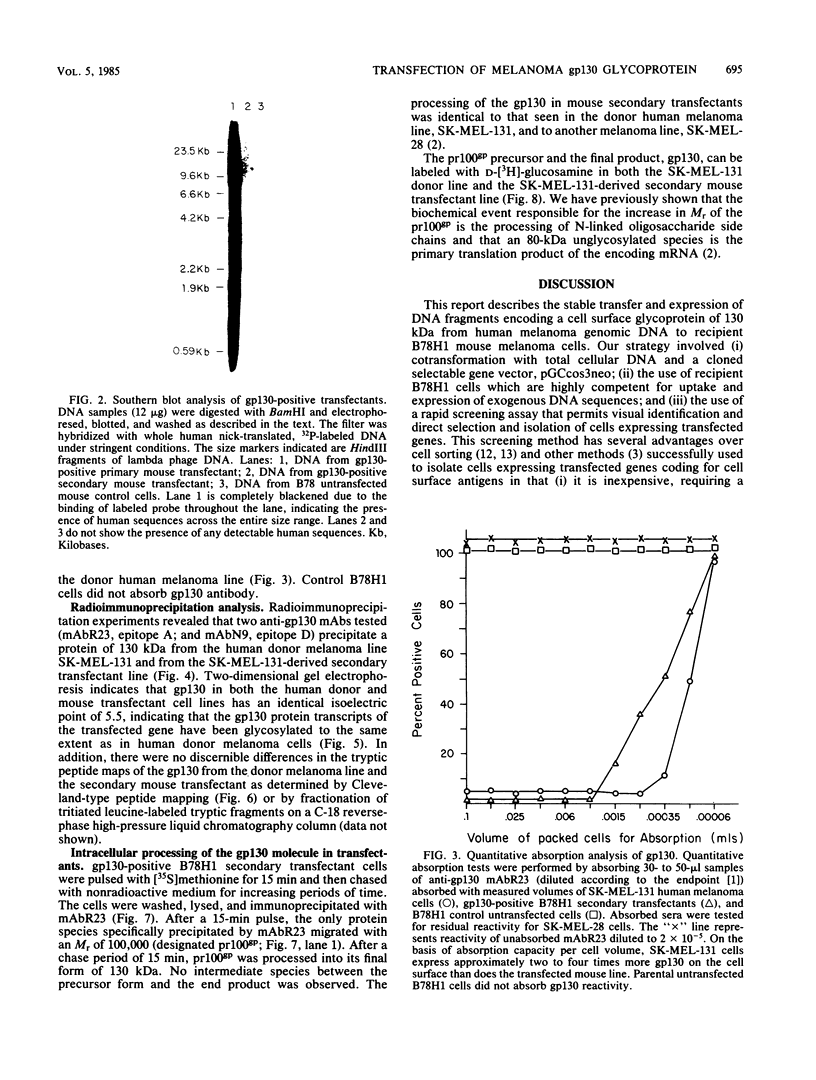
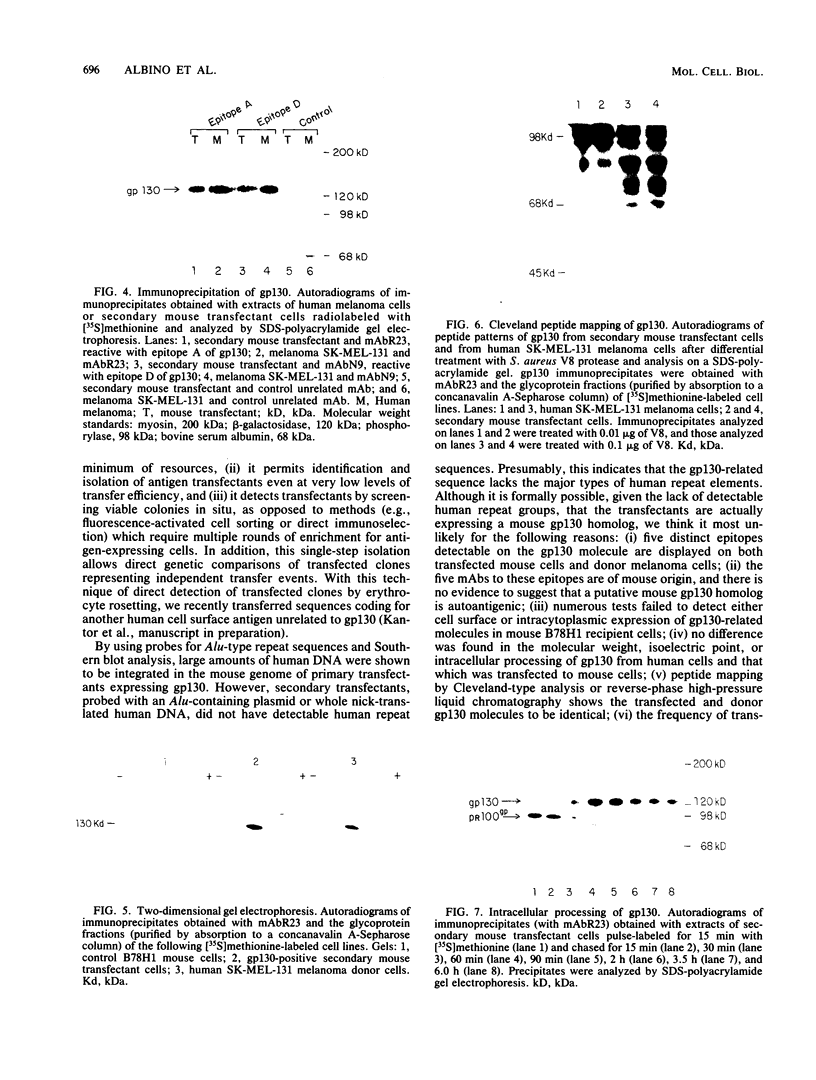
Abstract
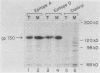
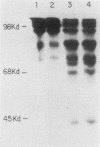
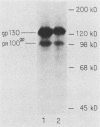
DNA sequences encoding a human melanoma membrane-bound sialoglycoprotein of 130,000 molecular weight (gp130) were introduced into a clonal derivative of mouse B-16 melanoma cells with the selectable neomycin resistance gene (aminoglycoside phosphotransferase). Mouse transfectants were identified by a rapid and precise screening method with mouse monoclonal antibodies and erythrocyte rosetting. The frequency of gp130 transfectants was approximately 1 in 2,000 to 5,000 colonies with neo+ cells. Analysis of secondary mouse transfectants has revealed that the transfected gp130 has a molecular weight, isoelectric point, intracellular processing, peptide map, and spatial orientation of surface-exposed epitopes indistinguishable from those seen with gp130 from human melanoma cells. In contrast to primary transfectants, secondary transfectants expressing gp130 lack demonstrable human repetitive sequences.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albino A. P., Lloyd K. O., Houghton A. N., Oettgen H. F., Old L. J. Heterogeneity in surface antigen and glycoprotein expression of cell lines derived from different melanoma metastases of the same patient. Implications for the study of tumor antigens. J Exp Med. 1981 Dec 1;154(6):1764–1778. doi: 10.1084/jem.154.6.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albino A. P., Lloyd K. O., Ikeda H., Old L. J. Biochemical analysis of a 130,000 molecular weight glycoprotein on human melanoma cells. J Immunol. 1983 Sep;131(3):1595–1599. [PubMed] [Google Scholar]
- Basch R. S., Berman J. W., Lakow E. Cell separation using positive immunoselective techniques. J Immunol Methods. 1983 Feb 11;56(3):269–280. doi: 10.1016/s0022-1759(83)80016-7. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Corsaro C. M., Pearson M. L. Enhancing the efficiency of DNA-mediated gene transfer in mammalian cells. Somatic Cell Genet. 1981 Sep;7(5):603–616. doi: 10.1007/BF01549662. [DOI] [PubMed] [Google Scholar]
- Dippold W. G., Lloyd K. O., Li L. T., Ikeda H., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma: definition of six antigenic systems with mouse monoclonal antibodies. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6114–6118. doi: 10.1073/pnas.77.10.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf L. H., Jr, Kaplan P., Silagi S. Efficient DNA-mediated transfer of selectable genes and unselected sequences into differentiated and undifferentiated mouse melanoma clones. Somat Cell Mol Genet. 1984 Mar;10(2):139–151. doi: 10.1007/BF01534903. [DOI] [PubMed] [Google Scholar]
- Houghton A. N., Eisinger M., Albino A. P., Cairncross J. G., Old L. J. Surface antigens of melanocytes and melanomas. Markers of melanocyte differentiation and melanoma subsets. J Exp Med. 1982 Dec 1;156(6):1755–1766. doi: 10.1084/jem.156.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W. R., Toomey T. P., Leinwand L., Duncan C. H., Biro P. A., Choudary P. V., Weissman S. M., Rubin C. M., Houck C. M., Deininger P. L. Ubiquitous, interspersed repeated sequences in mammalian genomes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1398–1402. doi: 10.1073/pnas.77.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavathas P., Herzenberg L. A. Stable transformation of mouse L cells for human membrane T-cell differentiation antigens, HLA and beta 2-microglobulin: selection by fluorescence-activated cell sorting. Proc Natl Acad Sci U S A. 1983 Jan;80(2):524–528. doi: 10.1073/pnas.80.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn L. C., McClelland A., Ruddle F. H. Gene transfer, expression, and molecular cloning of the human transferrin receptor gene. Cell. 1984 May;37(1):95–103. doi: 10.1016/0092-8674(84)90304-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowy D. R. Infectious murine leukemia virus from DNA of virus-negative AKR mouse embryo cells. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5539–5543. doi: 10.1073/pnas.75.11.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pellicer A., Wigler M., Axel R., Silverstein S. The transfer and stable integration of the HSV thymidine kinase gene into mouse cells. Cell. 1978 May;14(1):133–141. doi: 10.1016/0092-8674(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Rettig W. J., Dracopoli N. C., Goetzger T. A., Spengler B. A., Biedler J. L., Oettgen H. F., Old L. J. Somatic cell genetic analysis of human cell surface antigens: chromosomal assignments and regulation of expression in rodent-human hybrid cells. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6437–6441. doi: 10.1073/pnas.81.20.6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]