Abstract
Epidemiology studies revealed the connection between several types of cancer and type 2 diabetes (T2D) and suggested that T2D is both a symptom and a risk factor of pancreatic cancer. High level of circulating insulin (hyperinsulinemia) in obesity has been implicated in promoting aggressive types of cancers. Insulin resistance, a symptom of T2D, pressures pancreatic β-cells to increase insulin secretion, leading to hyperinsulinemia, which in turn leads to a gradual loss of functional β-cell mass, thus indicating a fine balance and interplay between β-cell function and mass. While the mechanisms of these connections are unclear, the mTORC1-Akt signaling pathway has been implicated in controlling β-cell function and mass, and in mediating the link of cancer and T2D. However, incomplete understating of how the pathway is regulated and how it integrates body metabolism has hindered its efficacy as a clinical target. The IQ motif containing GTPase activating protein 1 (IQGAP1)-Exocyst axis is a growth factor- and nutrient-sensor that couples cell growth and division. Here we discuss how IQGAP1-Exocyst, through differential interactions with Rho-type of small guanosine triphosphatases (GTPases), acts as a rheostat that modulates the mTORC1-Akt and MAPK signals, and integrates β-cell function and mass with insulin signaling, thus providing a molecular mechanism for cancer initiation in diabetes. Delineating this regulatory pathway may have the potential of contributing to optimizing the efficacy and selectivity of future therapies for cancer and diabetes.
Keywords: Cancer, Diabetes, Signaling, Metabolism
1. Introduction
The link between type 2 diabetes (T2D) and several types of human cancer has long been recognized, from epidemiological studies [1], and prospective studies support the concept that T2D represents both a symptom and a risk factor of pancreatic cancer [2, 3]. While altered metabolism has gained prominence as a “hallmark” of cancer, the molecular mechanisms of the link between metabolic diseases such as T2D and cancer have only now begun to unfold [4]. Nutrient overload in obesity leads to increased insulin secretion and consequent hyperinsulinemia [5], a symptom of T2D, and this high level of circulating insulin has been implicated in promoting more aggressive cancer phenotypes, often with poor prognosis [6]. Accordingly, the mammalian target of rapamycin (mTOR) pathway, which regulates insulin signaling and has been shown to link cancer with the nutritional status [7, 8], has been subject of intensive research as well as a clinical target and a focus of drug discovery for treating both cancer and diabetes [9]. Unfortunately, clinical trials of mTOR inhibitors produced weak results [8,10] that have been attributed to two main factors; first, the mTOR signaling pathway exhibits complicated and poorly defined feedback regulatory mechanisms [8, 10], and second, the mechanism by which the pathway coordinates body metabolism is unclear [8, 11]. This has led to the call for identifying the upstream regulators of the pathway and the molecules at the crossroads of coordinating body metabolism with mTOR in different tissues [10, 11]. Towards this end, recent studies revealed an emerging theme that signaling networks that couple pancreatic β-cell function and proliferation to regulate cell homeostasis, might impart on regulating the mTOR pathway, and underlie the link of cancer and diabetes. However, as discussed in the next section, the mechanisms that couple β-cell function and proliferation are far from being understood. The growth factor- and nutrient-sensor Cdc42-IQGAP1-Exocyst pathway serves like a rheostat to fine-tune the signals of mTOR and the mitogen activated protein kinase (MAPK) pathways, and has a role in coupling β-cell function and proliferation with glucose metabolism, and thus it represents one of the key pathways that link cell proliferation to metabolism. Moreover, deregulation of the Cdc42-IQGAP1 axis has oncogenic properties and associates with several types of human carcinomas, and thus it can link cancer to metabolic diseases. Therefore, the focus of this article will be on how the signaling components of this pathway connect β-cell function and mass in the pancreas with insulin signaling in target tissues, and how their dysfunction underlies the link of cancer and diabetes.
1.2. Loss of pancreatic β-cell mass as a major driver of diabetes
T2D is a complex disease that arises in obesity from a combination of defects in insulin signaling in target tissues and dysregulation of insulin secretion in pancreatic β-cells [5, 12, 13]. Failure of insulin-responsive tissues, such as the liver and the skeletal muscle, to respond adequately to insulin signaling, termed “insulin resistance”, prompts β-cells to compensate by increasing insulin secretion (hyperinsulinemia), a demand that ultimately triggers a progressive loss of β-cell mass, the failure to compensate, and thus the manifestation of T2D [12-16]. While insulin resistance can be reversible therapeutically, the loss of functional β-cell mass cannot at present be reversed, which has led to the concept that loss of β-cell mass is a major driver of T2D [12-17].
While much knowledge has been accumulated about the mechanisms of glucose-stimulated insulin secretion (GSIS), little is known about the mechanisms behind β-cell proliferation. The molecular mechanisms responsible for deficient insulin secretion have been extensively analyzed, revealing the involvement of diverse signals in GSIS and both a positive and a negative feedback action of secreted insulin on β-cells [18-20]. These mechanisms have been subject of recent excellent reviews [18-22], and therefore will not be covered here. By contrast, the mechanisms behind the loss of β-cell mass have only recently gained attention but remain debated as to whether β-cell division or differentiation is impaired in T2D and with evidence supporting both [12-17, 23]. Several studies implicated apoptosis and aberrations in β-cell viability in T2D, and suggested stimulating β-cell division for treatment [reviewed in 13]. However, recent evidence shows that in vitro induction of human β-cell division constitutes a rare event [17], thus utilizing it as a therapeutic approach, while avoiding the risk of neoplasia, will require full understanding of the mechanisms behind β-cell regeneration. On the other hand, dedifferentiation of diabetic β-cells has also been implicated as a factor in the pathogenesis of T2D, thus presenting stimulating differentiation as therapy [23]. The unifying thread in these studies is that loss of functional β-cell mass, be it by apoptosis or dedifferentiation, and a crucial interplay of β-cell function and mass with insulin signaling in peripheral tissues, underlie the pathology of T2D. While the exact molecular mechanisms underpinning these connections are still unclear, the role of the mTORC1 and the MAPK pathways as central players has been heavily investigated and is revealing the valuable insight that the signal-dynamics of these central pathways is crucial to homeostatic conditions, as discussed below.
2. Role of signal dynamics of mTOR and MAPK in cancer and diabetes
2.1. The mTOR pathway is regulated by negative feedback loops (NFLs) whose regulation is poorly defined
Dysfunction of mTOR-Akt signaling pathway associates with T2D and with the vast majority of the prevalent human cancers, and is currently a clinical target [8, 10]. The intracellular nutrient-sensor mTOR pathway is considered to be the hub for regulating insulin homeostasis, cell growth and proliferation, and is a major regulator of cell metabolism [8, 10]. As such mTOR signaling inputs into multiple cellular processes linked to appropriate cell growth control such as cell cycle progression, transcriptional control, ribosome biogenesis, protein translation and autophagy, essentially by monitoring energy status, nutrient availability and mitogen activity [8-11].
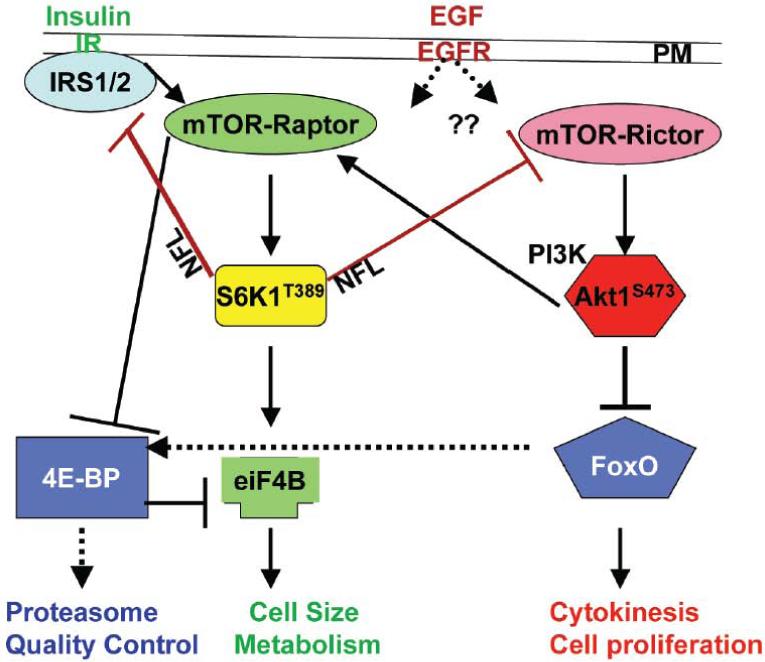
Because the mTOR pathway has been subject of intensive excellent reviews recently [8-11, 24], here we will only summarize information pertinent to the subject of this discussion (Fig. 1). The catalytic “mechanistic” TOR subunit (mTOR) is an evolutionarily conserved serine/threonine-specific protein kinase residing in two physically and functionally distinct, but tightly coordinated complexes; the rapamycin-sensitive mTOR complex 1(mTORC1), defined by the subunit Raptor and the rapamycin-resistant mTORC2, defined by the subunit Rictor [25-28]. In response to nutrient or growth factors, mTORC2 activates the protein kinase B (PKB/Akt), a serine/threonine-specific protein kinase, by phosphorylating it on Ser473. Activated pAktS473 inhibits the GTPase activating protein (GAP) Tsc1/2 complex, thus activating the Ras-like GTPase Rheb1/2 complex, which activates mTORC1 [29]. In turn, mTORC1 activates its target the ribosomal protein S6 kinase (pS6K1T389), a serine/threonine-protein kinase, and promotes insulin synthesis and signaling, leading to cell growth/size [30]. Thereafter, via known negative feedback loops (NFLs), pS6K1T389 reduces insulin sensitivity and synthesis by inactivating the insulin receptor substrate IRS1/2, an adaptor protein for the insulin receptor (IR), targeting it for proteasomal-mediated degradation, and thus terminating insulin signaling, or phosphorylates Rictor on Threonine 1135 to reduce mTORC2 signaling and inhibit Akt1S473 activity [31-33].
Figure 1.
An abbreviated diagram illustrating the mTORC1-S6K two negative feedback loops (NFLs) whose regulation is unknown. The mTORC2 (mTOR-Raptor) activates Akt by phosphorylating it on Serine 473. AktS473 then activates mTORC1 (mTOR-raptor), which activates its effector the ribosomal S6 kinase S6K by phosphorylating it on Threonin 389 to promote insulin secretion and cell size. AktS473 also promotes cell division by repressing and/or activating specific forkhead transcription factors box O (FoxO) in a process that may be mTORC2-independent [31-33]. S6KT389 then repress IRS and AktS473 to inhibit insulin signaling. In the fly (dotted lines) FoxO activates 4EBP, a diet-dependent repressor of protein translation to suppress insulin secretion and degrade excess proteins in the proteasome [36]. How the NFLs are regulated, how EGFR signals to mTOR, how Akt promotes cell division, are open questions.
Several questions regarding the mTOR-Akt regulations remain. First, how these two NFL themselves are regulated has been unclear. Second, whereas mTORC2 activation of Akt1S473 leads to activating mTORC1 and insulin secretion and is downregulated by S6K1T389 phosphorylation of RictorT1135, how Akt1S473 activity is directed towards cell division and survival is poorly defined. Particularly so because several recent studies demonstrated that mutation of RictorT1135 could not abolish the EGF-mediated phosphorylation of Akt1S473, which activates certain Forkhead box O transcription factors (FoxO) proteins, leading to promoting cell proliferation, and perhaps suggesting existence of alternative mechanism(s) to mTOR-Rictor, for activating Akt1S473 [31-33]. Later we will discuss the role of IQGAP1 as an alternative mechanism. Third, how mTOR impacts downstream effectors to terminate protein synthesis is unclear. mTORC1 promotes cap-dependent protein translation through its downstream effector the eukaryotic translation initiation factor 4E (eIF4E), by inhibiting the repressor eIF4E-binding protein (4E-BP), thus preventing eiF4E-4EBP binding. In response to nutrient, mTOR suppresses 4E-BP by phosphorylating it on Thr37 and/or Thr46 [34], but this crucial inhibitory phosphorylation event, which regulates cell growth, has turned out to be rapamycin-insensitive [35], suggesting that mTOR may not be the only factor regulating 4E-BP. Genetic evidence from the fly shows that FoxO signals to 4E-BP to inhibit insulin secretion and promote autophagy, and that constitutive signaling of FoxO-4E-BP axis mimics diet-restriction and increases lifespan [36]. Because FoxO proteins are evolutionarily conserved, it is curious whether they contribute to modulating 4E-BP activity in protein translation or termination of insulin signaling, thus explaining the rapamycin-insensitive inhibition of 4E-BP.
Because the mTORC1/S6K1 NFL generates a state of cellular insulin resistance, and S6K1 knockout in mice increases insulin sensitivity [37], several studies suggested that constitutive mTORC1 signaling in obesity, contributes to T2D [37-39]. However, genetic and pharmacologic studies revealed the complexity with which the pathway operates that prevents it from being a simple target, which is discussed below with focus on the role of mTORC1/S6K-Akt in the pancreas.
2.2. Targeting mTORC1 and Akt in research or clinic highlights importance of signal dynamics
The mTORC1 pathway plays a crucial role in the pancreas by regulating β-cell size and insulin secretion [8-11]. However, studies using genetic gain and loss of function of the mTORC1 components in the pancreas of animal models produced several contradictions [40-45]. In some studies, activation of mTORC1 in β-cells improved glucose tolerance and increased β-cell size and number [41, 42], and loss of S6K1, the target of mTORC1 widely used a marker of its activity, resulted in small β-cells and glucose intolerance in mice [43], which is a symptom of T2D. By contrast, other studies reported that constitutive activity of S6K1 in mice led to increased insulin secretion without increasing β-cell mass [44], and that loss of S6K improved insulin-sensitivity and protected against diet-induced obesity [37]. However, while constitutive activity of mTORC1 increased β-cell mass, eventually it led to insulin resistance and T2D [42], that has been attributed to activating the mTORC1-S6K1-mediated NFL and consequent inhibition of IRS1/2, which is suggested to lead to increased β-cell apoptosis [44], one of the underlying mechanisms that lead to T2D [13]. Therefore, these studies demonstrate that prolonged inhibition or activation of mTORC1 has detrimental outcome and may not be useful as long-term therapy.
Similarly, pharmacologic inhibition of mTORC1 by the specific inhibitor, rapamycin, exacerbated the symptoms of T2D both in rodents and humans [45-48]. Systemic administration of rapamycin in animals produced results varying from inconsistent insulin levels to hyperglycemia and hypoglycemia [47, 48], and clinical trials of rapamycin or its structurally related analogs “Rapalogs” resulted in hyperlipidemia and glucose intolerance in humans [10, 47, 48]. For this reason efforts were directed towards developing specific kinase inhibitors of mTOR, and met with the same fate; treatment with the mTOR kinase inhibitor AZD8055 also worsened the symptoms of T2D in mice [49].
Genetic studies in animal models paint a similar picture for Akt, which resides both upstream and downstream of mTORC1, and influences insulin signaling, glucose metabolism and cell survival. Akt signaling has been implicated also in cell-cycle progression and survival of pancreatic β-cells as well as in modulating β-cell mass and function [50-52]. Constitutive loss of Akt kinase activity in mice impaired β-cell adaptation to peripheral insulin resistance via defects in insulin secretion, rather than a decrease in β-cell mass or an increase in apoptosis [52]. However, more recent evidence reveal that maintaining functional β-cell mass in animal models requires Akt phosphorylation at Serine 473 and de-phosphorylation at Threonine 308 [51], suggesting that Akt-signal dynamics represents a crucial factor in regulating β-cell mass and function. Indeed, prolonged Akt activity, which is a known oncogenic signal, leads to hyper-proliferation and transformed phenotypes in cell culture [53], supporting the notion that T2D also arises from increasing non-functional β-cell mass due to loss of cell differentiation [23], which in turn predisposes to cancer at least in a subset of diabetic cases. To regulate cell differentiation, the mTORC1-Akt pathway cross-talks with the MAPK pathway via shared components.
2.3. Role of the MAPK pathway in cancer and diabetes
The MAPK cascade receives signal from the small GTPase Ras and transmits it through a triple MAPK cascade MAP3K (MAPKKK) Raf, which activates MAP2K (MAPKK) MEK1/2, and in turn activates several MAPKs such as ERK1/2, p38 and JNK/SAPK [54]. The oncogenic Ras-MAPK pathway is deregulated in many human cancers, including 90% of pancreatic cancers [55] and contributes to the pathogenesis of T2D [56]. Animal studies revealed that the different kinases of the MAPK cascade exhibit signal specificity and have distinct cellular functions. Mice lacking p38δ displayed improved glucose tolerance, insulin secretion and β-cell survival by relieving an inhibition on the phosphoinositide-dependent kinase-1 (PDK1), which activates Akt1 by phosphorylating it on Threonine 308 [57]. Similarly, loss of the activity of the stress-regulated c-Jun N-terminal kinases (JNKs/SAPK), which suppresses insulin signaling by phosphorylating IRS1 on serine 307 [58], improved insulin sensitivity in mice and decreased fat deposition [59]. However, the prototypical MAPK extracellular signal-regulated kinase, (ERK1/2), appears to exhibit a paradoxical response to glucose. ERK1/2 is required both for glucose-mediated induction [60] and chronic-glucose-mediated inhibition of insulin gene expression in β-cells [61], suggesting, and reminiscent of the case with mTORC1-Akt, that signal dynamics of MAPK is crucial to the normal functioning of the pathway and to homeostatic metabolism. Therefore, dominant inhibition or activation of this pathway would also be detrimental.
Underscoring the importance of signal dynamics in regulating cell and body homeostasis, are several recent studies implicating chronic administration of antidiabetic drugs, such as several types of Glucagon-like peptide-1 (GLP-1) receptor agonists, in promoting several types of cancer in human and in animal models through chronic deregulation of mTORC1-Akt and/or MAPK signals [62-64]. This molecular link between cancer and diabetes has also been emphasized by a study of human breast cancer in which the antidiabetic drug Metformin was associated with a lower rate of cell proliferation only in diabetic, but not in non-diabetic, patients [65]. Metformin mimics calorie restriction by improving insulin sensitivity and lowering insulin levels through activation of AMP-activated protein kinase (AMPK), a mitochondrial sensor that is activated by an increase in the AMP/ATP ratio [66], down regulation of c-MYC, IRS2 and inhibition of mTORC1 [67]. The caveat, however, is that prolonged inhibition of mTOR can exacerbate T2D in human and rodents [45, 46]. The systemic administration of these inhibitors may also play as a contributing factor to their serious side effects; however addressing this will require understanding how the functions of mTOR in the pancreas and the target tissues are coordinated. Collectively, genetic, pharmacologic and pharmaceutical evidence discussed here, underscore the delicate balance by which the mTORC1-MAPK pathway operates, and provides the insight that dysregulation of its signal dynamics can explain cancer development in diabetes. Therefore, mechanistic knowledge of how mTORC1-MAPK signal is modulated will be paramount to developing selective antidiabetic drugs while avoiding cancer risk. One such modulator of mTORC1-MAPK; IQGAP1, is discussed below
3. IQGAP1 as a rheostat of mTOR-Akt-MAPK signal and β-cell homeostasis
3.1. IQGAP1 is a growth factor- and nutrient-sensor scaffold that integrate cell signaling
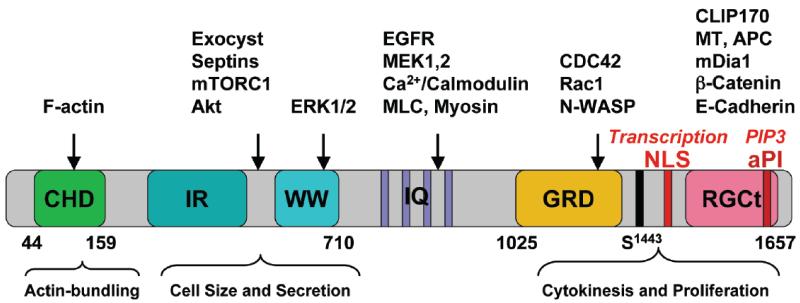
The widely conserved IQ motif containing GTPase activating protein (IQGAP) family of proteins resides at the interface of several eukaryotic signaling pathways and the cytoskeleton to influence cellular homeostasis [68]. There are three human IQGAP isoforms, IQGAP1-3 with considerable sequence homology and similar domain structure, but they differ in tissue specificity and cellular functions. IQGAP1 is the most studied, and has wider tissue distribution, including in the pancreas, liver and skeletal muscle, whereas IQGAP2 is limited to the liver, and platelet, and IQGAP3 is restricted to the brain [68-72], therefore, for relevance, the focus will be on IQGAP1. The modular nature of IQGAP1 (Fig. 2) enables it to bind signaling molecules, receptor tyrosine kinase (RTK), glutamate receptors, adaptor molecules and calcium sensors [70]. The CHD directly binds, bundles and caps F-actin, and thus IQGAP1 modulates F-actin dynamics through cycles of assembly and disassembly, and interactions with Cdc42, N-WASP, Arp2/3 complex and the formin mDia1 that involves the IQGAP1 C-terminal domain [69, 73-75]. The IR-WW associates with ERK1/2, Exocyst complex, mTORC1 and Akt [53, 76-78]. The IQ motifs associate with calcium/calmodulin, myosin light chain, the MAPK b-Raf and MEK1/2 [71], whereas the GRD binds Rac1 and Cdc42. For substitution of the catalytic arginine finger with a threonine, the GRD domain lacks a true GAP activity, and rather acts as a decoy GAP to trap and lock active Cdc42 locally without catalyzing GTPase activity, thus increasing the level of Cdc42-GTP in cells [77, 79, 80]. This feature may allow IQGAP1 to specify regions of cell polarity, localized hot spots for signal-regulated protein transport, or spatially activating signaling pathways for directed cell migration and growth [68]. To modulate epithelial adhesion, the RGCt domain binds β-catenin and E-cadherin members of the Wnt signaling that has also been widely implicated in cancer and T2D [81], and the cytoplasmic linker protein 170 (CLIP170) and the tumor suppressor adenomatous polyposis coli (APC) to also modulate the microtubule cytoskeleton [68-72]. Whereas the nuclear localization signal (NLS) at the end of the RGCt domain mediates IQGAP1’s cell cycle-dependent entry into the nucleus [82], the atypical phosphoinositide (aPI)-binding domain, like in PI3K and mTOR, binds PIP3 [83]. Altogether, these features facilitate IQGAP1’s spatiotemporal control of cell signaling related to aspects of cell proliferation such as cell size, division, differentiation, epithelial cell polarity as well as migration and phagocytosis, apparently via an underlying essential role in signal-controlled vesicular transport [68] as is discussed in the following sections.
Figure 2. Schematic representation of human IQGAP1’s domain structure, some of its binding-partners, and cellular function.
CHD; calponin homology domain; IR-WW: IQGAP1-repeats (IR) and the tryptophan (WW) repeats; IQ: four isoleucine and glutamine rich motifs; GRD: Ras GTPase-activating protein-related domain; RGCT: RasGAP-C terminus (RGCT) domain; the critical Ser-1443 is indicated; NLS: nuclear localization signal; aPI: C2/PH-like domain.
3.2. IQGAP1 modulates the mTORC1-Akt and MAPK signals
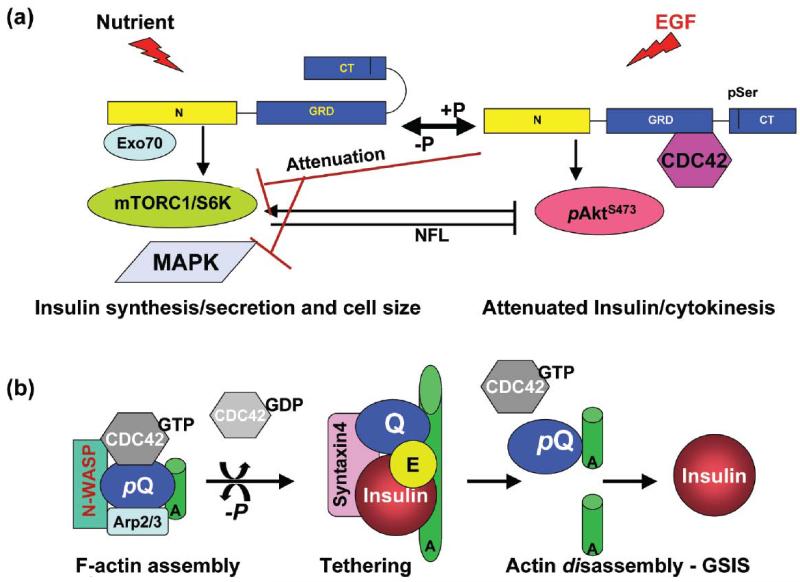
IQGAP1 appears to modulate the signal balance between mTORC1-Akt and MAPK, to regulate cell proliferation and differentiation in different tissues and cell types. In fibroblasts and epithelial cells, IQGAP1 serves as a scaffold for the MAPK cascade by binding b-Raf, MEK1/2 and ERK1/2, and regulating their activity [53, 84] apparently via direct binding to the epidermal growth factor receptor (EGFR) kinase domain [85]. In the heart, IQGAP1 integrates hypertrophy and survival signals by binding both c-Raf-MEK1/2-ERK1/2 and Akt [86]. In β-cells, it fine-tunes mTORC1-Akt1 vs. the MAPK ERK1/2 signaling to regulate cell proliferation [53, Fig. 3a]. Mechanistic analyses, using dominant mutants, revealed that in presence of epidermal growth factor (EGF), expression of IQGAP1-IR-WW, the mTORC1-binding domain, attenuates ERK1/2/GSK3α/β and mTORC1/S6K1T389 and robustly elevates the pAktS473 signal [53, 68, 77, 78]. These findings suggested that IQGAP1 relieves the mTORC1/S6K1T389 negative feedback (NFL) on pAktS473 [53, 68, 77, 78]. How IQGAP1 elevates AktS473 signal while attenuating S6K1T389 activity is being investigated on the premise that it involves a novel mTOR-IQGAP1 module parallel to mTOR-Rictor that specifies pAktS473 activity towards cell division. Indeed, mutation of Rictor Thr-1135, the site responsible for activating AktS473 and mediating the S6K-inhibition (NFL) of AktS473, does not alter the growth factor-dependent phosphorylation of Akt at Ser 473, suggesting either dispensability of that site for mTORC2 (mTOR-Rictor) kinase activity [31-33] or existence of alternative mechanisms (e.g. mTOR-IQGAP1) for activating AktS473 and diverting its activity towards cell proliferation instead of insulin secretion [53]. In support of this view, IQGAP1 binds both Akt and mTOR, and like the PI3K-mTOR family of proteins it has a PIP3-binding aPI (C2/PH-like) domain [83, Fig. 2], which could directly mediate the AktS473 activation we observed in several cell types [53]. This also provides explanation to why IQGAP1 exceeded TORC1 in sensitivity to rapamycin by several fold [53] and present the potential of its utility as a therapeutic predictor of rapamycin-sensitivity in a subset of T2D or cancer. Therefore, IQGAP1 resides both upstream and downstream of mTORC1-Akt-MAPK to couple cell growth and division [53, 78] by serving like a rheostat to adjust insulin secretion and β-cell mass, and thus linking cancer and diabetes [53], as discussed in the following sections.
Figure 3.
IQGAP1 as a phospho-serine-sensitive conformational-switch regulating β-cell function. (a) IQGAP1 couples cell size and division. Left, in response to nutrients (glucose), Exocyst-bound IQGAP1-N binds mTORC1 (and Sec61β translocon subunit, not shown) and promotes insulin synthesis and secretion, while auto-inhibition by folding of the C-terminus prevents Cdc42 from binding [53, 77, 78, 79]. Right, in response to EGF (or low nutrients), pIQGAP1S1443 has an open C-terminus, binds active Cdc42, and attenuates insulin synthesis and secretion by attenuating the S6K1T389 activity, and thus attenuating the negative feedback loop (NFL, black arrow) on AktS473 and diverting its activity towards cell division [53, 77, 78, 79]. (b) IQGAP1 known modulation of F-actin dynamics as applied to regulating glucose-stimulated insulin secretion (GSIS). Activated pIQGAP1S1443 (pQ) recruits active Cdc42 (Cdc42-GTP), N-WASP/Arp2/3 complex to assemble F-actin into meshwork [68, 69, 73-75] that help dock the vesicles, but inhibits secretion. Dephosphorylated IQGAP1 (Q) disengages Cdc42-GTP and the actin bundling machinery, and binds the exocyst and the t-SNARE syntaxin 1A, to tether the insulin vesicles/granules to the plasma membrane, culminating in actin disassembly [68, 69, 73-75] and insulin release (GSIS). A new cycle of actin assembly begins with IQGAP1 phosphorylation (pQ). P: phosphorylation by PKCε; E: Exocyst; A: actin filaments or cables.
3.3. Cdc42-IQGAP1-Exocyst modulates insulin secretion in pancreatic β-cells
IQGAP1 has a conserved intrinsic regulatory activity in secretion that different cell types from yeast to mammals utilize appropriately to regulate protein traffic [68]. In immune cells, IQGAP1-actin negatively regulates the delivery of secretory lysosomes to the target site at the plasma membrane [87], as well as the agonist-stimulated histamine secretion in mast cells [88]. In the budding yeast, IQGAP, Iqg1p, serves as a positional marker to select the bud-site, direct the exocytic secretory pathway first to that site to grow the bud and then redirect it to the cytokinetic plate to deposit the septum and bisect the daughter cells [89-91]. Mechanistic analyses of IQGAP1 role in insulin synthesis and secretion in pancreatic β-cells revealed that IQGAP1 is a regulator, playing both a negative and a positive role depending on phosphorylation and binding to Cdc42 [53, 77]. IQGAP1, and not IQGAP2 or IQGAP3, binds and co-localizes with the insulin vesicle-tethering machinery, exocyst and the t-SNARE Syntaxin 1A, to modulate GSIS [77]. Localizing to the endoplasmic reticulum, IQGAP1 binds the Sec61β translocon subunit, mTORC1 and Akt, and modulates insulin synthesis [53, 68, 77, 78]. Whereas IQGAP1-N domain that binds the exocyst and mTORC1 increases insulin synthesis, GSIS and the cell size, IQGAP1-C domain, which binds active Cdc42, reduces GSIS and the cell size, thus presenting IQGAP1 as a regulator of insulin secretion and cell size in concordance with mechanism by which it modulates mTORC1-Akt-MAPK signals [53, 77, 78], dysfunction of which could predispose to T2D and/or cancer. Indeed, the expression level of IQGAP1, and not Cdc42 or other IQGAP isoforms, is found to be significantly decreased in human β-cells isolated from patients with T2D (92, Osman, unpublished), thus supporting the results from cell culture, and highlighting the importance of IQGAP1-signal in insulin secretion and T2D.
This signal activity must involve IQGAP1’s modulation of F-actin dynamics. Total internal reflection fluorescence microscopy suggests that T2D is associated with fewer docked insulin vesicles [93] and that dynamic actin remodeling is crucial for exocytosis in two important steps. The step of docking and tethering of the vesicles requires the assembly of F-actin into meshwork [94], whereas the step of fusion and release of the vesicles requires the disassembly of the actin meshwork [95]. Cdc42 GTPase has been widely implicated both in mastoparan-induced insulin secretion via indirect interaction with Syntaxin 1A [96], and in GSIS via a presumptive role in F-actin dynamics [reviewed in 18-22]. However, Cdc42 does not bind F-actin directly and must do so through effector proteins. Clearly, IQGAP1, which is known as the master regulator of F-actin dynamics [69, 73-75], and serves both as an upstream regulator and a downstream effector of Cdc42, must be a direct player [77, 79]. IQGAP1 assembles the F-actin nucleation and capping machinery, N-WASP, Arp2/3 complex and the formin mDia1, to modulate F-actin dynamics in a Cdc42-regulated manner [69, 73, 74] that mirrors its modulation of GSIS [77]. Therefore, most probably this mechanism represents an important aspect of IQGAP1’s regulation of GSIS (Fig. 3b) and requires further studies. Further investigation is also required into how IQGAP1 downregulates insulin secretion and diverts Akt signal towards cell division. In the following sections, we discuss how IQGAP1 mediates the link between β-cell secretion and mass that underlie its potential mechanism in connecting cancer and diabetes.
3.4. IQGAP1 associates with human carcinomas and controls the cell division cycle
Several studies reported that changes in IQGAP1 expression or localization associates with colon cancer [97], metastatic melanoma [98], glioblastoma [99], gastric carcinoma [100] and thyroid cancer [101]. Furthermore, knockout of IQGAP1 in the mouse produced gastric hyperplasia and lung adenoma [102], and overexpression of IQGAP1 in cell culture caused transformed phenotypes, and induced tumors in mice [78, 103].
Several aspects of IQGAP1 cellular functions suggest that its association with carcinomas represents a cause and not a consequence of tumorigenesis. First, in response to EGF, IQGAP1 becomes serine-phosphorylated and binds active Cdc42-GTP [77, 79], leading to increased cell proliferation and transformed phenotypes [78]. Persistent Cdc42 activity, such as the expression of the constitutively active mutant Cdc42F28L, is oncogenic [104] and IQGAP1 is required for this activity [78]. Second, activated IQGAP1-Cdc42 dissociates epithelial adherens junctions by dissociating α-catenin from the E-cadherin-α-catenin-β-catenin complex, leading to the translocation of β-catenin into the nucleus and the initiation of oncogenic transcriptional events that sustain cell scattering, increased migration and invasion [reviewed in 105]. Third, IQGAP1 has a potential oncogenic transcriptional activity in the nucleus. We showed that expression of IQGAP1-F or IQGAP1-C, which binds active Cdc42-GTP, accelerates the cell cycle by increasing DNA synthesis as measured by the thymidine analog, bromodeoxyuridine (BrdU)-incorporation assay [78]. Indeed, a recent study revealed that IQGAP1 accumulates in the nucleus at G1/S phase where it associates with the DNA replication complex factors RPA32 and PCNA, and that RNAi-depletion of IQGAP1 delayed the cell cycle progression [82]. Fourth, IQGAP1 has an evolutionarily conserved regulatory role in cytokinesis, which we demonstrated first in yeast [91]. We showed that in several cell types, expression of IQGAP1-N or the internal IQGAP1-IR-WW arrests cytokinesis, producing multinucleated cells [78] and that IQGAP1 localizes as a ring in the midbody of dividing cells, suggesting a role in cell abscission, which is the final step in animal cytokinesis [53]. Several lines of evidence from different biological systems support this view. In Chinese hamster ovary (CHO) cells, a proteomic study identified IQGAP among the components of midbody proteins involved in cytokinesis [106]. In mouse oocyte and embryos, IQGAP1 localizes to the cleavage furrows, and disruption of Cdc42 function with Toxin B resulted in delocalizing IQGAP1 and cytokinesis failure [107]. In HeLa cells, IQGAP1 interacts with the endosomal sorting complex required for transport I (ESCRT-I) subunit TSG101, whose depletion inhibits cell abscission [108]. These observations suggest that IQGAP1’s role in directed secretion, which is required for bisecting the daughter cells in yeast [90, 91], is conserved in mammals. As polarized secretion and F-actin dynamics play major roles in directed cell migration, IQGAP1 has also been widely implicated in promoting cell migration and invasive growth of cancer cells, as discussed below
3.5. IQGAP1 controls cell migration and invasive growth of cancer cells
Several studies demonstrated that IQGAP1 promotes cell migration [73, 74, 78] and invasion of cancer cells [109-111]. However, mechanistic analysis of IQGAP1 control of cell migration and invasion, using deletion and phosphorylation point mutants revealed that the dynamics of IQGAP1, which determines its ability to cycle between a Cdc42-GTP-pIQGAP1 and an IQGAP1-exocyst bound forms is crucial for efficient migration and invasion [78]. Thus, unlike the case with its functions in secretion and cell proliferation that reside in opposite domains (see below), IQGAP1 controls cell migration/invasion through a concerted activity of both the C-terminal and the N-terminal domains [78]. Studies in human tumors and cell cultures suggest that this cycling might correlate with changes in the subcellular distribution of IQGAP1. Evidence for this was observed in human colorectal carcinomas where IQGAP1 overexpression and diffuse localization pattern associated with higher rates of distant metastasis and poor prognosis [112]. As discussed earlier overexpression of IQGAP1 leads to increased levels of pIQGAP1S1443 and binding to Cdc42-GTP, and activation of Akt, which could result in delocalizing a pool of IQGAP1 to sustain invasive growth. The molecular basis and exact locations of IQGAP1 aberrant redistribution in tumors requires further scrutiny.
Overall, the mechanisms discussed above underlie IQGAP1 generation of tumors and invasive growth through effector and signaling proteins, including mTORC1-Akt and the components of the canonical Wnt signaling pathway. Furthermore, our work in yeast provided the pivotal clue that IQGAP1 integrates these functions essentially through a signal-controlled coupling of cell growth/size and cytokinesis, which regulates cell homeostasis [68, 53], dysfunction of which underlies its mechanism in tumorigenesis and diabetes, as discussed below.
3. 6. IQGAP1 modulates β-cell proliferation
Mechanistic analyses of IQGAP1 function in the budding yeast revealed that yeast IQGAP1, Iqg1p; links cell growth (polarity) to cytokinesis by linking the bud-site selection and secretion machineries to Cdc42p signaling and to the actin cytoskeleton [89-91]. This function is conserved in mammals, where IQGAP1 promotes cell size by its N-terminal domain and cell proliferation by its C-terminal domain, thus regulating cell homeostasis in several cell types, including pancreatic β-cell lines [53, 77, 78]. This function is regulated in part by phosphorylation of Ser1443 at IQGAP1’s C-terminus, and differential binding to Cdc42-GTP, which in turn determines binding to appropriate partners to promote cell growth or division [53, 77, 78]. Expression of the phospho-mutant IQGAP1S1443A inhibits cell proliferation, whereas the phosphomimetic IQGAP1S1443E increases cell proliferation and induces transformed phenotypes [78]. This function requires the activities of Cdc42, mTORC1 and Akt, as tested by dominant point mutants, RNAi and specific inhibitors [78]. Thus, IQGAP1 fine-tunes mTORC1-Akt1 vs. the MAPK signaling to integrate β-cell proliferation and insulin homeostasis (Figs. 3 &4). Support for this model was evidenced by the finding that expression of the domain that binds mTORC1 and Exo70, and enhances insulin secretion and cell size under full nutrients, attenuates ERK1/2/GSK3α/β and mTORC1/S6K activity while markedly enhancing Akt activity in response to EGF [53, 77, 78]. This evidence supports the concept that IQGAP1 serves as a sensor that modulates mTORC1-MAPK signaling to regulate cell growth and division according to nutrient availability, and that its deregulated activity can bypass the action of nutrient or mitogens to induce cell proliferation, apoptosis or differentiation. This view is consistent with the opposing signals of ERK1/2/GSK3α/β and Akt [113], which IQGAP1 modulates. Whereas GSK3α/β controls apoptosis, lipid and fat metabolism and is a critical Akt substrate where active pAktS473 inhibits GSK3 by phosphorylating Ser21 on GSK3α and Ser9 on GSK3β [114], ERK1/2 promotes cell differentiation [115], thus attenuating their activity suppresses cell differentiation and apoptosis in concordance with the elevated pAktS473 signal that promotes cell proliferation [53]. Consistent with this notion targeted knockdown of GSK3β in remnant pancreas of a 90% pancreatectomized adult rats led to regeneration of β-cells by increasing proliferation, and prevented the acinar cell apoptosis [116]. These data also agree with the evidence that energy starvation activates MAPK p38-regulated/activated kinase (PRAK), which in turn suppresses mTORC1 activity [117]. Overall, these data demonstrate that IQGAP1 signaling integrates modulation of ERK1/2 and Akt1 signaling to appropriately control insulin secretion/cell size or cell proliferation.
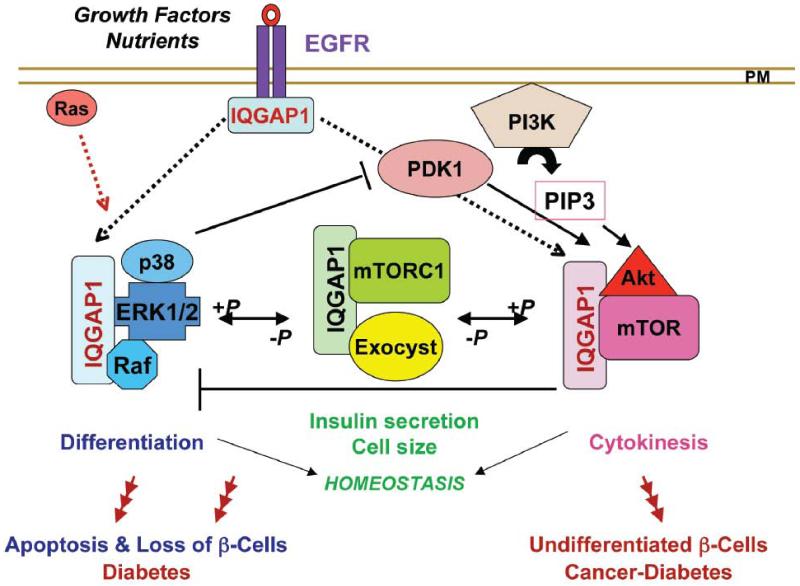
Figure 4. A Model of IQGAP1 Signaling in the link of cancer and diabetes.
IQGAP1 binds receptor tyrosine kinases (RTK) like EGFR [85] directly or indirectly to signal the formation of specialized scaffolds, which regulate cell homeostasis through dynamic phosphorylation [53, 68, 77, 78]. Deregulation of IQGAP1’s signal dynamics can tilt the IQGAP1-mTORC1-Akt1-MAPK pathway to the left or to the right (red arrows with multiple heads) [53, 68, 77, 78]. If tilted to the left via down regulation of IQGAP1 and/or persistence of the un-phosphorylated form and deregulation of the MAPK signal, it leads to increased β-cell size [78] and apoptosis and thus diabetes resulting from loss of β-cells, and explaining why IQGAP1 expression is down regulated in β-cells from a population of diabetic patients. If the pathway is tilted to the right via overexpression of IQGAP1 and/or persistence of the phospho-form and chronic Akt activity, it leads to transformed phenotypes [53, 78], leading to cancer initiation as well as diabetes, resulting from undifferentiated β-cells. Therefore, the nature of the deregulation in the pathway defines different subsets of patients and might be useful for developing personalized medicine.
Several lines of evidence support that elevated IQGAP1 signal, and not just mere expression, promotes cell proliferation and transformation [53, 78]. For example, while the expression of dominant-negative mutants such as IQGAP1IR-WW, the phospho-defective mutant IQGAP1S1443A or the IQGAP1ΔMK24, which disrupts Cdc42 binding, promotes insulin secretion and cell size, they inhibit cell proliferation and generation of transformed phenotypes [53, 77, 78]. By contrast, dominant-active mutants such as IQGAP1-C or the phosphomimetic IQGAP1S1443E, promotes transformed phenotypes and inhibits insulin secretion while reducing the cell size [77, 78]. Furthermore, functional insulinoma β-cells that secrete insulin, contain less pserine-IQGAP1 compared to non-functional undifferentiated insulinomas that are deficient in insulin secretion [53]. Therefore, a critical level of pIQGAP1 appears to be important for balanced cell proliferation, and raises curiosity about its potential utilization in therapeutic β-cell regeneration. Also, these findings highlight the notion of the dynamic link between β-cell function and mass, and support the concept of dedifferentiation as culprit in diabetic β-cell failure [23], at least in a subset of cases, denoted by elevated pIQGAP1.
In summary, the evidence discussed in these sections support the model that IQGAP1 normally serves like a rheostat for adjusting insulin and β-cell homeostasis via a two-step mechanism. At the plasma membrane it regulates GSIS by modulating F-actin-dynamics, interplaying with Cdc42-Arp2/3-N-WASP and Exo70 to control insulin tethering and release while increasing insulin synthesis and translocation by engaging mTORC1 (Fig. 3). Upon phosphorylation, it attenuates the mTORC1/S6K1→Akt1 NFL, disassembles actin meshwork, downregulates insulin secretion, and thus sustaining Akt1 signaling and diverting it towards cell proliferation while suppressing apoptosis and differentiation by attenuating ERK1/2-GSK3 signaling [53]. Factors causing significant shift of the pathway to the right or to the left would deregulate insulin level and result in cell apoptosis or lead to β-cell transformation (undifferentiated state), associated with the pathogenesis of T2D and/or cancer (Fig. 4) and would explain why IQGAP1 is decreased in a subset of human T2D. Moreover, like the case of mTORC1-Akt, IQGAP1-signal dynamics appears crucial to its differential interactions and function, and may have a role in regulating glucose metabolism in target tissues as is discussed below
4. IQGAP1-Exocyst axis as integrator of β-cell mass and function with insulin signaling
The current view that prolonged stress on β-cells associated with the increased insulin secretion accompanying insulin resistance contributes to loss of β-cell mass, and subsequent T2D, underscores the importance of identifying the points of crosstalk that connect β-cell mass/function to insulin signaling. Although mTORC1 has been implicated in both functions, how it coordinates the involved signals remains insufficiently defined [8-11]. Expression of IQGAP1 both in the pancreas and the target tissues, and its differential interactions with the exocyst and the GTPase Rac1, two proteins that have been widely implicated in glucose metabolism by regulating the trafficking of the glucose transporter GLUT4, provides a platform for such coordination. The exocyst is a widely conserved nutrient-regulated hetero-octameric vesicle targeting and membrane-tethering complex required for signal-dependent exocytosis that promotes cell growth [118] and cell abscission [119], thus it couples cell growth and cytokinesis. Serving as effectors for several small GTPases, including Cdc42, Rac1, Tc10, Rab and Rals [68, 119-120], the exocyst promotes insulin secretion [77, 122, 123], insulin synthesis [124-126] and insulin-induced glucose uptake by translocating the glucose transporter Glut4 to the surface [121]. Earlier studies have demonstrated the link of these functions with T2D, as defects in insulin signaling impair Glut4 translocation and lead to insulin resistance [127, 128], thus supporting a role for the exocyst in the pathology of T2D.
Mounting evidence supports that the exocyst collaborates with or antagonizes mTOR to promote anabolism or catabolism to support cell growth under variable nutrient conditions. The exocyst differentially engages the two Ral isoforms, RalA or RalB, to modulate epithelial cell polarity [129] and metabolism. Under nutrient conditions, the assembly of RalA-dependent exocyst is crucial for insulin-regulated delivery of Glut4 to the plasma membrane in adipocytes [121]. By contrast, under nutrient limitation, a RalB-exocyst initiates autophagosome biogenesis, thus triggering autophagy in human epithelial cells [130] to release nutrients. The Autophagy-lysosomal pathway is a central catabolic trafficking pathway responsible for recycling misfold proteins and damaged organelles, thus it impacts T2D, cancer, degenerative disease and aging [131-134]. Active mTORC1 directly suppresses autophagy in presence of nutrients, and thus under low nutrients, the induction of autophagy requires the inhibition of mTORC1 [135]. Because RalA promotes mTORC1 activation [136] and RalA-exocyst promotes glucose metabolism whereas RalB-exocyst initiates autophagy, it appears clear that interplay of RalB/exocyst/RalA/mTORC1 contributes to the mechanisms that integrate anabolism and catabolism in the regulation of body metabolism.
Additionally, because the exocyst subunits exhibit differential localization and distinct functions in different cell types [137, 138] and achieve functional diversity through interactions with different GTPases, they likely provide crucial points of feedback in coordinating body metabolism. Such mechanism likely will involve a Rac1-IQGAP1-exocyst axis, because Rac1, which also binds IQGAP1, has been extensively implicated in GLUT4 transport in the insulin-responsive peripheral tissues [139-141]. Therefore, it is possible to envision that differential engagement of IQGAP1-exocyst with Cdc42 and Rac1 modulates not only insulin synthesis and secretion in β-cells, but also glucose metabolism in the target tissues through modulating mTORC1-MAPK signal, and thus contributes to coordinating body metabolism. This, as these components couple cell growth and division with insulin signaling, the dysfunction of which can mechanistically explain the development of cancer in obesity (Fig. 4). Development of animal models of diabetes for the exocyst components and IQGAP1 should provide valuable insights into these connections.
5. Concluding Remarks
The intimate connection between cancer and diabetes is particularly troubling in light of rising population obesity, especially in children. Deciphering the signaling networks that couple β-cell mass and function with insulin signaling would open avenues for developing selective strategies for treating T2D and its associated cancers. Cell signaling is a dynamic process; therefore, chronically inhibiting or activating mTOR signaling as a therapeutic strategy has been proven ineffective. Identifying the modulators of the pathway and developing drugs accordingly would be an ideal approach. In this respect, the Cdc42-IQGAP1, which serves as a rheostat of the mTORC1-Akt-MAPK appears promising. However several questions remain to be answered before this axis can be targeted for effective therapy. First, the mechanism by which IQGAP1 inhibits insulin will have to be delineated in detail to identify the downstream effectors and whether it couples to the ubiquitin-proteasomal, the autophagy-lysosomal pathways or both. Second, the mechanism by which IQGAP1 specifies AktS743 activity towards cell division must be delineated. Indeed, mTORC2 activation of AktS743 appears to input into activating mTORC1, while IQGAP1, which binds both mTOR and Akt, and regulates cytokinesis, has the features of binding phosphoinositides that would allow it to specify Akt activity towards cytokinesis and cell proliferation through as yet to be identified effectors. Third, IQGAP1 interactions with the exocyst and Rac1, which are widely implicated in regulating glucose metabolism through trafficking of the glucose transporter, positions it as a plausible switch point for integrating insulin signaling in the pancreas and the target tissues such as the liver and the skeletal muscles, and our laboratory is currently exploring these questions. Delineating this pathway could provide valuable insights for ways to enhancing β-cell regeneration, and also for identifying critical points in the mechanisms of insulin action that could be exploited for treating insulin resistance.
In addition to cytoplasmic-nuclear shuttling, regulations by phosphoserine and phosphotyrosine sites, the Cdc42-IQGAP1-mTORC1-Akt pathway is likely to be fine-tuned by isoforms in targets tissues such as antagonism by IQGAP3 in the brain or by IQGAP2 in the liver and/or by non-coding RNAs such as microRNAs. Bioinformatics analyses predict that several microRNAs potentially regulate and be regulated by IQGAP1-mTORC1 pathway in a tissue- and cell cycle-specific manner (Osman, unpublished). These epigenetic mechanisms will have potential as targets for personalized medicine and will require analyses in animal models.
Of particular interest is how dynamic IQGAP1-mTORC1 subcellular localization responds to different stimuli and determines their function, and how deregulated co-localization might define cancer and/or diabetes. In different cell types, we found that mTORC1 and IQGAP1 colocalize to several intracellular compartments, including Golgi, the endoplasmic reticulum, perinuclear compartments and the lysosomes (Osman, unpublished. It is unclear whether these represent specific cellular functions or response to different cues. In response to amino acids mTORC1 distributes to the lysosomes surface [142] and amino acids withdrawal or treatment with Metformin, which mimics amino acid removal, causes the diffusion of mTORC1 throughout the cytoplasm, and thus suppresses mTORC1 signaling in an AMPK-independent manner [147]. Therefore, distinctive localization of IQGAP1 and mTORC1 in health and disease would likely be a marker of their activity and function and must be analyzed in detail.
Acknowledgements
We thank Dr. Gordon Weir, MD (Joslin Diabetes Center, Harvard Medical School) for insightful comments on an earlier draft. We acknowledge Dr. Alan Saltiel’s (University of Michigan) comments on an earlier draft and Dr. Rick Cerione (Cornell, Ithaca) for previous support. Work in the MO lab was supported by grants from NIH-NCI (CA104285) and from ACS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer. 2011;11:886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 2.Wang F, Herrington M, Larsson M, Permert J. The relationship between diabetes and pancreatic cancer. Molecular Cancer 2003. 2013;2(4) doi: 10.1186/1476-4598-2-4. http://www.molecular-cancer.com/content/2/1/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elena JW, Steplowski E, Yu K, Hartge P, et al. Diabetes and risk of pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Cancer Causes Control. 2013;24:13–25. doi: 10.1007/s10552-012-0078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward PS, Thompson CB. Metabolic Reprogramming: A Cancer Hallmark Even Warburg Did Not Anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unraveling the mechanism. Lancet. 2010;375:2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300:2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laplante M, Sabatini DM. mTOR Signaling in Growth Control and Disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 10.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nature Rev Cancer. 2012;12:159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 11.Howell JJ, Manning BD. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol. Metab. 2011;22:94–102. doi: 10.1016/j.tem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weir GC, Marselli L, Marchetti P, Katsuta H, Jung MH, Bonner-Weir S. Towards better understanding of the contributions of overwork and glucotoxicity to the β-cell inadequacy of type 2 diabetes. Diabetes, Obesity and Metabolism. 2009;11(Suppl. 4):82–90. doi: 10.1111/j.1463-1326.2009.01113.x. [DOI] [PubMed] [Google Scholar]
- 13.Bonner-Weir S, Li L, O-Yahalom W-C, Guo L, Weir GC, Sharma A. β-cell growth and regeneration: replication is only part of the story. Diabetes. 2010;59:2340–2348. doi: 10.2337/db10-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonas JC, Sharma A, Hasenkamp W, et al. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem. 1999;274:14112–14121. doi: 10.1074/jbc.274.20.14112. [DOI] [PubMed] [Google Scholar]
- 15.Donath MY, Halban PA. Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia. 2004;47:581–589. doi: 10.1007/s00125-004-1336-4. [DOI] [PubMed] [Google Scholar]
- 16.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutti S, Sauter NS, Bouzakri K, Prazak R, Halban PA, et al. In Vitro Proliferation of Adult Human Beta-Cells. PLoS ONE. 2012;7(4):e35801. doi: 10.1371/journal.pone.0035801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rorsman P, Braun M. Regulation of Insulin Secretion in Human Pancreatic Islets. Annual Rev. Physiol. 2013;75 doi: 10.1146/annurev-physiol-030212-183754. DOI: 10.1146/annurev-physiol-030212-183754. [DOI] [PubMed] [Google Scholar]
- 19.Ingo B, Leibiger B, Per-Olof Berggren. Insulin Signaling in the Pancreatic β-Cell. Annual Review of Nutrition. 2008;28:233–251. doi: 10.1146/annurev.nutr.28.061807.155530. [DOI] [PubMed] [Google Scholar]
- 20.Seino S, Shibasaki T, Minami K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J Clin Invest. 2011;121:2118–2125. doi: 10.1172/JCI45680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Thurmond DC. Mechanisms of biphasic insulin-granule exocytosis-roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci. 2009;122:893–903. doi: 10.1242/jcs.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowluru A. Small G proteins in islet beta-cell function. Endocr Rev. 2010;31:52–78. doi: 10.1210/er.2009-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talchai CS, Xuan HV, Lin L, Sussel L, Accili D. Pancreatic β-Cell Dedifferentiation as a Mechanism of Diabetic β-Cell Failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Zhong J, Inuzuka H, Gao D, Shaik S, Sarkar FH, Wei W. An Evolving Role for DEPTOR in Tumor Development and Progression. Neoplasia. 2012;14:368–375. doi: 10.1593/neo.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 26.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 27.Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 28.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 29.Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem. Soc. Trans. 2009;37:217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Boulbes D, Chen CH, Shaikenov T, Agarwal NK, Peterson TR, Addona TA, Keshishian H, Carr SA, Magnuson MA, Sabatini DM, Sarbassov DD. Rictor phosphorylation on the Thr-1135 site does not require mammalian target of rapamycin complex 2. Mol Cancer Research. 2010;8:896–906. doi: 10.1158/1541-7786.MCR-09-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dibble CC, Asara JM, Manning BD. Characterization of Rictor Phosphorylation Sites Reveals Direct Regulation of mTOR Complex 2 by S6K1. Mol Cell Biol. 2009;29:5657–5670. doi: 10.1128/MCB.00735-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Julien L-A, Carriere A, Moreau J, Roux PP. mTORC1-Activated S6K1 Phosphorylates Rictor on Threonine 1135 and Regulates mTORC2 Signaling. Mol. Cell. Biol. 2010;30:908–921. doi: 10.1128/MCB.00601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gingras A-C, Steven P, Gygi B, Raught RD, Polakiewicz RT, Abraham MF, Hoekstra R. Aebersold, and N Sonenberg, Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes and Development. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livingstone M, Bidinosti M. Rapamycin-insensitive mTORC1 activity controls eIF4E:4E-BP1 binding. [v1; ref status: Indexed http://f1000r.es/NM6hpo] F1000Research. 1(2012) doi: 10.12688/f1000research.1-4.v1. http://f1000r.es/NM6hpo (doi: 10.3410/f1000research.1-4.v1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143:813–25. doi: 10.1016/j.cell.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 38.Polak P, Hall MN. mTOR and the control of whole body metabolism. Curr. Opin. Cell Biol. 2009;21:209–218. doi: 10.1016/j.ceb.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 39.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR Complex 1 Pathway by Nutrients, Growth Factors, and Stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mori H, Inoki K, Opland D, Münzberg H, Villanueva EC, Faouzi M, Ikenoue T, Kwiatkowski DJ, Macdougald OA, Myers MG, Jr, Guan KL. Crucial roles for the TSC-mTOR pathway in b-cell function. Am. J. Physiol. Endocrinol. Metab. 2009;297:E1013–1022. doi: 10.1152/ajpendo.00262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rachdi L, Balcazar N, Osorio-Duque F, Elghazi L, Weiss A, Gould A, Chang-Chen KJ, Gambello MJ, Bernal-Mizrachi E. Disruption of Tsc2 in pancreatic beta cells induces beta cell mass expansion and improved glucose tolerance in a TORC1-dependent manner. Proc. Natl. Acad. Sci. USA. 2008;105:9250–9255. doi: 10.1073/pnas.0803047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shigeyama Y, Kobayashi T, Kido Y, Hashimoto N, Asahara S, Matsuda T, Takeda A, Inoue T, Shibutani Y, Koyanagi M, et al. Biphasic response of pancreatic beta-cell mass to ablation of tuberous sclerosis complex 2 in mice. Mol. Cell. Biol. 2008;28:2971–2979. doi: 10.1128/MCB.01695-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, Klumperman J, Thorens B, Thomas G. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature. 2000;408:994–997. doi: 10.1038/35050135. [DOI] [PubMed] [Google Scholar]
- 44.Elghazi L, Balcazar N, Blandino-Rosano M, Cras-Méneur C, Fatrai S, Gould AP, Chi MM, Moley K>H>, Bernal-Mizrachi E. Decreased IRS signaling impairs beta-cell cycle progression and survival in transgenic mice overexpressing S6K in beta-cells. Diabetes. 2010;59:2390–2399. doi: 10.2337/db09-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fraenkel M, Ketzinel-Gilad Y, Ariav O, Pappo M, Karaca J, Castel M-F, Berthault C, Magnan C, Cerasi E, Kaiser N, Leibowitz G. mTOR inhibition by rapamycin prevents β-Cell adaptation to hyperglycemia and exacerbates the metabolic state in Type 2 Diabetes. Diabetes. 2008;57:945–957. doi: 10.2337/db07-0922. [DOI] [PubMed] [Google Scholar]
- 46.Stallone G, Infante B, Grandaliano G, Gesualdo L. Management of side effects of sirolimus therapy. Transplantation. 2009;87(8, Suppl):S23–S26. doi: 10.1097/TP.0b013e3181a05b7a. [DOI] [PubMed] [Google Scholar]
- 47.Houde VP, Brûlé S, Festuccia WT, Blanchard PG, Bellmann K, Deshaies Y, Marette A. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes. 2010;59:1338–1348. doi: 10.2337/db09-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang GR, Wu YY, Chiu YS, Chen WY, Liao JW, Hsu HM, Chao TH, Hung SW, Mao FC. Long-term Administration of Rapamycin Reduces Adiposity, but Impairs Glucose Tolerance in High-Fat Diet fed KK/HlJ Mice. Blackwell Publishing Ltd.; 2009. pp. 188–198. [DOI] [PubMed] [Google Scholar]
- 49.Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, Vincent JP, Ellston R, Jones D, Sini P, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70:288–298. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 50.Elghazi L, Bernal-Mizrachi E. Akt and PTEN: beta-cell mass and pancreas plasticity. Trends Endocrinol Metab. 2009;20(5):243–51. doi: 10.1016/j.tem.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gu Y, Lindner J, Kumar A, Yuan W, Magnuson MA. Rictor/mTORC2 is essential for maintaining a balance of beta cell proliferation and size. Diabetes. 2011;60:827–837. doi: 10.2337/db10-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernal-Mizrachi E, et al. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet beta cells. J Clin Invest. 2004;114:928–36. doi: 10.1172/JCI20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tekletsadik YK, Sonn R, Osman MA. A Conserved Role for IQGAP1 in Regulating TOR Complex 1. J. Cell Sci. 2012;125:2041–2052. doi: 10.1242/jcs.098947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uhlik MT, Abell AN, Cuevas BD, Nakamura K, Johnson GL. Wiring diagrams of MAPK regulation by MEKK1, 2, and 3. Biochem Cell Biol. 2004;82:658–6. doi: 10.1139/o04-114. [DOI] [PubMed] [Google Scholar]
- 55.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 56.Lim AK, Nikolic-Paterson DJ, Ma FY, Ozols E, Thomas MC, Flavell RA, Davis RJ, Tesch GH. Role of MKK3-p38 MAPK signalling in the development of type 2 diabetes and renal injury in obese db/db mice. Diabetologia. 2009;52(2):347–58. doi: 10.1007/s00125-008-1215-5. [DOI] [PubMed] [Google Scholar]
- 57.Sumara G, Formentini I, Collins S, Sumara I, Windak R, Bodenmiller B, Ramracheya R, Caille D, Jiang H, Platt KA, Meda P, Aebersold R, Rorsman P, Ricci R. Regulation of PKD by the MAPK p38delta in insulin secretion and glucose homeostasis. Cell. 2009;23:235–48. doi: 10.1016/j.cell.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee YH, Giraud J, Davis RJ, White MF. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J Biol Chem. 2003;278:2896–2902. doi: 10.1074/jbc.M208359200. [DOI] [PubMed] [Google Scholar]
- 59.Hirosumi J, Tuncman G,, Chang L,, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 60.Khoo S, Griffen SC, Xia Y, Baer R, German MS, Cobb MH. Regulation of insulin gene transcription by extracellular-signal regulated protein kinases (ERK) 1 and 2 in pancreatic beta cells. J Biol Chem. 2003;278:32969–32977. doi: 10.1074/jbc.M301198200. [DOI] [PubMed] [Google Scholar]
- 61.Lawrence MC, McGlynn K, Park BH, Cobb MH. ERK1/2-dependent activation of transcription factors required for acute and chronic effects of glucose on the insulin gene promoter. J Biol Chem. 2005;280:26751–26759. doi: 10.1074/jbc.M503158200. [DOI] [PubMed] [Google Scholar]
- 62.Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141:150–156. doi: 10.1053/j.gastro.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Madsen LW, Knauf JA, Gotfredsen C, Pilling A, Sjögren I, Andersen S, Andersen L, De Boer AS, Manova K, Barlas A, et al. GLP-1 Receptor Agonists and the Thyroid: C-Cell Effects in Mice Are Mediated via the GLP-1 Receptor and not Associated with RET Activation. Endocrinology. 2012;153:1538–1547. doi: 10.1210/en.2011-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hillaire-Buys J-L, et al. Pioglitazone and bladder cancer. The Lancet. 2011;378:1543–1544. doi: 10.1016/S0140-6736(11)61662-0. [DOI] [PubMed] [Google Scholar]
- 65.Bonanni B, Puntoni M, Cazzaniga M, Pruneri G, Serrano D, Guerrieri-Gonzaga A, Gennari A, Stella M, Trabacca V, Galimberti P, Veronesi H, Johansson V, Aristarco F, Bassi A, Luini M, Lazzeroni C, Varricchio G, Viale P, Bruzzi A. Decensi, Dual effect of metformin on breast cancer proliferation in a randomized presurgical trial. J Clin Oncol. 2012;30:2593–2600. doi: 10.1200/JCO.2011.39.3769. [DOI] [PubMed] [Google Scholar]
- 66.Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, Foskett JK. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bladino GM, Valero M, Cioce F, Mori L, Casadei C, Pulito A, Sacconi F, Biagioni F, Cortese G, Galanti S, Menetti C, Citro G, Muti P, Strano S. Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nature Comm. 2012;3:1859. doi: 10.1038/ncomms1859. doi:10.1038/ncomms1859. [DOI] [PubMed] [Google Scholar]
- 68.Osman MA. An emerging role for IQGAP1 in regulating protein traffic. The Scientific World JOURNAL. 2010;10:944–953. doi: 10.1100/tsw.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brandt DT, Grosse R. Get to grips: steering local actin dynamics with IQGAPs. EMBO Rep. 2007;8:1019–23. doi: 10.1038/sj.embor.7401089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malarkannan S, Awasthi A, Rajasekaran K, Kumar P, Schuldt KM, Bartoszek A, Manoharan N, Goldner NK, Umhoefer CM, Thakar MS. IQGAP1: a regulator of intracellular spacetime relativity. J Immunol. 2012;188:2057–2063. doi: 10.4049/jimmunol.1102439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.White CD, Brown MD, Sacks DB. IQGAPs in Cancer: A Family of Scaffold Proteins Underlying Tumorigenesis. FEBS Lett. 2009;583:1817–1824. doi: 10.1016/j.febslet.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnson M, Sharma M, Henderson BR. IQGAP1 regulation and roles in cancer. Cell Signal. 2009;10:1471–1478. doi: 10.1016/j.cellsig.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 73.Benseñor LB, Kan H-M, Wang N, Wallrabe H, Davidson L, Cai Y, Schafer DA, Bloom GS. IQGAP1 Regulates Cell Motility by Linking Growth Factor Signaling to Actin Assembly. J. Cell Sci. 2007;120:658–669. doi: 10.1242/jcs.03376. [DOI] [PubMed] [Google Scholar]
- 74.Le Clainche C, Schlaepfer D, Ferrari A, Klingauf M, Grohmanova K, Veligodskiy A, Didry D, Le D, Egile C, Carlier M-F, Kroschewski R. IQGAP1 stimulates actin assembly through the N-Wasp-Arp2/3 pathway. J. Biol. Chem. 2007;282:426–435. doi: 10.1074/jbc.M607711200. [DOI] [PubMed] [Google Scholar]
- 75.Pelikan-Conchaudron AC, Clainche Le, Didry D, Carlier M-F. The IQGAP1 Protein is a calmodulin-regulated barbed end capper of actin filaments. J. Biol. Chem. 2011;286:35119–35128. doi: 10.1074/jbc.M111.258772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roy M, Li Z, Sacks DB. IQGAP1 Binds ERK2 and Modulates Its activity. J. Biol Chem. 2004;279:17329–17337. doi: 10.1074/jbc.M308405200. [DOI] [PubMed] [Google Scholar]
- 77.Rittmeyer EN, Daniel S, Hsu SC, Osman MA. A dual role for IQGAP1 in regulating exocytosis. J. Cell Sci. 2008;121:391–408. doi: 10.1242/jcs.016881. [DOI] [PubMed] [Google Scholar]
- 78.Wang J-B, Sonn R, Tekletsadik YK, Samorodnitsky D, Osman MA. IQGAP1 regulates cell proliferation through a novel CDC42-mTOR pathway. J Cell Sci. 2009;122:2024–2033. doi: 10.1242/jcs.044644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grohmanova K, Schlaepfer D, Hess D, Gutierrez P, Beck M, Kroschewski R. Phosphorylation of IQGAP1 modulates its binding to Cdc42, revealing a new type of Rho-GTPase regulator. J. Biol. Chem. 2004;279:48495–48504. doi: 10.1074/jbc.M408113200. [DOI] [PubMed] [Google Scholar]
- 80.Kurella VB, Richard JM, Parke CL, LeCour LF, Jr, Bellamy HD, Worthylake DK. Crystal Structure of the GTPase-activating Protein-related Domain from IQGAP1. J. of Biol Chem. 2009;284:14857–14865. doi: 10.1074/jbc.M808974200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clevers H, Nusse R. Wnt/β-Catenin Signaling and Disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 82.Johnson M, Sharma M, Brocardo MG, Henderson BR. IQGAP1 translocates to the nucleus in early S-phase and contributes to cell cycle progression after DNA replication arrest. Intl J. of Biochem and Cell Biol. 2011;43:65–73. doi: 10.1016/j.biocel.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 83.Dixon MJ, Gray A, Schenning M, Agacan M, Tempel W, Tong Y, Nedyalkova L, Park H-W, Leslie NR, van Aalten DMF, Downes CP, Batty IH. IQGAP Proteins Reveal An Atypical Phosphoinositide (aPI) Binding Domain With a Pseudo C2 Domain Fold. J. Biol. Chem. 2012;287:22483–22496. doi: 10.1074/jbc.M112.352773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown MD, Sacks DB. Protein scaffolds in MAP kinase signaling. Cell Signal. 2009;21:462–469. doi: 10.1016/j.cellsig.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McNulty DE, Li Z, White CD, Sacks DB, Annan RS. MAPK scaffold IQGAP1 binds the EGF receptor and modulates its activation. J Biol Chem. 2011;286:15010–15021. doi: 10.1074/jbc.M111.227694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sbroggiò M, Carnevaleb D, Berteroa A, Cifelli G, Blasioa ED, Mascio G, Hirsch E, Bahou WF, Turco E, Silengo L, Brancaccio M, Lembo G, Tarone G. IQGAP1 regulates ERK1/2 and AKT signaling in the heart and sustains functional remodeling upon pressure overload. Cardiovasc Res. 2011;91:456–464. doi: 10.1093/cvr/cvr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 88.Psatha MI, Razi M, Koffer A, Moss SE, Sacks DB, Bolsover SR. Targeting of calcium:calmodulin signals to the cytoskeleton by IQGAP1. Cell Calc. 2007;41:593–605. doi: 10.1016/j.ceca.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 89.Osman MA, Cerione RA. Iqg1p, a yeast homologue of the mammalian IQGAPs, mediates cdc42p effects on the actin cytoskeleton. J. Cell Biol. 1998;142:443–455. doi: 10.1083/jcb.142.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Osman MA, Cerione RA. Actin doesn’t do the locomotion. Secretion drives cell polarization. In: Segev N, editor. Protein Trafficking: Mechanisms & Regulation. Landes Bioscience/Eurekah.com; Georgetown, Texas: 2006. Eurekah.com [Google Scholar]
- 91.Osman MA, Konopka JB, Cerione RA. Iqg1p links spatial and secretion landmarks to polarity and cytokinesis. J. Cell Biol. 2002;159:601–611. doi: 10.1083/jcb.200205084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marselli L, Thorne J, Ahn YB, Omer A, Sgroi DC, Libermann T,H, Otu H, Sharma A, Bonner-Weir S, Weir GC. Gene expression of purified beta-cell tissue obtained from human pancreas with laser capture microdissection. J Clin Endocrinol Metab. 2008;93:1046–53. doi: 10.1210/jc.2007-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ohara-Imaizumi M, Nishiwaki C, Kikuta T, Nagai S, Nakamichi Y, Nagamatsu S. TIRF imaging of docking and fusion of single insulin granule motion in primary rat pancreatic β-cells: different behaviour of granule motion between normal and Goto–Kakizaki diabetic rat β-cells. Biochem. J. 2004;381:13–18. doi: 10.1042/BJ20040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lopez JA, Burchfield JG, Blair DH, Mele K, Ng Y, Vallotton P, James DE, Hughes WE. Identification of a distal GLUT4 trafficking event controlled by actin polymerization. Mol Biol Cell. 2009;20:3918–29. doi: 10.1091/mbc.E09-03-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vardjan N, Stenovec M, Jorgacevski J, Kreft M, Grilc S, Zorec R. The fusion pore and vesicle cargo discharge modulation. Ann NY Acad Sci. 2009;1152:135–44. doi: 10.1111/j.1749-6632.2008.04007.x. [DOI] [PubMed] [Google Scholar]
- 96.Daniel S, Noda M, Cerione RA, Sharp GW. A link between Cdc42 and syntaxin is involved in mastoparan-stimulated insulin release. Biochemistry. 2002;41:9663–9671. doi: 10.1021/bi025604p. [DOI] [PubMed] [Google Scholar]
- 97.Nabeshima K, Shimao Y, Inoue T, Koono M. Immunohistochemical analysis of IQGAP1 expression in human colorectal carcinomas: its overexpression in carcinomas and association with invasion fronts. Cancer Let. 2002;176:101–109. doi: 10.1016/s0304-3835(01)00742-x. [DOI] [PubMed] [Google Scholar]
- 98.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 99.Balenci L, Clarke ID, Dirks PB, Assard N, Ducray F, Jouvet A, Belin MF, Honnor J. IQGAP1 protein specifies amplifying cancer cells in glioblastoma multiforme. Cancer Res. 2006;66:9074–82. doi: 10.1158/0008-5472.CAN-06-0761. [DOI] [PubMed] [Google Scholar]
- 100.Takemoto H, Yuchiro D, Shiozaki H, Imamura H, Utsunomya T, Miyata H, Yano M, Inoue M, Fujiwara Y, Monden M. Localization of IQGAP1 is inversely correlated with intercellular adhesion mediated by E-cadherin in gastric cancers. Int. J. Cancer. 2001;91:783–788. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1121>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 101.Liu Z, Liu D, Bojdani E, EEl-Naggar AK, Vasko V, Xing M. IQGAP1 plays an important role in the invasiveness of thyroid cancer. Clin Cancer Res. 2010;15(16):6009–6018. doi: 10.1158/1078-0432.CCR-10-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li S, Wang Q, Chakladar A, Bronson RT, Bernards A. Gastric hyperplasia in mice lacking the putative Cdc42 effector IQGAP1. Mol. Bio Cell. 2000;20:697–701. doi: 10.1128/mcb.20.2.697-701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jadeski L, Mataraza JM, Jeong H-W, Li Z, Sacks DB. IQGAP1 stimulates proliferation and enhances tumorigenesis of human breast epithelial Cells. J. Bio.l Chem. 2007;283:1008–1017. doi: 10.1074/jbc.M708466200. [DOI] [PubMed] [Google Scholar]
- 104.Lin R, Bagrodia S, Cerione RA, Manor D. A novel Cdc42Hs mutant induces cellular transformation. Curr. Biol. 1997;7:794–797. doi: 10.1016/s0960-9822(06)00338-1. [DOI] [PubMed] [Google Scholar]
- 105.Noritake U, Watanabe T, Sato K, Wang S, Kaibuchi K. IQGAP1: a key regulator of adhesion and migration. J. Cell Sci. 2005;118:2085–2092. doi: 10.1242/jcs.02379. [DOI] [PubMed] [Google Scholar]
- 106.Skop AR, Liu H, Yates J, Meyer BJ, Heald R. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science. 2004;305:61–66. doi: 10.1126/science.1097931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bielak-Zmijewska A, Kolano A, Szczepanska K, Maleszewski M, Borsuk E. Cdc42 protein acts upstream of IQGAP1 and regulates cytokinesis in mouse oocytes and embryos. Developmental Biology. 2008;322:21–32. doi: 10.1016/j.ydbio.2008.06.039. [DOI] [PubMed] [Google Scholar]
- 108.Morita E, Sandrin V, Chung HY, et al. Human ESCRT and ALIX proteins interact with proteins of themidbody and function in cytokinesis. EMBO Journal. 2007;26:4215–4227. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mataraza JM, Briggs MW, Li Z, Entwistle A, Ridley AJ, Sacks DB. IQGAP1 Promotes Cell Motility and Invasion. J. Biol. Chem. 2003;278:41237–41245. doi: 10.1074/jbc.M304838200. [DOI] [PubMed] [Google Scholar]
- 110.Sakurai-Yageta M, Recchi C, Le Dez G, Sibarita J-B, Daviet L, Camonis C, D’Souza-Schorey C, Chavrier P. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J. Cell Biol. 2008;181:986–998. doi: 10.1083/jcb.200709076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Casteel DE, Turner S, Schwappacher R, Rangaswami H, Su-You J, Zhuang S, Booss GR, B R. Pilz, Rho Isoform-specific Interaction with IQGAP1 Promotes Breast Cancer Cell Proliferation and Migration. J. Biol. Chem. 2012;287:38367–38378. doi: 10.1074/jbc.M112.377499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hayashi H, Nabeshima K, Aoki M, Hamasaki M, Enatsu S, Yamauchi Y, Yamashita Y, Iwasaki H. Overexpression of IQGAP1 in advanced colorectal cancer correlates with poor prognosi-critical role in tumor invasion. Int. J. Cancer. 2010;126:2563–2574. doi: 10.1002/ijc.24987. [DOI] [PubMed] [Google Scholar]
- 113.Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, Bargou RC, Qin J, Lai CC, Tsai FJ, Tsai CH, Hung MC. Erk Associates with and Primes GSK-3 for Its Inactivation Resulting in upregulation of β-Catenin. Mol. Cell. 2005;19:159–170. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 114.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 115.Minden A, Karin M. Regulation and function of the JNK subgroup of MAP kinases. Biochim Biophys Acta. 1997;1333:F85–104. doi: 10.1016/s0304-419x(97)00018-8. [DOI] [PubMed] [Google Scholar]
- 116.Figeac F, Ilias A, Bailbe D,, Portha B, Movassat J. Local In Vivo GSK3β Knockdown Promotes Pancreatic β-Cell and Acinar Cell Regeneration in 90% Pancreatectomized Rat. Mol Ther. 2012;20:1944–1952. doi: 10.1038/mt.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zheng M, Wang Y-H, Wu X-N, Wu S-Q, Lu B-Ju, Dong M-Q, Zhang H, Sun P/, Lin S-C, Guan K-L, Han J. Inactivation of Rheb by PRAK-mediated phosphorylation is essential for energy-depletion-induced suppression of mTORC1. Nat Cell Biol. 2011;13:263–272. doi: 10.1038/ncb2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.He B, Guo W. The exocyst complex in polarized exocytosis. Curr Opin Cell Biol. 2009;21(4):537–42. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gromley A, Yeaman C, Rosa J, Redick S, Chen C-T, Mirabelle S, Guha M, Sillibourne J, Doxsey SJ. Centriolin anchoring of exocyst and SNARE complexes at midbody is required for secretory vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 120.Essida M, Gopaldassa N, Yoshidab K, Merrifieldb C, Soldati T. Rab8a regulates the exocyst-mediated kiss-and-run discharge of the Dictyostelium contractile vacuole. Mol. Biol. Cell. 2012;23:1267–1282. doi: 10.1091/mbc.E11-06-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen XW, Saltiel AR. Ral’s engagement with the exocyst: breaking up is hard to do. Cell Cycle. 2011;10:2299–304. doi: 10.4161/cc.10.14.16524. [DOI] [PubMed] [Google Scholar]
- 122.Tsuboi T, Ravier MA, Xie H, Ewart MA, Gould GW, et al. Mammalian exocyst complex is required for the docking step of insulin vesicle exocytosis. J. Biol. Chem. 2005;280:25565–25570. doi: 10.1074/jbc.M501674200. [DOI] [PubMed] [Google Scholar]
- 123.Saito T, Shibasaki T, Seino S. Involvement of Exoc3l, a protein structurally related to the exocyst subunit Sec6, in insulin secretion. Biomed Res. 2008;29:85–91. doi: 10.2220/biomedres.29.85. [DOI] [PubMed] [Google Scholar]
- 124.Lipschutz JH, Lingappa VR, Mostov KE. The exocyst affects protein synthesis by acting on the translocation machinery of the endoplasmic reticulum. J. Biol. Chem. 2003;278:20954–20960. doi: 10.1074/jbc.M213210200. [DOI] [PubMed] [Google Scholar]
- 125.Toikkanen JH, Miller KJ, Soderlund H, Jantti, Keranen S. The β subunit of the Sec61p endoplasmic reticulum translocon interacts with the exocyst complex in Saccharomyces cerevisiae. J. Biol. Chem. 2003;278:20946–20953. doi: 10.1074/jbc.M213111200. [DOI] [PubMed] [Google Scholar]
- 126.Wiederkehr A, Du Y, Pypaert M, Ferro-Novick S, Novick P. Sec3p is needed for the spatial regulation of secretion and for the inheritance of the cortical endoplasmic reticulum. Mol. Biol. Cell. 2003;14:4770–4782. doi: 10.1091/mbc.E03-04-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ciaraldi TP, Abrams L, Nikoulina S, Mudaliar S, Henry RR. Glucose transport in cultured human skeletal muscle cells. Regulation by insulin and glucose in nondiabetic and non-insulin-dependent diabetes mellitus subjects. J. Clin. Invest. 1995;96:2820–2827. doi: 10.1172/JCI118352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Garvey WT, Maianu L, Zhu JH, Brechtel-Hook G, Wallace P, Baron AD. Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J. Clin. Invest. 1998;101:2377–2386. doi: 10.1172/JCI1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hazelett CC, Sheffb D, Yeaman C. RalA and RalB differentially regulate development of epithelial tight junctions. Mol. Biol. Cell. 2011;22:4787–4800. doi: 10.1091/mbc.E11-07-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bodemann BO, Orvedah A, Cheng T, Ram RR, Yi-Hung Ou, Formstecher E, Maiti M, Hazelett CC, Wauson EM, Balakireva M, Camonis JH, Yeaman C, Levine B, White MA. RalB and the Exocyst Mediate the Cellular Starvation Response by Direct Activation of Autophagosome Assembly. Cell. 2011;144:253–267. doi: 10.1016/j.cell.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Longatti A, Tooze SA. Vesicular trafficking and autophagosome formation. Cell Death Differ. 2009;16:956–965. doi: 10.1038/cdd.2009.39. [DOI] [PubMed] [Google Scholar]
- 133.Samue VT, Shulman GI. Mechanisms for Insulin Resistance: Common Threads and Missing Links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Alers S, SLöffler A, Wesselborg S, Stork B. The incredible ULKs. Cell Comm. and Signal. 2012;10(7) doi: 10.1186/1478-811X-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Junget CH, et al. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maehama T, Tanaka M, Nishina H, Murakami M, Kanaho Y, Hanada K. RalA functions as an indispensable signal mediator for the nutrient sensing system. J. Biol. Chem. 2008;283:35053–35059. doi: 10.1074/jbc.M805822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gerges NZ, Bakos DS, Rupasinghe CN, Spaller MR, Estepan JA. Dual role of the exocyst in AMPA receptor targeting and insertion into the postsynaptic membrane. EMBO J. 2006;25:1623–1634. doi: 10.1038/sj.emboj.7601065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hazelett CC, Yeaman C. Sec5 and Exo84 Mediate Distinct Aspects of RalA-Dependent Cell Polarization. PLoS ONE. 2012;7(6):e39602. doi: 10.1371/journal.pone.0039602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ueda S, Kitazawa S, Ishida K, Nishikawa Y, Matsui M, Matsumuto H, Aoki T, Nozaki S, Takeda T, Tamori Y, Aiba A, Kahn CR, Kataoka T, Satoh T. Crucial role of the small GTPase Rac1 in insulin-stimulated translocation of glucose transporter 4 to the mouse skeletal muscle sarcolemma. FASEB J. 2010;24:2254–61. doi: 10.1096/fj.09-137380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chiu TT, Jensen TE, Sylow L, Richter EA, Klip A. Rac1 signalling towards GLUT4/glucose uptake in skeletal muscle. Cell Signal. 2011;23:1546–54. doi: 10.1016/j.cellsig.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 141.Balamatsias D, Kong AM, Waters JE, Sriratana A, Gurung R, Bailey CG, Rasko JE, Tiganis T, Macaulay SL, Mitchell CA. Identification of P-Rex1 as a novel Rac1-guanine nucleotide exchange factor (GEF) that promotes actin remodeling and GLUT4 protein trafficking in adipocytes. J Biol Chem. 2011;286:43229–40. doi: 10.1074/jbc.M111.306621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]