Abstract
Nitric oxide (NO) has many important physiological roles in the body. Since NO electrodes can directly measure NO concentration in the nM range and in real time, NO electrode methods have been generally used in laboratories for measuring NO concentration in vivo and in vitro. This review focuses on the application of electrode methods in studies of NO diffusion and metabolic kinetics. We have described the physical and chemical properties that need to be considered in the preparation of NO stock solution, discussed the effect of several interfering factors on the measured curves of NO concentration that need to be eliminated in the experimental setup for NO measurements, and provided an overview of the application of NO electrode methods in measuring NO diffusion and metabolic kinetics in solution and in biological systems. This overview covers NO metabolism by oxygen (O2), superoxide, heme proteins, cells and tissues. Important conclusions and physiological implication of these studies are discussed.
Keywords: NO kinetics, NO electrode, NO metabolism and diffusion, nitrosothiol, superoxide, peroxynitrite
1. Introduction
Nitric oxide (NO) is a potent vasodilator and plays an important physiological role in the body [1, 2]. NO can be endogenously generated from NO synthases (NOS) or through other enzyme [3-5] and non-enzyme pathways [6, 7] in tissues. The physiological role of NO is dependent on the effective NO concentration or NO bioavailability. Lack of NO bioavailability is associated with certain cardiovascular diseases, while excess NO production may also cause tissue injury [8-10]. NO can be oxidized by O2 in solution, and the rate of NO autoxidation is first-order with respect to [O2] and second-order with respect to [NO] [11-14]. The half-life of NO in solution under a constant O2 concentration is inversely proportional to the initial NO concentration. Based on the measured rate constant of NO autoxidation, it can be predicted that the half-life of NO with a concentration below 1 μM should be longer than 10 minutes. However, NO half-life in tissues or in the body is much shorter (0.09-2 seconds) [15-18], indicating that NO is very unstable in biological systems. It is well known that NO can rapidly react with superoxide, hemoglobin (Hb), myoglobin (Mb), and some other heme proteins in the body [9, 19-23]. Therefore, the NO bioavailability in the body is not only dependent on the rate of NO generation, but also greatly affected by the rate of NO metabolism. In some cardiovascular diseases, lack of NO bioavailability is mainly caused by the increased rate of NO consumption in the vascular wall [24]. Studies of NO consumption and metabolism kinetics are critical for better understanding the causes resulting in lack of NO bioavailability in cardiovascular diseases.
Various detection methods and techniques such as the Griess method, the fluorimetric method, EPR, chemiluminescence and electrochemical sensing have been used in studies of NO reaction kinetics [25-27]. The limits of different NO detection techniques have been summarized in a recent review article [28]. Each technique has its own advantages and disadvantages. The advantage of NO electrochemical sensors is the ability to directly detect NO concentration in solution or in biological samples with the lowest detection limit of nM or below [29]. This advantage allows NO electrodes to be an excellent tool for directly measuring NO concentration. Previous review articles have described the design and fabrication of NO electrodes and their applications in detections of NO generation in biological systems [30]. This review will focus on the application of NO electrodes in measuring NO metabolism kinetics. Accordingly, the experimental setup, possible interference, and analysis of experimental data will be discussed.
2. Physical and chemical properties of NO in water and preparation of NO stock solution
2.1. Solubility of NO in buffer and the concentration equilibrium between solution and gas phase
NO is a colorless diatomic free radical. Its saturation concentration in water at 20-25 °C is ~2 mM [31] and 1.7-1.8 mM in buffer solution [31, 32] under 1 atm NO pressure (PNO). Since NO can react with O2, it is important to remove O2 from the solution before preparation of NO stock solution. In the absence of O2, the saturated NO concentration is stable in a closed glass container [33] for several months without a significant decrease in concentration. As a gas in the closed container, PNO in solution always tends to reach equilibrium with PNO in the gas phase. If NO in gas phase is removed, there will be a net rate of NO diffusion from the solution into the gas phase. In contrast, if PNO in the gas phase increases, there will be a net rate of NO dissolution into the solution. Thus, even if NO is not consumed in solution by a chemical reaction, NO concentration will change if PNO in the gas phase is different from PNO in the solution.
2.2. Autoxidation of NO
When NO reacts with O2 in the gas phase, the final product is •NO2, as seen below [34]:
This reaction rate follows third-order kinetics:
ONOO• and N2O2 are involved as intermediates and other intermediates, such as N2O3, ONOONO and N2O4 may be also formed [34, 35]. The final product of NO autoxidation in aerated solution is nitrite. The overall reaction is:
This reaction also follows third-order kinetics [11-14, 36]:
| (1) |
The intermediates ONOO•, •NO2, ONOONO, N2O4 and N2O3 are involved or suggested in the reaction[14, 35]. In the presence of a constant concentration of O2, the rate of NO decay is second-order with respect to [NO], so the relationship of [NO] with time t can be obtained from Eq. (1):
| (2) |
The reported kNO at 37 °C is ~1×107 M-2s-1 at 37 °C [12, 33]. Assuming that O2 concentration is 200 μM, the apparent rate constant of NO autoxidation will be 2×103 M-1s-1. The half-life of NO autoxidation can be derived from Eq. (2):
| (3) |
The half-life of NO follows second-order kinetics and therefore, is inversely proportional to the initial NO concentration. This means that NO half-life increases 10-fold when the initial NO concentration decreases 10 times. If initial NO concentrations are 10 and 0.1 μM, their half-life in the solution will be 50 and 5000 seconds, respectively. However, in actual measurements, the half-life of 0.1 μM NO in the solution is much shorter than the predicted half-life because NO can diffuse out of the solution.
2.3. Preparation of NO stock solution
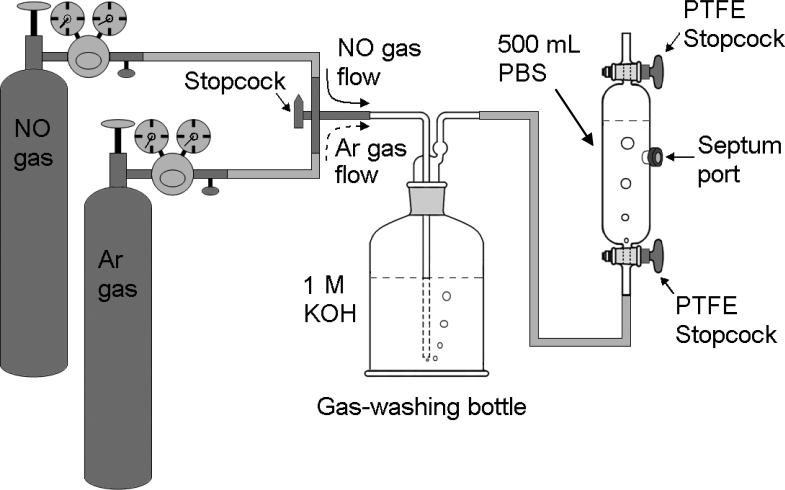
While NO can be generated from NO donors [37, 38] or by mixing nitrite with sulfuric acid [32] in the presence of KI [29], it is convenient to make NO stock solution from commercial NO gas [16, 29, 39-41]. The setup for preparation of NO stock solution consists of a container holding buffer solution and a bottle containing concentrated alkaline solution for removing other nitrogen oxides from the commercial NO gas. The container and the bottle are connected with a tank of NO gas and a tank of N2 or argon (Ar) gas as shown in Fig. 1. The whole system is flashed by nitrogen or argon gas for 30 minutes to remove O2 from the connecting tubing, the alkaline solution (NO-prewashing solution) in the bottle, and the buffer solution in the container. Then, the gas switch is turned from nitrogen or argon gas to NO gas allowing NO gas to flow through the alkaline solution and the buffer solution for about 15-30 minutes. The commercial NO gas usually contains some other nitrogen oxides such as NO2, N2O4 and N2O3 that can be dissolved into the solution to form nitrite (NO2-), nitrate (NO3-) and proton (H+) to acidify the solution. In acidic solution, nitrite forms the volatile nitrous acid HNO2, which will be carried away with the washed NO gas to enter the NO stock solution and raise the nitrite level in the NO stock solution. Therefore, the highly concentrated alkaline solution is required as the NO-prewashing solution to neutralize the released H+. However, the NO-prewashing process cannot remove all other nitrogen oxides from each NO gas bubble through the NO-washing solution. The remaining nitrogen oxides may acidify the NO stock solution if distilled water (no buffer) is used for preparation of the NO stock solution. Therefore, if one desires the NO stock solution to be in the neutral pH range, it is better to use a buffer solution instead of distilled water. The proper NO bubbling time needs to be considered in the preparation of NO solution. If the bubbling time is too short, NO is not saturated in the solution; however, if the bubbling time is too long, nitrite and nitrate will be accumulated in the NO stock solution. Preparation of saturated NO stock concentration is convenient for experiments because the concentration of NO in a saturated solution at room temperature is known. In some cases, to minimize nitrite contamination in the NO stock solution, the NO bubbling time can be reduced to 1 or 2 minutes to make an unsaturated NO stock solution. The concentration of the unsaturated NO stock solution can be calibrated with a known concentration of Hb or Mb on a UV/Vis spectrophotometer [42].
Fig. 1.
Illustration of the setup for preparation of NO stock solution in the Septum Port Gas Sampling Tube. A gas-washing bottle containing alkaline solution is placed between the gas tanks and the gas sampling tube. The whole system is flushed with N2 or Ar gas for 30 minutes to remove O2 from connecting tubing, the alkaline solution (NO-prewashing solution) in the bottle and the buffer solution in the gas sampling tube. Then the gas switch is turned from N2 or Ar gas to NO gas allowing NO gas to go through the alkaline solution in the gas-washing bottle and the buffer solution in the gas-sampling tube for about 15-30 minutes.
3. In vitro NO measurements: experimental setups and interfering factors
3.1. Experimental setups
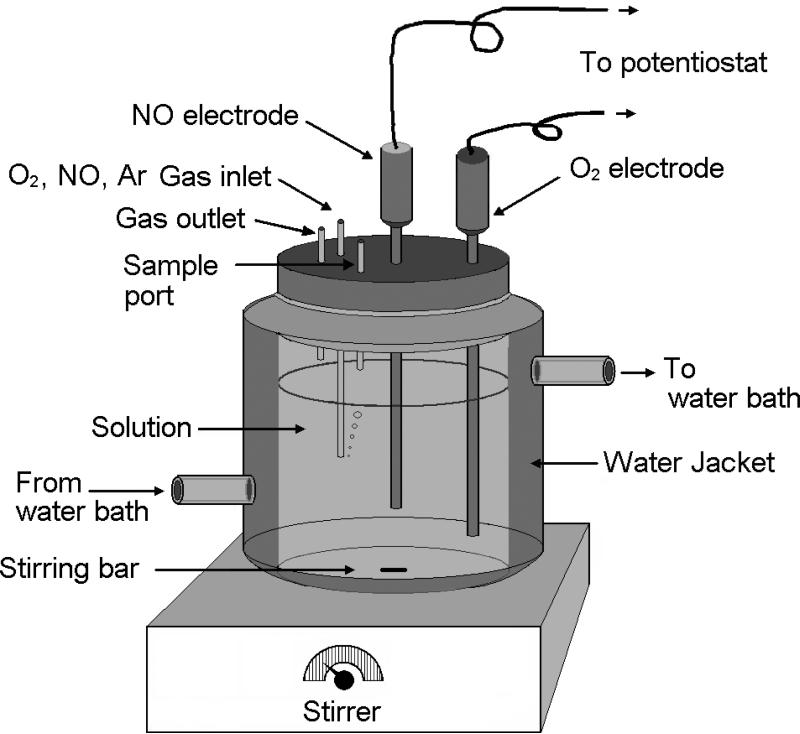
Several experimental setups for measurements of NO reaction and/or diffusion in vitro [43-47] and in vivo [48] have been illustrated in the literature. Fig. 2 shows a typical experimental setup for measuring NO metabolism kinetics in vitro. This setup has a magnetic stirrer that can stir the solution at a constant and controlled speed, a water circulation bath to control the temperature of the solution, two ports for installing NO and O2 electrodes, and three small tubes on the cap for sample injection, gas inlet and gas outlet. Since O2 can penetrate most kinds of polymer tubing, it is better to use only glass or stainless steel tubing and vessels and stainless steel tubing should be connected to glass components in the experimental setup. Three-electrode systems have also been used in the measurements of NO concentrations [39, 49, 50]. Design, fabrication, and analytical performance characteristics of NO electrodes are not described here. Interested readers can refer other review articles [29, 30, 51].
Fig. 2.
Illustration of the water-jacketed chamber for measuring NO and O2 concentration in the solution. The solution is stirred with a stirring bar that is controlled by a magnetic stirrer underneath. A water circulation bath connects to the chamber to control and maintain a constant temperature of the test solution. O2 gas, N2 gas or O2/N2 gas mixture can be introduced into the chamber through the gas inlet on the cap of the chamber to control and maintain a certain O2 partial pressure in the chamber. An O2 electrode and a NO electrode are inserted in the test solution to monitor the O2 and NO concentrations in the solution.
3.2. Interference factors in NO measurements
Constant potential amperometry is the main method used for continuously recording the change of NO concentration with time. At this constant potential, NO is oxidized into nitrite and then further oxidized into nitrate. Several different factors can interfere with the detected currents. The interfering factors for NO detection in living systems have been summarized in a review article [51]. The pitfalls and perils of applying electrochemistry to measure another similar gas molecule, O2, in biological systems were discussed in a classic reference [52]. When measurements of NO metabolism kinetics are performed in a chamber in vitro, additional interfering factors may apply. To obtain good experimental data from an in vitro experiment recording NO generation or decay in the solution, knowledge on how to minimize these interfering factors needs to be acquired.
3.2.1. Background currents
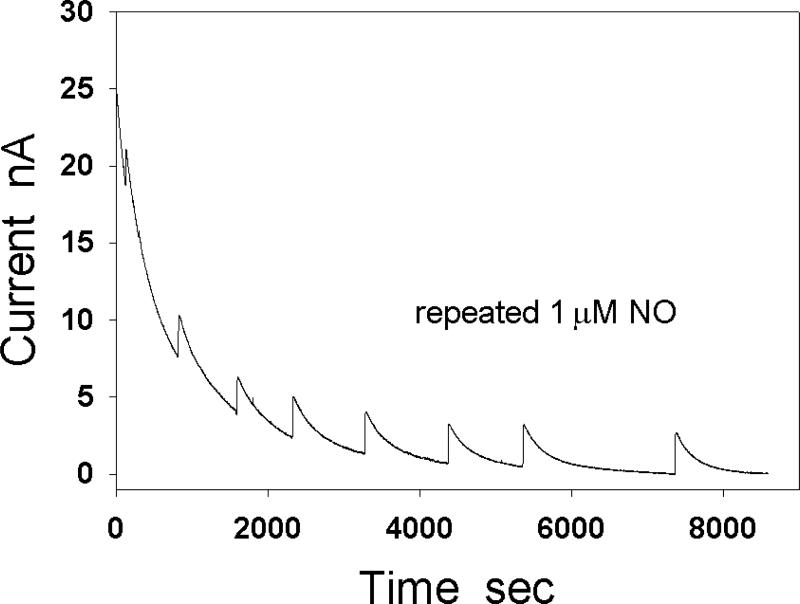
When a constant potential is applied to a NO electrode, it causes a large background current at the electrode even when there is no NO in the solution. The background current consists of non-Faradaic currents such as capacitive charging currents and Faradaic currents such as electrolysis of contaminates on the electrodes or in the solution. This current rapidly decreases at the beginning, and then gradually slows down its pace. After NO is added into the solution, the electrode current will be the sum of the NO oxidation current and the background current. If the background current is nearly a constant, the change in the electrode current will be mainly dependent on the changes of NO concentration in the solution. However, if the change in magnitude of background current is comparable to or greater than the change in magnitude of NO oxidation current, the recorded change in electrode current may seriously deviate from the NO oxidation current. In this case, it may not be possible to obtain correct reaction kinetic constants from the recorded current curves. To record a qualified NO oxidation current, the slope of the baseline needs to approach zero. Since the size of the NO electrode is small and the physiological NO concentration is usually below μM, the NO oxidation current at the NO electrode is usually in the range of low nA or sub nA (Fig. 3). The process of stabilizing the electrode for the first measurement of NO concentration on each day requires ten minutes to a several hours depending on the electrodes used.
Fig. 3.
Effect of background current on the detected NO oxidation current at a NO electrode. NO (1 μM) was repeatedly injected into the test solution during the baseline stabilization.
3.2.2. Stirring and sample injection in test solutions
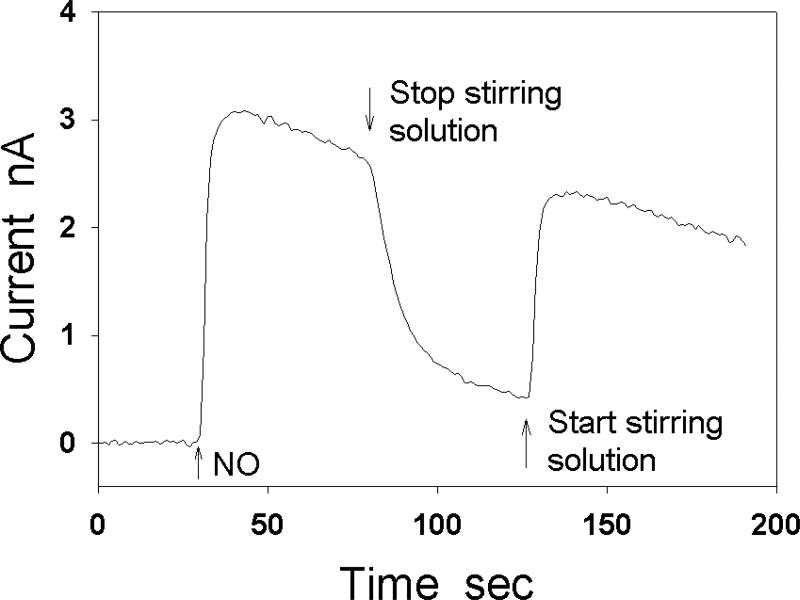
When injecting a sample solution into the chamber containing the test solution, the injected solution causes a local and transient convection in the test solution. This transient convection can affect the thickness of the effective diffusion layer surrounding the electrode and may generate an artificial current peak in amperometric measurements, especially when the electrode response time is quick. If NO stock solution is slowly injected into the aerated test solution at a location far from the detection electrode without significantly stirring the test solution, the injected NO will have a high local concentration in the test solution that can be quickly oxidized by O2 before reaching the NO electrode. Thus, the injected NO may not be detectable by the NO electrode unless the injection location is close to the detection electrode [53]. Rapidly stirring the solution with a magnetic bar at a constant speed can largely reduce the interference of sample injection. The rapid stirring not only quickly and uniformly distributes the added sample in the whole solution, but also forces the solution to move at a relatively high constant speed so that the solution movement caused by the injection of samples can be ignored [33]. Maintaining a constant stirring speed during measurements of NO is important [47, 51, 54, 55], because the stirring speed can affect the thickness of the diffusion layer in the test solution adjunct to the electrode, which may significantly change the amplitude of electrode current (Fig. 4).
Fig. 4.
Effect of stirring on the detected NO oxidation current at a carbon cylindrical electrode (200 μm in diameter). After the stirring is stopped, the detected current is reduced by 80%.
3.2.3. NO volatilization from the solution
To obtain a stable NO concentration in the solution, one needs not only to remove O2 from the chamber including the solution and the gas phase but also to maintain a constant PNO in the gas phase or eliminate the space of the gas phase in the chamber [33, 56]. When PNO in the gas phase is smaller than PNO in the solution, more NO will volatilize into the gas phase. The rate constant (kd) of NO volatilization out of the solution is proportional to the surface of the solution and inversely proportional to volume of the solution [12]. Stirring the solution can increase kd. NO volatilization may become the main cause of NO loss in the solution when there is no O2 and other NO scavengers in the solution or when low concentration of NO (below μM) is in aerated solution. In stirred aerated solution, the half-life of NO volatilization may be in the low minutes [33], but the half-life of 100 μM NO autoxidation is less than 10 seconds. In this situation, the main NO consumption is attributed to NO autoxidation. However, if NO concentration is in the μM range or below, the rate of NO autoxidation will be close to or even slower than the rate of NO volatilization. In this case, NO volatilization will have a significant effect on the concentration curve of NO decay. NO volatilization can be largely prevented by eliminating the headspace above the test solution [33].
3.2.4. Temperature Changes
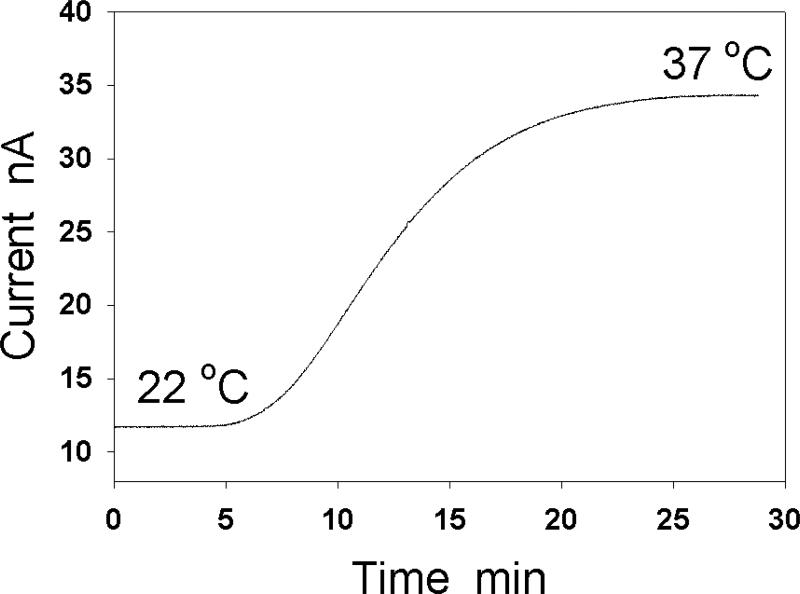
Electrode current is sensitive to temperature change. If temperature in a test chamber is held at a value different from room temperature by a circulating water bath, replacing solution in the chamber after a measurement may cause temperature changes in the test solution. Additionally, adding a large amount of sample solution to the chamber and/or if the sample solution has a large temperature difference from the test solution in the chamber may also cause temperature changes. (Fig. 5).
Fig. 5.
Effect of temperature on the detected current by a NO electrode. When the temperature of the test solution increases from 22 °C to 37 °C, the electrode current is raised by nearly 3 fold.
4. Measurements of NO diffusion and metabolism kinetics
4.1. Measurements of NO autoxidation in solution and biological membrane
Shibuki reported the measurement of NO autoxidation in aerated solution using an electrochemical microprobe similar to a miniature O2 electrode [43]. In this experiment, NO was pre-dissolved in a syringe at a given concentration and then pumped into a chamber. This flowing system is not suitable for a long time recording because the volume of the syringe that is pumped to the chamber limits the recording time. The NO concentration in the chamber was measured every few minutes. This was the first measurement of NO autoxidation in an aerated solution by an electrode method. However, even though the autoxidation-induced NO consumption was observed, the experimental data was not suitable for determining the kinetics and rate constants of most NO reactions because of the large time intervals. Taha, et al., used a porphyrin-coated microelectrode to measure NO consumption in solution in a chamber [39]. The whole NO decay curve can be recorded after NO is injected into the chamber. Importantly, it was found that more NO was consumed by cells in the presence of O2. However, the stirring process in the test solution was not described and emphasized. If the test solution is not stirred quickly in the experiment or if the injection is too close to the NO electrode, a transient current of NO oxidation at the electrode will be detected and the amplitude of the detected current is dependent on the injection location. Stirring the test solution during the measurements of NO reaction kinetics has been emphasized in later studies [40, 54, 57]. After carefully considering the effect of sample injection and NO volatilization on the measured currents, electrode techniques can be used to measure autoxidation kinetic curves of NO in the low μM range or even in the sub μM range [56]. The experimental results show that the decay of low NO concentrations (low μM or sub μM) in aerated water are caused by NO autoxidation and NO volatilization. If NO volatilization is blocked or the rate of NO volatilization in the total NO decay is corrected, NO autoxidation retains its third-order kinetic characteristics. In biological systems, NO autoxidation not only occurs in the extracellular and intracellular fluid, but the main sites of NO autoxidation are in cellular and intracellular membranes [58, 59], suggesting that biological membranes are important locations for nitrosative chemistry, which occurs upon NO autooxidation, such as formation of nitrosothiol, DNA damage, and genotoxicity, as well as formation of nitrosamine and lipid peroxide [60-63].
4.2. The effect of superoxide on NO decay kinetics
Superoxide (O2•-), the one electron reduction product of O2, can be generated in the body from different sources. For example, after O2 is breathed into the lungs and is bound by Hb in red blood cells, a small portion of the bound O2 can accept one electron from Hb to form O2•- that is released from the heme [64]. In the blood, the activated leukocyte NADPH oxidases use NADPH as the electron donor to donate an electron to O2, generating a large amount of O2•- [65, 66]; in the vascular wall and tissues, many flavin enzymes, such as vascular NAD(P)H- oxidases [67, 68], xanthine oxidase [69, 70] and NO synthases [71], can reduce O2 into O2•-. In mitochondria, a large amount of O2 is metabolized to H2O but a small portion (1-2%) of O2 is converted to O2•- [72]. An excess O2•- production is associated with cardiovascular disease and many other diseases. O2•- is unstable in solution. Its decay follows second-order self-dismutation kinetics with a rate constant ksd ≈5×105 M-1s-1 at pH 7.4 and room temperature [73]. The half-life of O2•- self-dismutation is inversely proportional to its decay rate constant and initial concentration as follows:
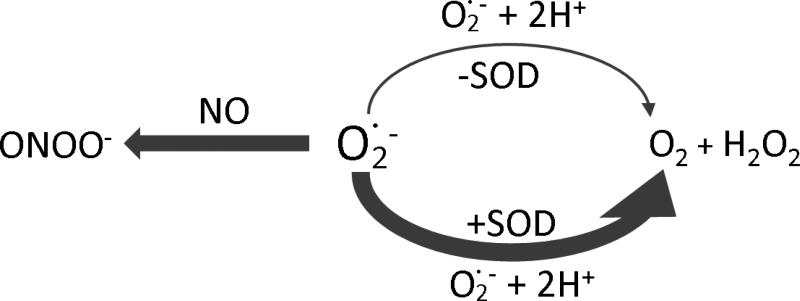
If the initial superoxide concentration , its half-life in the solution will be t1/2=20 seconds, implying that superoxide concentration decreases to 50 nM in 20 seconds. Superoxide dismutase (SOD) plays an important role in tissues to very efficiently remove excess superoxide. In the presence of SOD, kinetics of superoxide decay converts from second-order with respect to into first-order with respect to both and SOD with a rate constant ksod≈2×109 M-1s-1 [73, 74]. The half-life of superoxide is inversely proportional to SOD concentration. When SOD concentration is as low as 12 nM, the half-life of superoxide is <30 ms. For initial superoxide concentration at 100 nM, it will decrease to 1.2×10-8 nM in 1 second. SOD concentration in tissues is in the μM range [75, 76], so superoxide concentration in the body must be very low under normal physiological conditions. The reaction of NO with superoxide is extremely rapid with a rate constant 4.3-6.7×109 M-1s-1 [77, 78]. The product of this reaction is peroxynitrite, which is a potent oxidant (Fig. 6). Using a Clark-type NO electrode, Mayer and Schmidt, et al., demonstrated the effect of superoxide on NO concentrations generated from NO synthases (NOS) [79].
Fig. 6.
The reaction scheme of superoxide in the presence of NO and SOD. Reactions of superoxide with NO and SOD are extremely quick and much greater than the self-dismutation of superoxide.
4.3. NO metabolism by Hb
On the inner surface of blood vessels, eNOS generates NO that diffuses into the vascular wall to regulate vascular tone and maintain vascular homeostasis [80, 81]. One of the main pathways for NO metabolism in the body is the reaction of NO with different heme proteins. Blood contains nearly 8 mM heme in the form of Hb. Each heme contains an iron ion normally in the ferrous form Fe2+ (>99%) or the ferric form Fe3+ (<1%) [82]. Both O2 and NO can bind on the ferrous deoxyHb (or Hb(Fe2+)) to form Hb(Fe2+-O2) (oxy form) and Hb(Fe2+-NO) respectively at a rate of 1-10×107 M-1s-1 at room temperature[83-86]. It has been reported that NO can be also transported on Cys93 of Hb β-chain to form S-nitrosohemoglobin (SNO) [87]. The reaction of NO with Hb(Fe2+-O2) has a rate constant on the same order of magnitude as NO binding to ferrous Hb [22, 23]. Products of this reaction are ferric Hb (metHb) and nitrate, shown as follows:
This reaction is called NO dioxygenation, which is very rapid with a rate constant in the range from 2.5×107 to 8.9×107 M-1s-1 at 20 °C [22, 23, 83]. In contrast, the reaction of O2 with Hb(Fe2+-NO) is very slow, which mainly depends on the rate of NO dissociation from the heme [88]. Since deoxyHb or Hb(Fe2+-O2) reaction with NO is very rapid , almost all the endothelium-generated NO will be scavenged if the 8 mM heme directly exists in the blood plasma. However, enough endothelium-generated NO actually diffuses from the endothelium to its target cells in the vascular wall. One can questions why deoxyHb or Hb(Fe2+-O2) is less efficient in trapping NO in blood compared to its in vitro effects in solution? OxyHb in blood is not free in the blood plasma but is enclosed in red blood cells in the blood, NO consumption by the Hb enclosed in RBCs is much slower than by cell-free Hb because the process of extracellular diffusion limits the rate of NO consumption by RBC-enclosed Hb. This has been tested in experiments using a NO microelectrode technique [57]. It was observed that NO consumption by RBC-enclosed Hb is nearly 3 orders of magnitude slower than that by cell-free Hb. This slower rate of NO consumption by RBC-enclosed Hb has also been explained in other ways [89, 90], but lines of convincing evidence have shown that the slow rate of NO consumption by RBCs is largely due to the limit of NO diffusion in the extracellular solution while other sources of resistance may have some small contributions [91-94].
4.4. NO consumption by other heme proteins
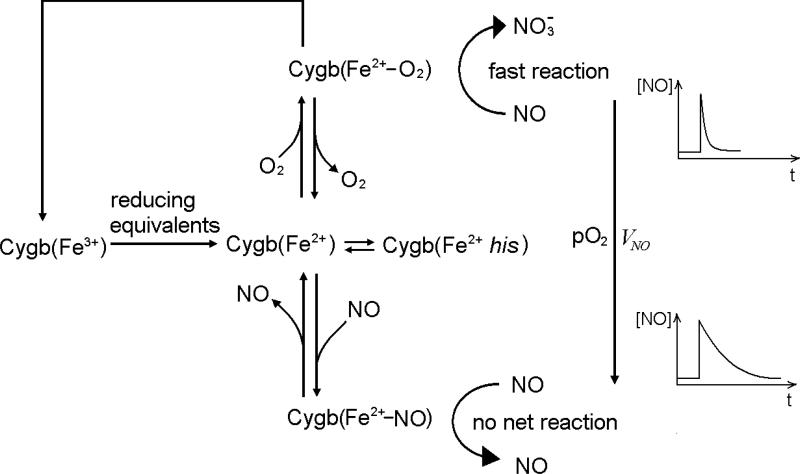
Besides oxyHb and oxyMb, other oxy-globins such as oxy-neuroglobin (oxyNgb) and oxy-cytoglobin (oxyCygb) can also dioxygenize NO at a rate constant similar to oxyHb and oxyMb [95]. Hb mainly exists in red blood cells. Mb is mainly expressed in cardiac myocytes and oxidative skeletal muscle and its concentration is in hundreds of μM. Ngb is mainly expressed in brain and retina, and Cygb is mainly expressed in fibroblasts and related cell types [96, 97]. Unlike Hb and Mb, both Ngb and Cygb have low (μM) cellular concentrations except in retina where Ngb concentration is ~100 μM. Thus, Ngb and Cygb may not function as O2 carriers for delivering O2 from blood to tissue like Hb or for the storage of O2 like Mb. Recent evidence shows that Cygb is expressed in vascular adventitial fibroblasts and in vascular smooth muscle cells [98]. Considering that Cygb at low concentration in the presence of cellular reductants (such as ascorbate or cytochrome P450 reductase with NADPH) and O2 can continuously consume NO in an O2-dependent manner and at a much slower rate that is limited by the reduction of the ferric Cygb to ferrous Cygb [98-100], Cygb may function as an O2 sensor to regulate the rate of NO consumption in an O2-dependent manner. By measuring the rate of NO consumption at different O2 concentrations using an NO electrode and an O2 electrode, it was observed that the rate of NO consumption by Cygb is largely regulated by O2 when [O2] is less than 50 μM. This result suggests that Cygb may efficiently regulate the vascular tone for controlling blood flow by adjusting the rate of NO consumption in response to changes of O2 concentration [101]. A proposed reaction scheme for the Cygb-mediated O2-dependent NO metabolism is shown in Fig. 7.
Fig. 7.
The reaction scheme for the oxygen-dependent NO consumption mediated by Cygb. The reaction is limited by the reduction of Cygb(Fe3+) into Cygb(Fe2+). O2 and NO compete with each other to bind on Cygb(Fe2+). More Cygb(Fe2+-O2) will be formed at high O2 concentration, and more Cygb(Fe2+-NO) will be formed at low O2 concentration. Since the reaction of Cygb(Fe2+-O2) with NO is very quick and the reaction of Cygb(Fe2+-NO) with NO is very slow, the oxygen concentration can significantly regulate the rate of NO consumption by Cygb in the presence of a cell reductant.
4.5. Hydrogen peroxide-induced NO consumption in biological systems
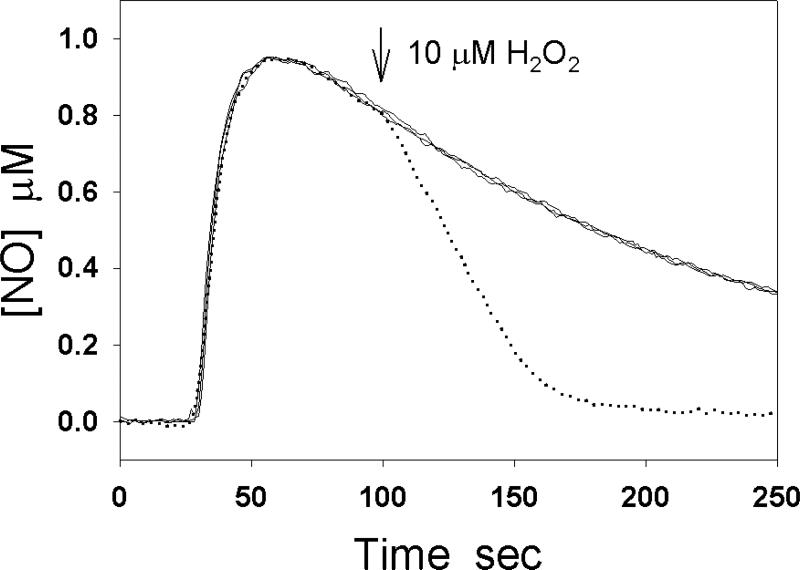
NO does not directly react with hydrogen peroxide in water. However, if heme proteins such as Hb and Mb are present in the solution, the heme proteins may mediate NO oxidation by H2O2 [102-104]. For example, H2O2 can oxidize deoxyMb to ferryl Mb (MbFeIV=O) and oxidize metMb to MbFeIV=O and ferryl porphyrin cation radical [105-107]. The latter is unstable, which can convert to MbFeIV=O. The reaction of NO with MbFeIV=O forms ferric Mb and nitrite. This reaction is very fast with a rate constant 1.8×107 M-1s-1 at pH 7.5 and 20 °C [104]. The reaction of NO with HbFeIV=O has a similar rate constant [103]. The H2O2-induced NO oxidation by heme globins can be easily measured by a NO electrode. Fig. 8 demonstrates four typical experimental curves. The three solid lines are the NO decay curves after 1 μM NO is injected into the buffer solution alone, or containing 0.5 μM Mb, or containing 10 μM H2O2. The dotted curve is obtained by adding 1 μM NO into the buffer solution containing 0.5 μM Mb and 10 μM H2O2 is added after the peak of NO oxidation current. H2O2 markedly increases NO decay in the presence of Mb. Catalase is a heme protein whose primary role is to break down H2O2 into O2 and H2O. The reaction of H2O2 with catalase forms an intermediate heme(FeIV=O). By using an NO sensor, it was demonstrated that H2O2 induces NO consumption by catalase due to the reaction of NO with heme(FeIV=O) [108].
Fig. 8.
H2O2-induced NO consumption in the presence of Mb. The solid lines represent the NO decay curves after 1 μM NO was injected into the buffer solution, in the buffer solution containing 0.5 μM Mb, and in the buffer solution containing 10 μM H2O2. When 1 μM NO was injected into the solution in the presence of 0.5 μM Mb and the peak of the NO oxidation current was reached, the injection of 10 μM H2O2 greatly increased the rate of NO decay (dotted line).
4.6. NO diffusion and metabolism in cells and tissues
4.6.1. Earlier studies on the NO diffusion-reaction transport in the aortic wall
It has been well documented that NO is consumed by cells and tissues [16, 109-111]. Therefore, the rate of biotransport of NO in the tissue will not be only affected by the NO diffusion rate but also by the NO consumption rate in the body [112, 113]. Using an NO microelectrode, Taha et. al. examined NO diffusion in the aortic wall. In their experiments, a segment of rabbit aortic ring was placed in an organ chamber vertically. The tip of the NO microelectrode was put down and inserted in the aortic wall in the direction parallel to the inner and outer surface of the aortic ring at the location ~100 μm from the inner surface of the aortic ring. The thickness of the rabbit aortic wall is ~200 μm [114]. The NO concentration distribution between inner surface and the microelectrode was simulated by the Fick's equation. The NO diffusion coefficient (DNO) in the aortic wall was assumed as 3.3×10-5 cm2s-1. By comparing the measured time course of NO concentration with the simulated time course of NO concentration, the authors observed a delay in the measured NO concentration, suggesting that NO is consumed in the aortic wall.
4.6.2. Measurement of NO diffusion and reaction kinetic parameters in brain
Using o-phenylenediamine-modified carbon fiber electrodes, Friedemann et. al measured DNO in rat brain [115]. In the measurements, NO stock solution was loaded into a single-barrel micropipette. The micropipette was attached to an o-PD electrode with a tip separation (d) of ~350 μm. The electrode-pipette assembly was then inserted into discrete areas of the cortex and striatum of the rat brain. After a small volume of NO solution was ejected from the micropipette, the current at the electrode was recorded. DNO was calculated from the measured d and the time (T) for the measured current to reach maximal amplitude based on a theoretical equation assuming that the volume of the injected NO solution is as small as a dimensionless point. The measured DNO in brain is 1.2×10-5 cm2s-1, which is ~40% of DNO (3.0-3.4×10-5 cm2s-1) measured in water [31, 93, 116]. A similar detection technique has been also used to measure the rate of NO consumption and DNO in brain in vivo recently [18, 48]. The measured rate constant of NO decay k in brain is 0.67-0.84 s-1. A theoretical equation considering the actual volume of the injected NO solution was applied to analyze experimental data. The equation assumes that DNO and the NO decay rate constant in the injected NO solution and in the surrounding brain tissue are the same. In the data analysis, it was also assumed that the injected NO solution forms a spherical volume surrounding (model A) or beneath (model B) the tip of the pipette. Computer simulations showed that the values of DNO determined from the two models have some differences. The value of DNO in agarose gel determined from model A is a constant, but DNO determined from model B increases with the injected volume of NO solution. In brain, DNO determined from the model A decreases as the injected NO volume increases, but DNO determined from model B is found to be constant. Existence of these differences between the two models is reasonable if it is considered that (i) the distance from the electrode to the center of the spherical solution increases with the volume of the spherical solution in model B but not in model A, and (ii) DNO and k in the injected spherical solution and the surrounding brain tissues may be markedly different. The diffusion reaction kinetic parameters reported in these studies are important for computer simulations of NO biotransport in the brain.
4.6.3. Oxygen-dependent NO metabolism by cells
Thomas et al measured the rate of NO metabolism by parenchymal cells (hepatocytes) using an NO electrode method [16]. It was demonstrated that the rate of NO metabolism by hepatocytes is regulated by both the NO concentration and O2 concentration. With a mathematical model considering the O2-dependent NO consumption in cells and the NO-inhibited O2 consumption by mitochondria, they predicted the distribution of NO and O2 concentrations in the extravascular tissue space and found that the interaction between NO and O2 in parenchymal cells extends the diffusion distance of NO and O2 from the vessel and dramatically blunts the effects of increased tissue work on the development of hypoxic distal regions. Gardner et al examined NO consumption by different mammalian cells [109]. They observed that NO metabolism by mammalian cells is O2-dependent. Since the NO consumption by these cells is inhibited by heme and flavoenzyme inhibitors, they suggested that the NO consumption by these mammalian cells was caused by an efficient mammalian heme and flavin-dependent NO dioxygenase.
4.6.4. Measurement of NO diffusion and metabolism in the vascular wall
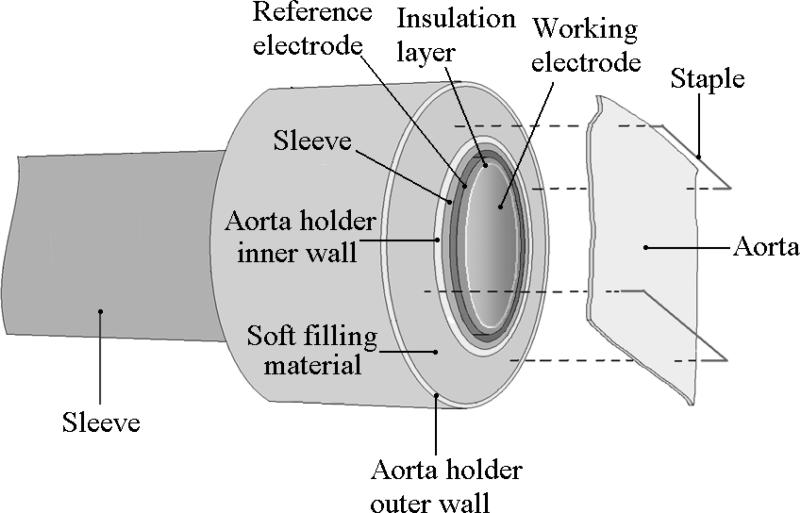
Recently,an electrode method was developed to measure NO diffusion coefficient in the aortic wall in our laboratory. The aorta is the largest artery in the body. To measure the NO diffusion coefficient, an aorta holder was installed outside of a Clark-type electrode (Fig. 9). The tip surface of the Clark-type electrode was aligned on the same plane with the surface of the aorta holder to form a large planar surface. A segment of aortic ring was longitudinally opened and flatly placed on the co-plane of the electrode tip, and metal pins used to fix the aortic wall. It was observed that the NO diffusion coefficient in the aortic wall is 0.85×10-5 cm2s-1, which is about ~25% of the NO diffusion coefficient in water [117]. It was also found that NO is consumed in the aortic wall in an O2-dependent manner and that the rate constant of the vascular NO consumption is 4×103 M-1s-1 [17]. Computer simulations show that this O2-dependent vascular NO consumption is large enough to regulate vascular NO levels in response to the change in O2 concentration.
Fig. 9.
The Clark-type NO electrode with an aorta holder as attachment for measuring the NO diffusion coefficient in the aortic wall. A segment of aortic ring is longitudinally opened and flatly placed on the co-plane of the electrode tip, and metal pins are used to fix the aortic wall.
5. Summary
The NO electrode technique is a unique tool in measurements of NO diffusion and metabolism kinetics because this technique can directly measure NO concentration in solution with a detection limit in the nM range and a response time in under seconds. However, to obtain a high quality experimental curve of NO concentration change, several interfering factors such as background currents, injection-induced solution convection, and temperature must be carefully eliminated. Using NO electrode techniques, researchers have measured NO reaction and diffusion kinetics in solution, in biological membrane, in cells, and in tissues. These studies are very helpful to better understand how blood, heme proteins, O2 and reactive oxygen species regulate NO bioavailability in the body. Combining mathematical models and novel NO electrode techniques, researchers are able to directly examine NO diffusion and metabolism kinetics within the vascular wall and other tissues.
Overview of electrode measurements of NO diffusion & metabolism kinetics is provided.
Important conclusions and physiological implications of these studies are discussed.
Methods for eliminating interfering factors in electrode measurements are proposed.
ACKNOWLEDGEMENT
This work was supported by National Institutes of Health grants HL063744, HL065608, and HL38324.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–42. [PubMed] [Google Scholar]
- 2.Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–95. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 3.Castello PR, David PS, McClure T, Crook Z, Poyton RO. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 2006;3:277–87. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Godber BL, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, Harrison R. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J Biol Chem. 2000;275:7757–63. doi: 10.1074/jbc.275.11.7757. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem. 2001;276:24482–9. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- 6.Paunel AN, Dejam A, Thelen S, Kirsch M, Horstjann M, Gharini P, Murtz M, Kelm M, de Groot H, Kolb-Bachofen V, Suschek CV. Enzyme-independent nitric oxide formation during UVA challenge of human skin: characterization, molecular sources, and mechanisms. Free Radic Biol Med. 2005;38:606–15. doi: 10.1016/j.freeradbiomed.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–9. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 8.Napoli C, Ignarro LJ. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch Pharm Res. 2009;32:1103–8. doi: 10.1007/s12272-009-1801-1. [DOI] [PubMed] [Google Scholar]
- 9.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–4. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Wink DA, Darbyshire JF, Nims RW, Saavedra JE, Ford PC. Reactions of the bioregulatory agent nitric oxide in oxygenated aqueous media: determination of the kinetics for oxidation and nitrosation by intermediates generated in the NO/O2 reaction. Chem. Res. Toxicol. 1993;6:23–7. doi: 10.1021/tx00031a003. [DOI] [PubMed] [Google Scholar]
- 12.Lewis RS, Deen WM. Kinetics of the reaction of nitric oxide with oxygen in aqueous solutions. Chem. Res. Toxicol. 1994;7:568–74. doi: 10.1021/tx00040a013. [DOI] [PubMed] [Google Scholar]
- 13.Kharitonov VG, Sundquist AR, Sharma VS. Kinetics of nitric oxide autoxidation in aqueous solution. J Biol Chem. 1994;269:5881–3. [PubMed] [Google Scholar]
- 14.Goldstein S, Czapski G. Kinetics of Nitric Oxide Autoxidation in Aqueous Solution in the Absence and Presence of Various Reductants. The Nature of the Oxidizing Intermediates. Journal of the American Chemical Society. 1995;117:12078–84. [Google Scholar]
- 15.Kelm M, Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res. 1990;66:1561–75. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- 16.Thomas DD, Liu X, Kantrow SP, Lancaster JR., Jr. The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc. Natl. Acad. Sci. U. S. A. 2001;98:355–60. doi: 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Srinivasan P, Collard E, Grajdeanu P, Lok K, Boyle SE, Friedman A, Zweier JL. Oxygen regulates the effective diffusion distance of nitric oxide in the aortic wall. Free Radic Biol Med. 2010;48:554–9. doi: 10.1016/j.freeradbiomed.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos RM, Lourenco CF, Pomerleau F, Huettl P, Gerhardt GA, Laranjinha J, Barbosa RM. Brain nitric oxide inactivation is governed by the vasculature. Antioxid Redox Signal. 2011;14:1011–21. doi: 10.1089/ars.2010.3297. [DOI] [PubMed] [Google Scholar]
- 19.McCall TB, Boughton-Smith NK, Palmer RM, Whittle BJ, Moncada S. Synthesis of nitric oxide from L-arginine by neutrophils. Release and interaction with superoxide anion. Biochem J. 1989;261:293–6. doi: 10.1042/bj2610293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas DD, Miranda KM, Colton CA, Citrin D, Espey MG, Wink DA. Heme proteins and nitric oxide (NO): the neglected, eloquent chemistry in NO redox signaling and regulation. Antioxid Redox Signal. 2003;5:307–17. doi: 10.1089/152308603322110887. [DOI] [PubMed] [Google Scholar]
- 21.Anson ML, Mirsky AE. On the combination of nitric oxide with haemoglobin. J Physiol. 1925;60:100–2. doi: 10.1113/jphysiol.1925.sp002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Jr., Olson JS. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–83. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 23.Herold S, Exner M, Nauser T. Kinetic and mechanistic studies of the NO*-mediated oxidation of oxymyoglobin and oxyhemoglobin. Biochemistry. 2001;40:3385–95. doi: 10.1021/bi002407m. [DOI] [PubMed] [Google Scholar]
- 24.Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;31(Suppl 2):S170–80. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 25.Nagano T. Practical methods for detection of nitric oxide. Luminescence. 1999;14:283–90. doi: 10.1002/(SICI)1522-7243(199911/12)14:6<283::AID-BIO572>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 26.Tarpey MM, Fridovich I. Methods of detection of vascular reactive species: nitric oxide, superoxide, hydrogen peroxide, and peroxynitrite. Circ Res. 2001;89:224–36. doi: 10.1161/hh1501.094365. [DOI] [PubMed] [Google Scholar]
- 27.Mur LA, Mandon J, Cristescu SM, Harren FJ, Prats E. Methods of nitric oxide detection in plants: a commentary. Plant Sci. 2011;181:509–19. doi: 10.1016/j.plantsci.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Hetrick EM, Schoenfisch MH. Analytical chemistry of nitric oxide. Annu Rev Anal Chem (Palo Alto Calif) 2009;2:409–33. doi: 10.1146/annurev-anchem-060908-155146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies IR, Zhang X. Nitric oxide selective electrodes. Methods Enzymol. 2008;436:63–95. doi: 10.1016/S0076-6879(08)36005-4. [DOI] [PubMed] [Google Scholar]
- 30.Privett BJ, Shin JH, Schoenfisch MH. Electrochemical nitric oxide sensors for physiological measurements. Chem Soc Rev. 2010;39:1925–35. doi: 10.1039/b701906h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zacharia IG, Deen WM. Diffusivity and solubility of nitric oxide in water and saline. Ann. Biomed. Eng. 2005;33:214–22. doi: 10.1007/s10439-005-8980-9. [DOI] [PubMed] [Google Scholar]
- 32.Mesaros S, Grunfeld S, Mesarosova A, Bustin D, Malinski T. Determination of nitric oxide saturated (stock) solution by chronoamperometry on a porphyrine microelectrode. Analytica Chimica Acta. 1997;339:265–70. [Google Scholar]
- 33.Liu X, Liu Q, Gupta E, Zorko N, Brownlee E, Zweier JL. Quantitative measurements of NO reaction kinetics with a Clark-type electrode. Nitric Oxide. 2005;13:68–77. doi: 10.1016/j.niox.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Tsukahara H, Ishida T, Mayumi M. Gas-phase oxidation of nitric oxide: chemical kinetics and rate constant. Nitric Oxide. 1999;3:191–8. doi: 10.1006/niox.1999.0232. [DOI] [PubMed] [Google Scholar]
- 35.Galliker B, Kissner R, Nauser T, Koppenol WH. Intermediates in the autoxidation of nitrogen monoxide. Chemistry. 2009;15:6161–8. doi: 10.1002/chem.200801819. [DOI] [PubMed] [Google Scholar]
- 36.Pires M, Rossi MJ, Ross DS. Kinetic and mechanistic aspects of the NO oxidation by O2 in aqueous-phase. Int. J. Chem. Kinet. 1994;26:1207–27. [Google Scholar]
- 37.Artz JD, Thatcher GR. NO release from NO donors and nitrovasodilators: comparisons between oxyhemoglobin and potentiometric assays. Chem Res Toxicol. 1998;11:1393–7. doi: 10.1021/tx980205+. [DOI] [PubMed] [Google Scholar]
- 38.Feelisch M. The use of nitric oxide donors in pharmacological studies. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:113–22. doi: 10.1007/pl00005231. [DOI] [PubMed] [Google Scholar]
- 39.Taha Z, Kiechle F, Malinski T. Oxidation of nitric oxide by oxygen in biological systems monitored by porphyrinic sensor. Biochem Biophys Res Commun. 1992;188:734–9. doi: 10.1016/0006-291x(92)91117-9. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt K, Klatt P, Mayer B. Reaction of peroxynitrite with oxyhaemoglobin: interference with photometrical determination of nitric oxide. Biochem J. 1994;301(Pt 3):645–7. doi: 10.1042/bj3010645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wink DA, Christodoulou D, Ho M, Krishna MC, Cook JA, Haut H, Randolph JK, Sullivan M, Coia G, Murry R, Meyer T. A discussion of electrochemical techniques for the detection of nitric oxide. Methods: A companion to Methods in Enzymology. 1995;7:71–7. [Google Scholar]
- 42.Feelisch M, Noack EA. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur J Pharmacol. 1987;139:19–30. doi: 10.1016/0014-2999(87)90493-6. [DOI] [PubMed] [Google Scholar]
- 43.Shibuki K. An electrochemical microprobe for detecting nitric oxide release in brain tissue. Neurosci Res. 1990;9:69–76. doi: 10.1016/0168-0102(90)90048-j. [DOI] [PubMed] [Google Scholar]
- 44.Thomson MJ, Stevanin TM, Moir JW. Measuring nitric oxide metabolism in the pathogen Neisseria meningitidis. Methods Enzymol. 2008;437:539–60. doi: 10.1016/S0076-6879(07)37027-4. [DOI] [PubMed] [Google Scholar]
- 45.Kilinc E, Yetik G, Dalbasti T, Ozsoz M. Comparison of electrochemical detection of acetylcholine-induced nitric oxide release (NO) and contractile force measurement of rabbit isolated carotid artery endothelium. J Pharm Biomed Anal. 2002;28:345–54. doi: 10.1016/s0731-7085(01)00621-5. [DOI] [PubMed] [Google Scholar]
- 46.Allen BW, Piantadosi CA, Coury LA., Jr. Electrode materials for nitric oxide detection. Nitric Oxide. 2000;4:75–84. doi: 10.1006/niox.2000.0273. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt K, Mayer B. Determination of NO with a Clark-type electrode. Methods Mol Biol. 1998;100:101–9. doi: 10.1385/1-59259-749-1:101. [DOI] [PubMed] [Google Scholar]
- 48.Santos RM, Lourenco CF, Gerhardt GA, Cadenas E, Laranjinha J, Barbosa RM. Evidence for a pathway that facilitates nitric oxide diffusion in the brain. Neurochem Int. 2011;59:90–6. doi: 10.1016/j.neuint.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 49.Shin JH, Privett BJ, Kita JM, Wightman RM, Schoenfisch MH. Fluorinated xerogel-derived microelectrodes for amperometric nitric oxide sensing. Anal Chem. 2008;80:6850–9. doi: 10.1021/ac800185x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park JK, Tran PH, Chao JK, Ghodadra R, Rangarajan R, Thakor NV. In vivo nitric oxide sensor using non-conducting polymer-modified carbon fiber. Biosens Bioelectron. 1998;13:1187–95. doi: 10.1016/s0956-5663(98)00078-5. [DOI] [PubMed] [Google Scholar]
- 51.Allen BW, Coury LA, Jr., Piantadosi CA. Electrochemical detection of physiological nitric oxide: materials and methods. Methods Enzymol. 2002;359:125–34. doi: 10.1016/s0076-6879(02)59177-1. [DOI] [PubMed] [Google Scholar]
- 52.Fatt I. Polarographic Oxygen Sensors. CRC Press; Cleveland: 1976. [Google Scholar]
- 53.Yan Q, Liu Q, Zweier JL, Liu X. Potency of authentic nitric oxide in inducing aortic relaxation. Pharmacol Res. 2007;55:329–34. doi: 10.1016/j.phrs.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Christodoulou D, Kudo S, Cook JA, Krishna MC, Miles A, Grisham MB, Murugesan M, Ford PC, Wink DA. Electrochemical methods for detection of nitric oxide. Methods Enzymol. 1996;268:69–83. doi: 10.1016/s0076-6879(96)68010-0. [DOI] [PubMed] [Google Scholar]
- 55.Malinski T, Mesaros S, Tomboulian P. Nitric oxide measurement using electrochemical methods. Methods Enzymol. 1996;268:58–69. doi: 10.1016/s0076-6879(96)68009-4. [DOI] [PubMed] [Google Scholar]
- 56.Liu X, El-Sherbiny GA, Collard E, Huang X, Follmer D, El-Mahdy M, Zweier JL. Application of carbon fiber composite minielectrodes for measurement of kinetic constants of nitric oxide decay in solution. Nitric Oxide. 2010;23:311–8. doi: 10.1016/j.niox.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu X, Miller MJ, Joshi MS, Sadowska-Krowicka H, Clark DA, Lancaster JR., Jr. Diffusion-limited reaction of free nitric oxide with erythrocytes. J Biol Chem. 1998;273:18709–13. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 58.Liu X, Miller MJ, Joshi MS, Thomas DD, Lancaster JR., Jr. Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc Natl Acad Sci U S A. 1998;95:2175–9. doi: 10.1073/pnas.95.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shiva S, Brookes PS, Patel RP, Anderson PG, Darley-Usmar VM. Nitric oxide partitioning into mitochondrial membranes and the control of respiration at cytochrome c oxidase. Proc Natl Acad Sci U S A. 2001;98:7212–7. doi: 10.1073/pnas.131128898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bartsch H, Ohshima H, Pignatelli B, Calmels S. Endogenously formed N-nitroso compounds and nitrosating agents in human cancer etiology. Pharmacogenetics. 1992;2:272–7. doi: 10.1097/00008571-199212000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Pryor WA, Lightsey JW. Mechanisms of nitrogen dioxide reactions: initiation of lipid peroxidation and the production of nitrous Acid. Science. 1981;214:435–7. doi: 10.1126/science.214.4519.435. [DOI] [PubMed] [Google Scholar]
- 62.Tamir S, deRojas-Walker T, Wishnok JS, Tannenbaum SR. DNA damage and genotoxicity by nitric oxide. Methods Enzymol. 1996;269:230–43. doi: 10.1016/s0076-6879(96)69025-9. [DOI] [PubMed] [Google Scholar]
- 63.Wink DA, Kasprzak KS, Maragos CM, Elespuru RK, Misra M, Dunams TM, Cebula TA, Koch WH, Andrews AW, Allen JS, et al. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science. 1991;254:1001–3. doi: 10.1126/science.1948068. [DOI] [PubMed] [Google Scholar]
- 64.Misra HP, Fridovich I. The generation of superoxide radical during the autoxidation of hemoglobin. J Biol Chem. 1972;247:6960–2. [PubMed] [Google Scholar]
- 65.Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–76. [PubMed] [Google Scholar]
- 66.Lee C, Miura K, Liu X, Zweier JL. Biphasic regulation of leukocyte superoxide generation by nitric oxide and peroxynitrite. J Biol Chem. 2000;275:38965–72. doi: 10.1074/jbc.M006341200. [DOI] [PubMed] [Google Scholar]
- 67.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 68.Souza HP, Liu X, Samouilov A, Kuppusamy P, Laurindo FR, Zweier JL. Quantitation of superoxide generation and substrate utilization by vascular NAD(P)H oxidase. Am J Physiol Heart Circ Physiol. 2002;282:H466–74. doi: 10.1152/ajpheart.00482.2001. [DOI] [PubMed] [Google Scholar]
- 69.Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993;91:2546–51. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zweier JL, Kuppusamy P, Thompson-Gorman S, Klunk D, Lutty GA. Measurement and characterization of free radical generation in reoxygenated human endothelial cells. Am J Physiol. 1994;266:C700–8. doi: 10.1152/ajpcell.1994.266.3.C700. [DOI] [PubMed] [Google Scholar]
- 71.Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci U S A. 1996;93:6770–4. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Turrens JF. Superoxide production by the mitochondrial respiratory chain. Biosci Rep. 1997;17:3–8. doi: 10.1023/a:1027374931887. [DOI] [PubMed] [Google Scholar]
- 73.Riley DP, Rivers WJ, Weiss RH. Stopped-flow kinetic analysis for monitoring superoxide decay in aqueous systems. Anal Biochem. 1991;196:344–9. doi: 10.1016/0003-2697(91)90476-a. [DOI] [PubMed] [Google Scholar]
- 74.Forman HJ, Fridovich I. Superoxide dismutase: a comparison of rate constants. Arch Biochem Biophys. 1973;158:396–400. doi: 10.1016/0003-9861(73)90636-x. [DOI] [PubMed] [Google Scholar]
- 75.Marklund SL. Human copper-containing superoxide dismutase of high molecular weight. Proc Natl Acad Sci U S A. 1982;79:7634–8. doi: 10.1073/pnas.79.24.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liochev SI, Fridovich I. Superoxide and nitric oxide: consequences of varying rates of production and consumption: a theoretical treatment. Free Radic Biol Med. 2002;33:137–41. doi: 10.1016/s0891-5849(02)00864-x. [DOI] [PubMed] [Google Scholar]
- 77.Huie RE, Padmaja S. The reaction of no with superoxide. Free Radic Res Commun. 1993;18:195–9. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 78.Goldstein S, Czapski G. The reaction of NO. with O2.- and HO2.: a pulse radiolysis study. Free Radic Biol Med. 1995;19:505–10. doi: 10.1016/0891-5849(95)00034-u. [DOI] [PubMed] [Google Scholar]
- 79.Mayer B, Klatt P, Werner ER, Schmidt K. Kinetics and mechanism of tetrahydrobiopterin-induced oxidation of nitric oxide. J Biol Chem. 1995;270:655–9. doi: 10.1074/jbc.270.2.655. [DOI] [PubMed] [Google Scholar]
- 80.Rees DD, Palmer RM, Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989;86:3375–8. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Radomski MW, Moncada S. Regulation of vascular homeostasis by nitric oxide. Thromb Haemost. 1993;70:36–41. [PubMed] [Google Scholar]
- 82.Wilburn-Goo D, Lloyd LM. When patients become cyanotic: acquired methemoglobinemia. J Am Dent Assoc. 1999;130:826–31. doi: 10.14219/jada.archive.1999.0306. [DOI] [PubMed] [Google Scholar]
- 83.Cassoly R, Gibson Q. Conformation, co-operativity and ligand binding in human hemoglobin. J Mol Biol. 1975;91:301–13. doi: 10.1016/0022-2836(75)90382-4. [DOI] [PubMed] [Google Scholar]
- 84.Sharma VS, Ranney HM. The dissociation of NO from nitrosylhemoglobin. J Biol Chem. 1978;253:6467–72. [PubMed] [Google Scholar]
- 85.Moore EG, Gibson QH. Cooperativity in the dissociation of nitric oxide from hemoglobin. J Biol Chem. 1976;251:2788–94. [PubMed] [Google Scholar]
- 86.Kharitonov VG, Sharma VS, Magde D, Koesling D. Kinetics of nitric oxide dissociation from five- and six-coordinate nitrosyl hemes and heme proteins, including soluble guanylate cyclase. Biochemistry. 1997;36:6814–8. doi: 10.1021/bi970201o. [DOI] [PubMed] [Google Scholar]
- 87.Anand P, Stamler JS. Enzymatic mechanisms regulating protein S-nitrosylation: implications in health and disease. J Mol Med (Berl) 2012;90:233–44. doi: 10.1007/s00109-012-0878-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Herold S, Rock G. Mechanistic studies of the oxygen-mediated oxidation of nitrosylhemoglobin. Biochemistry. 2005;44:6223–31. doi: 10.1021/bi0475929. [DOI] [PubMed] [Google Scholar]
- 89.Vaughn MW, Huang KT, Kuo L, Liao JC. Erythrocytes possess an intrinsic barrier to nitric oxide consumption. J. Biol. Chem. 2000;275:2342–8. doi: 10.1074/jbc.275.4.2342. [DOI] [PubMed] [Google Scholar]
- 90.Sakai H, Sato A, Masuda K, Takeoka S, Tsuchida E. Encapsulation of concentrated hemoglobin solution in phospholipid vesicles retards the reaction with NO, but not CO, by intracellular diffusion barrier. J Biol Chem. 2008;283:1508–17. doi: 10.1074/jbc.M707660200. [DOI] [PubMed] [Google Scholar]
- 91.Liu X, Samouilov A, Lancaster JR, Jr., Zweier JL. Nitric oxide uptake by erythrocytes is primarily limited by extracellular diffusion not membrane resistance. J Biol Chem. 2002;277:26194–9. doi: 10.1074/jbc.M201939200. [DOI] [PubMed] [Google Scholar]
- 92.Azarov I, Liu C, Reynolds H, Tsekouras Z, Lee JS, Gladwin MT, Kim-Shapiro DB. Mechanisms of slower nitric oxide uptake by red blood cells and other hemoglobin-containing vesicles. J Biol Chem. 2011;286:33567–79. doi: 10.1074/jbc.M111.228650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu X, Yan Q, Baskerville KL, Zweier JL. Estimation of nitric oxide concentration in blood for different rates of generation. Evidence that intravascular nitric oxide levels are too low to exert physiological effects. J Biol Chem. 2007;282:8831–6. doi: 10.1074/jbc.M611684200. [DOI] [PubMed] [Google Scholar]
- 94.Tsoukias NM, Popel AS. Erythrocyte consumption of nitric oxide in presence and absence of plasma-based hemoglobin. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H2265–77. doi: 10.1152/ajpheart.01080.2001. [DOI] [PubMed] [Google Scholar]
- 95.Gardner PR, Gardner AM, Brashear WT, Suzuki T, Hvitved AN, Setchell KD, Olson JS. Hemoglobins dioxygenate nitric oxide with high fidelity. J Inorg Biochem. 2006;100:542–50. doi: 10.1016/j.jinorgbio.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 96.Hankeln T, Ebner B, Fuchs C, Gerlach F, Haberkamp M, Laufs TL, Roesner A, Schmidt M, Weich B, Wystub S, Saaler-Reinhardt S, Reuss S, Bolognesi M, De Sanctis D, Marden MC, Kiger L, Moens L, Dewilde S, Nevo E, Avivi A, Weber RE, Fago A, Burmester T. Neuroglobin and cytoglobin in search of their role in the vertebrate globin family. J Inorg Biochem. 2005;99:110–9. doi: 10.1016/j.jinorgbio.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 97.Fago A, Hundahl C, Malte H, Weber RE. Functional properties of neuroglobin and cytoglobin. Insights into the ancestral physiological roles of globins. IUBMB Life. 2004;56:689–96. doi: 10.1080/15216540500037299. [DOI] [PubMed] [Google Scholar]
- 98.Halligan KE, Jourd'heuil FL, Jourd'heuil D. Cytoglobin is expressed in the vasculature and regulates cell respiration and proliferation via nitric oxide dioxygenation. J Biol Chem. 2009;284:8539–47. doi: 10.1074/jbc.M808231200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smagghe BJ, Trent JT, 3rd, Hargrove MS. NO dioxygenase activity in hemoglobins is ubiquitous in vitro, but limited by reduction in vivo. PLoS One. 2008;3:e2039. doi: 10.1371/journal.pone.0002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gardner AM, Cook MR, Gardner PR. Nitric-oxide dioxygenase function of human cytoglobin with cellular reductants and in rat hepatocytes. J Biol Chem. 2010;285:23850–7. doi: 10.1074/jbc.M110.132340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu X, Huang X, Follmer D, Liu K, Hemann C, Druhan L, Zweier JL. Cytoglobin Effectively Regulates the Oxygen-Dependent Consumption of Nitric Oxide in the Vascular Wall. Circulation. 2010;122:A16226. [Google Scholar]
- 102.Gorbunov NV, Osipov AN, Day BW, Zayas-Rivera B, Kagan VE, Elsayed NM. Reduction of ferrylmyoglobin and ferrylhemoglobin by nitric oxide: a protective mechanism against ferryl hemoprotein-induced oxidations. Biochemistry. 1995;34:6689–99. doi: 10.1021/bi00020a014. [DOI] [PubMed] [Google Scholar]
- 103.Herold S, Rehmann FJ. Kinetics of the reactions of nitrogen monoxide and nitrite with ferryl hemoglobin. Free Radic Biol Med. 2003;34:531–45. doi: 10.1016/s0891-5849(02)01355-2. [DOI] [PubMed] [Google Scholar]
- 104.Herold S, Rehmann FJ. Kinetic and mechanistic studies of the reactions of nitrogen monoxide and nitrite with ferryl myoglobin. J Biol Inorg Chem. 2001;6:543–55. doi: 10.1007/s007750100231. [DOI] [PubMed] [Google Scholar]
- 105.Matsui T, Ozaki S, Liong E, Phillips GN, Jr., Watanabe Y. Effects of the location of distal histidine in the reaction of myoglobin with hydrogen peroxide. J Biol Chem. 1999;274:2838–44. doi: 10.1074/jbc.274.5.2838. [DOI] [PubMed] [Google Scholar]
- 106.Alayash AI, Ryan BA, Eich RF, Olson JS, Cashon RE. Reactions of sperm whale myoglobin with hydrogen peroxide. Effects of distal pocket mutations on the formation and stability of the ferryl intermediate. J Biol Chem. 1999;274:2029–37. doi: 10.1074/jbc.274.4.2029. [DOI] [PubMed] [Google Scholar]
- 107.Reeder BJ, Svistunenko DA, Sharpe MA, Wilson MT. Characteristics and mechanism of formation of peroxide-induced heme to protein cross-linking in myoglobin. Biochemistry. 2002;41:367–75. doi: 10.1021/bi011335b. [DOI] [PubMed] [Google Scholar]
- 108.Brown GC. Reversible binding and inhibition of catalase by nitric oxide. Eur J Biochem. 1995;232:188–91. doi: 10.1111/j.1432-1033.1995.tb20798.x. [DOI] [PubMed] [Google Scholar]
- 109.Gardner PR, Martin LA, Hall D, Gardner AM. Dioxygen-dependent metabolism of nitric oxide in mammalian cells. Free Radic Biol Med. 2001;31:191–204. doi: 10.1016/s0891-5849(01)00569-x. [DOI] [PubMed] [Google Scholar]
- 110.Schmidt K, Mayer B. Consumption of nitric oxide by endothelial cells: Evidence for the involvement of a NAD(P)H-, flavin- and heme-dependent dioxygenase reaction. FEBS Letters. 2004;577:199–204. doi: 10.1016/j.febslet.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 111.Hall CN, Keynes RG, Garthwaite J. Cytochrome P450 oxidoreductase participates in nitric oxide consumption by rat brain. Biochem J. 2009;419:411–8. doi: 10.1042/BJ20082419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lancaster JR., Jr. Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8137–41. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wood J, Garthwaite J. Models of the diffusional spread of nitric oxide: implications for neural nitric oxide signalling and its pharmacological properties. Neuropharmacology. 1994;33:1235–44. doi: 10.1016/0028-3908(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 114.Schneiderman G, Pritchard WF, Ramirez CA, Colton CK, Smith KA, Stemerman MB. Rabbit aortic medial thickness under relaxed and specified simulated in vivo conditions. Am J Physiol. 1983;245:H623–7. doi: 10.1152/ajpheart.1983.245.4.H623. [DOI] [PubMed] [Google Scholar]
- 115.Friedemann MN, Robinson SW, Gerhardt GA. o-Phenylenediamine-modified carbon fiber electrodes for the detection of nitric oxide. Anal Chem. 1996;68:2621–8. doi: 10.1021/ac960093w. [DOI] [PubMed] [Google Scholar]
- 116.Malinski T, Taha Z, Grunfeld S, Patton S, Kapturczak M, Tomboulian P. Diffusion of nitric oxide in the aorta wall monitored in situ by porphyrinic microsensors. Biochem. Biophys. Res. Commun. 1993;193:1076–82. doi: 10.1006/bbrc.1993.1735. [DOI] [PubMed] [Google Scholar]
- 117.Liu X, Srinivasan P, Collard E, Grajdeanu P, Zweier JL, Friedman A. Nitric Oxide Diffusion Rate is Reduced in the Aortic Wall. Biophys J. 2008:1880–9. doi: 10.1529/biophysj.107.120626. [DOI] [PMC free article] [PubMed] [Google Scholar]