth Factor | PIGF | Interferon-inducible T Cell Alpha Chemoattractant | I-TAC |
| Regulated upon activation, normal T-cell expressed, and presumably secreted | RANTES | Monocyte Chemoattractant Protein 3 | MCP-3 |
| Transforming Growth Factor beta 1 | TGF-β1 | Monocyte Chemoattractant Protein 4 | MCP-4 |
| Tissue Inhibitor of Metalloproteinases-1 | TIMP-1 | Matrix Metalloproteinase 1 | MMP-1 |
| Tissue Inhibitor of Metalloproteinases-2 | TIMP-2 | Matrix Metalloproteinase 9 | MMP-9 |
| Thrombopoeitin | TPO | Platelet Endothelial Cell Adhesion Molecule | PECAM-1 |
| Vascular Endothelial Growth Factor | VEGF | Tyrosine Kinase with Immunoglobulin and Epidermal Growth Factor Homology Domain 2 | Tie-2 |
| Transforming Growth Factor beta 1 | RANTES | Tumor Necrosis Factor-alpha | TNF-α |
| Urokinase Plasminogen Activator Receptor | uPAR | ||
| Vascular Endothelial Growth Factor Receptor 2 | VEGF-R2 | ||
| Vascular Endothelial Growth Factor Receptor 3 | VEGF-R3 | ||
Statistical Analysis
Statistical analysis in this study was designed to assess whether the differences in protein levels between pre- and post-treatment were statistically significant. The Statistical Analysis of Microarrays (SAM) method performs a modified t-test to determine whether two sample populations of measurements are significantly different. Significance of the SAM result is determined by permutation analysis that accounts for the number of comparisons performed and typically reported as a q-value, which indicates the expected percentage of false (Type One) discoveries which would be made if the analyst rejects the null hypothesis for all results with that degree of statistical significance.
RESULTS
During a 26-month period, four patients were identified that met our criteria of a new diagnosis of OCP as defined by a positive biopsy showing linear junctional deposition of IgG and complement component C3 on direct immunofluorescence. A summary of each OCP patient’s ocular exam findings, associated systemic disease history and course of immuno-modulatory therapy (IMT) is provided in Table 2. Three patients (OCP1, OCP3, OCP4) had bulbar, intrapalpebral, conjunctival biopsies and one patient (OCP2) had a skin biopsy for pathologic analysis. All four patients showed linear junctional deposition of IgG and complement component C3 on direct immunofluorescence consistent with a diagnosis of ocular cicatricial pemphigoid. Tear samples were collected prior to examination and biopsy. Control patients ranged in age from 57 years to 77 years, had negative histories for prior ocular or systemic disease, and showed no signs of conjunctival or other ocular inflammation on biomicroscopic examination.
Table 2.
Clinical Summary of Ocular Cicatricial Pemphigoid Patients.
| Patient No./Sex/Age, y | Clinical Findings | Systemic Disease | IMT | Time on IMT prior to follow- up tear collection (months) | Schirmer’s Strip Results (mm) Pre-IMT (OD, OS) and Post-IMT (OD, OS) |
|---|---|---|---|---|---|
| OCP1/F/84 | Diffuse bulbar conjunctival injection, subepithelial scarring | Oral mucous membrane pemphigoid | Cyclophosphamide | 2 | 16, 16 (Pre) 26, 25 (Post) |
| OCP2/M/69 | Conjunctival bullae, fornix shortening, inferior symblepharon | Non-small cell lung carcinoma | Radiation, Cisplatin, Etoposide, Prednisone, Mycophenolate mofetil | 4 | 5, 8 (Pre) 35, 12 (Post) |
| OCP3/M/58 | Irregular corneal epithelium, fornix shortening, symblepharon | None | Cyclophosphamide, oral prednisone, topical prednisolone | 1 and 2 | 15, 12 (Pre) 20, 1 (1 m Post) 5, 5 (2 m Post) |
| OCP4/F/77 | Diffuse bulbar conjunctival injection, subepithelial scarring, symblepharon | Rheumatoid arthritis, chronic myelogenous leukemia (CML) | Prednisone, Methotrexate | 2 | 8, 6 (Pre) 10, 6 (Post) |
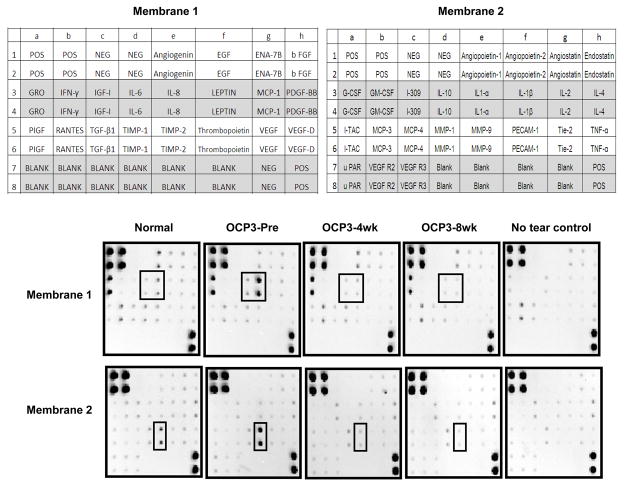
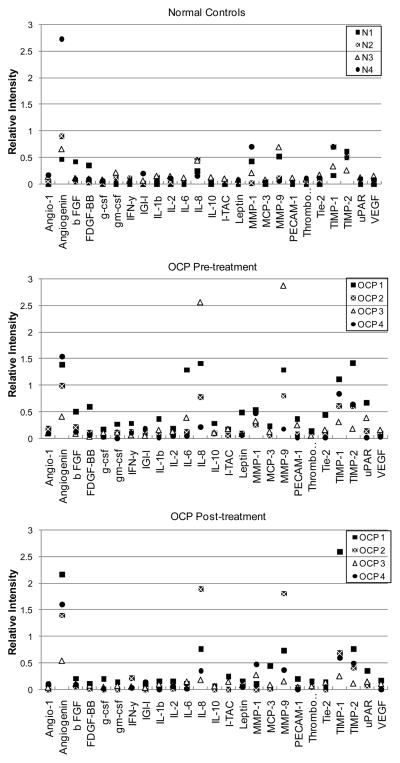
Figure 1 shows representative membrane arrays comparing normal tears to patient OCP3’s tears collected before (pre) and following 4- and 8-weeks of IMT. Control membranes run in tandem with each tear sample to elicit background signal are shown (No tear control). The two post-treatment measurements for OCP3 had a correlation coefficient of 0.97 (Pearson correlation, 95% confidence interval 0.95 to 0.99), demonstrating reproducibility and stability of the post-treatment assay. Compared to normal controls, OCP patients showed moderately increased levels of angiogenin, IL-6, IL-8, MMP-9, TIMP-1 and TIMP-2 (Figure 2). IL-8 and MMP-9 were significantly elevated in three out of four OCP patients (OCP1, OCP2, OCP3) with active disease (Pre-Treatment), with the mean fold-increases reaching 4.6 and 4.2, respectively, (False Discovery Rate q-value < 0.001). IL-8 and MMP-9 levels were reduced following IMT (Post-treatment), except in the case of OCP2, where paraneoplastic syndrome associated with non-small cell lung carcinoma required an alternative treatment regimen consisting of radiation, cisplatin, etoposide, prednisone and Mycophenolate mofetil.
Figure 1.
Duplicate dots of capture antibodies specific for each protein as well as positive (pos) and negative (neg) controls are shown for a representative normal (normal 3) and OCP patient (OCP 3). Low to moderate signals for angiogenin, growth related oncogene (GRO), IL-6, IL-8, TIMP-1 and TIMP-2, MMP1, and MMP9 are detected in normal tears. Tears from the OCP patient were collected at the time of presentation (Pre) and after 4 and 8 weeks of immuno-modulatory therapy (IMT). Boxes denote the decrease in signal for IL-6, IL-8, and MMP-9 with treatment.
Figure 2.
Graphical comparison of background-subtracted densitometric values (Y-axis) for tear proteins measured in four normal (N) patients and four OCP patients before and after treatment.
DISCUSSION
Previous studies have focused specifically on mRNA expression and protein analysis of individual cytokines in OCP conjunctival biopsy specimens. Because tear proteomics has been shown to reflect the dynamics of ocular surface processes occurring in various ocular diseases, including vernal keratoconjunctivitis (VKC), atopic keratoconjunctivitis (AKC), or Sjögren’s syndrome (SS), 4–8 we decided to study the tears of patients with OCP.
Using membrane array technology, we identified specific tear proteins present in a single OCP tear sample and how these proteins change in response to IMT. Over a 26-month period, we identified four patients with active and currently untreated OCP in our tertiary referral clinic. IL-8 and MMP-9 were significantly elevated in three out of the four patients and decreased with the resolution of active, clinical inflammation in as little as one month of IMT. An increase in IL-8 and MMP-9 in active OCP may result from the activation of infiltrating neutrophils by IL-8, which is known to induce the release of MMP-9. The reduction in levels of IL-8 and MMP-9 to near baseline in patients OCP1 and OCP3 following systemic IMT correlated with resolution of active inflammation by clinical examination.
To our knowledge, this is the first report demonstrating the use of novel, tear-based membrane array technology to assess ocular inflammation in patients with active OCP both naïve to specific treatment and following IMT. Relative tear protein levels were reported as we found it particularly difficult to accurately quantify the specific concentration of low abundant tear proteins due to tear specific matrix effects arising, in part, from the variable presence of a highly “sticky” confounding factor, which we have elaborated upon elsewhere. 8 Data obtained using membrane array serves a valuable purpose in that by using dilute blocked samples and incubation wells for membranes with large plastic surfaces, the highly “sticky” tear factor can selectively bind to the plastic and not the membrane and thereby provide a more accurate set of signals. The obtained data is critical for selecting targets for later quantitative assays. For this reason membrane arrays are of particular importance for selecting biomarkers for further more expensive quantitative evaluation.
Acknowledgments
This research was supported by NIH/NEI grants EY016203 (NAM), EY02162 (NAM), EY018858 (MFC), seed funds from ALTA California (NAM, MFC), and RPB James S. Adams Scholar Award (ECS).
References
- 1.Foster CS, Sainz De La Maza M. Ocular cicatricial pemphigoid review. Curr Opin Allergy Clin Immunol. 2004;4:435–9. doi: 10.1097/00130832-200410000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Sack RA, Conradi L, Krumholz D, Beaton A, Sathe S, Morris C. Membrane array characterization of 80 chemokines, cytokines, and growth factors in open- and closed-eye tears: angiogenin and other defense system constituents. Invest Ophthalmol Vis Sci. 2005;46:1228–38. doi: 10.1167/iovs.04-0760. [DOI] [PubMed] [Google Scholar]
- 3.Team RDC. [Accessed March 30. 2011];R: A Language and Environment for Statistical Computing. Available at: http://www.R-project.org.
- 4.Shoji J, Inada N, Sawa M. Antibody array-generated cytokine profiles of tears of patients with vernal keratoconjunctivitis or giant papillary conjunctivitis. Jpn J Ophthalmol. 2006;50:195–204. doi: 10.1007/s10384-005-0319-4. [DOI] [PubMed] [Google Scholar]
- 5.Shoji J, Kawaguchi A, Gotoh A, Inada N, Sawa M. Concentration of soluble interleukin-6 receptors in tears of allergic conjunctival disease patients. Jpn J Ophthalmol. 2007;51:332–7. doi: 10.1007/s10384-007-0461-2. [DOI] [PubMed] [Google Scholar]
- 6.Li S, Sack R, Vijmasi T, Sathe S, Beaton A, Quigley D, Gallup M, McNamara NA. Antibody protein array analysis of the tear film cytokines. Optom Vis Sci. 2008;85:653–60. doi: 10.1097/OPX.0b013e3181824e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonardi A, Sathe S, Bortolotti M, Beaton A, Sack R. Cytokines, matrix metalloproteases, angiogenic and growth factors in tears of normal subjects and vernal keratoconjunctivitis patients. Allergy. 2009;64:710–7. doi: 10.1111/j.1398-9995.2008.01858.x. [DOI] [PubMed] [Google Scholar]
- 8.Sack R, Conradi L, Beaton A, Sathe S, McNamara N, Leonardi A. Antibody array characterization of inflammatory mediators in allergic and normal tears in the open and closed eye environments. Exp Eye Res. 2007;85:528–38. doi: 10.1016/j.exer.2007.07.004. [DOI] [PubMed] [Google Scholar]