Abstract
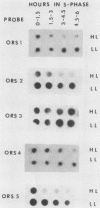
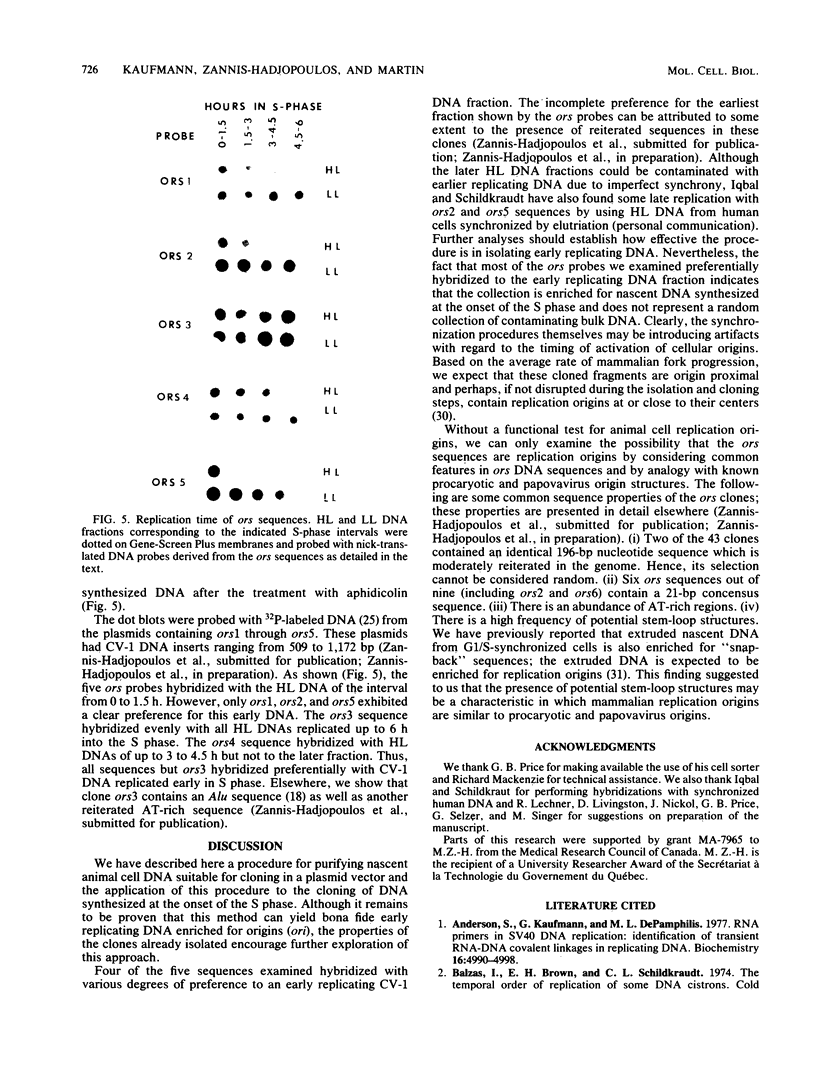
To study the structure and complexity of animal cell replication origins, we have isolated and cloned nascent DNA from the onset of S phase as follows: African green monkey kidney cells arrested in G1 phase were serum stimulated in the presence of the DNA replication inhibitor aphidicolin. After 18 h, the drug was removed, and DNA synthesis was allowed to proceed in vivo for 1 min. Nuclei were then prepared, and DNA synthesis was briefly continued in the presence of Hg-dCTP. The mercury-labeled nascent DNA was purified in double-stranded form by extrusion (M. Zannis-Hadjopoulos, M. Perisco, and R. G. Martin, Cell 27:155-163, 1981) followed by sulfhydryl-agarose affinity chromatography. Purified nascent DNA (ca. 500 to 2,000 base pairs) was treated with mung bean nuclease to remove single-stranded ends and inserted into the NruI site of plasmid pBR322. The cloned fragments were examined for their time of replication by hybridization to cellular DNA fractions synthesized at various intervals of the S phase. Among five clones examined, four hybridized preferentially with early replicating fractions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Kaufman G., DePamphilis M. L. RNA primers in SV40 DNA replication: identification of transient RNA-DNA covalent linkages in replicating DNA. Biochemistry. 1977 Nov 15;16(23):4990–4998. doi: 10.1021/bi00642a009. [DOI] [PubMed] [Google Scholar]
- Balazs I., Brown E. H., Schildkraut C. L. The temporal order of replication of some DNA cistrons. Cold Spring Harb Symp Quant Biol. 1974;38:239–245. doi: 10.1101/sqb.1974.038.01.027. [DOI] [PubMed] [Google Scholar]
- Braun R., Wili H. Time sequence of DNA replication in Physarum. Biochim Biophys Acta. 1969 Jan 21;174(1):246–252. doi: 10.1016/0005-2787(69)90248-2. [DOI] [PubMed] [Google Scholar]
- Braunstein J. D., Schulze D., DelGiudice T., Furst A., Schildkraut C. L. The temporal order of replication of murine immunoglobulin heavy chain constant region sequences corresponds to their linear order in the genome. Nucleic Acids Res. 1982 Nov 11;10(21):6887–6902. doi: 10.1093/nar/10.21.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calza R. E., Eckhardt L. A., DelGiudice T., Schildkraut C. L. Changes in gene position are accompanied by a change in time of replication. Cell. 1984 Mar;36(3):689–696. doi: 10.1016/0092-8674(84)90349-0. [DOI] [PubMed] [Google Scholar]
- Chan C. S., Tye B. K. A family of Saccharomyces cerevisiae repetitive autonomously replicating sequences that have very similar genomic environments. J Mol Biol. 1983 Aug 15;168(3):505–523. doi: 10.1016/s0022-2836(83)80299-x. [DOI] [PubMed] [Google Scholar]
- Chan C. S., Tye B. K. Organization of DNA sequences and replication origins at yeast telomeres. Cell. 1983 Jun;33(2):563–573. doi: 10.1016/0092-8674(83)90437-3. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Ward D. C. Mercurated polynucleotides: new probes for hybridization and selective polymer fractionation. Biochemistry. 1975 Jun 3;14(11):2458–2469. doi: 10.1021/bi00682a028. [DOI] [PubMed] [Google Scholar]
- Dooley D. C., Ozer H. L. Ordered replication of DNA sequences: synthesis of mouse satellite and adjacent main band sequences. J Cell Physiol. 1979 Mar;98(3):515–526. doi: 10.1002/jcp.1040980310. [DOI] [PubMed] [Google Scholar]
- Fangman W. L., Hice R. H., Chlebowicz-Sledziewska E. ARS replication during the yeast S phase. Cell. 1983 Mar;32(3):831–838. doi: 10.1016/0092-8674(83)90069-7. [DOI] [PubMed] [Google Scholar]
- Furst A., Brown E. H., Braunstein J. D., Schildkraut C. L. alpha-Globulin sequences are located in a region of early-replicating DNA in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1023–1027. doi: 10.1073/pnas.78.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J. L. Effect of damage to early, middle, and late-replicating DNA on progress through the S period in Chinese hamster ovary cells. Exp Cell Res. 1978 Mar 15;112(2):225–232. doi: 10.1016/0014-4827(78)90204-5. [DOI] [PubMed] [Google Scholar]
- Hand R. Eucaryotic DNA: organization of the genome for replication. Cell. 1978 Oct;15(2):317–325. doi: 10.1016/0092-8674(78)90001-6. [DOI] [PubMed] [Google Scholar]
- Heintz N. H., Hamlin J. L. An amplified chromosomal sequence that includes the gene for dihydrofolate reductase initiates replication within specific restriction fragments. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4083–4087. doi: 10.1073/pnas.79.13.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A., Riggs A. D. On the mechanism of DNA replication in mammalian chromosomes. J Mol Biol. 1968 Mar 14;32(2):327–341. doi: 10.1016/0022-2836(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Jelinek W. R., Toomey T. P., Leinwand L., Duncan C. H., Biro P. A., Choudary P. V., Weissman S. M., Rubin C. M., Houck C. M., Deininger P. L. Ubiquitous, interspersed repeated sequences in mammalian genomes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1398–1402. doi: 10.1073/pnas.77.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann G. Characterization of initiator RNA from replicating simian virus 40 DNA synthesized in isolated nuclei. J Mol Biol. 1981 Mar 25;147(1):25–39. doi: 10.1016/0022-2836(81)90077-2. [DOI] [PubMed] [Google Scholar]
- Marchionni M. A., Roufa D. J. Replication of viral DNA sequences integrated within the chromatin of SV40-transformed Chinese hamster lung cells. Cell. 1981 Oct;26(2 Pt 2):245–258. doi: 10.1016/0092-8674(81)90307-x. [DOI] [PubMed] [Google Scholar]
- Martin R. G. The transformation of cell growth and transmogrification of DNA synthesis by simian virus 40. Adv Cancer Res. 1981;34:1–68. doi: 10.1016/s0065-230x(08)60238-9. [DOI] [PubMed] [Google Scholar]
- Montiel J. F., Norbury C. J., Tuite M. F., Dobson M. J., Mills J. S., Kingsman A. J., Kingsman S. M. Characterization of human chromosomal DNA sequences which replicate autonomously in Saccharomyces cerevisiae. Nucleic Acids Res. 1984 Jan 25;12(2):1049–1068. doi: 10.1093/nar/12.2.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrali-Noy G., Spadari S., Miller-Faurès A., Miller A. O., Kruppa J., Koch G. Synchronization of HeLa cell cultures by inhibition of DNA polymerase alpha with aphidicolin. Nucleic Acids Res. 1980 Jan 25;8(2):377–387. doi: 10.1093/nar/8.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford I. R., Martin R. F., Finch L. R., Hodgson G. S. Inhibition of DNA synthesis and cell death. Biochim Biophys Acta. 1982 Feb 26;696(2):154–162. doi: 10.1016/0167-4781(82)90023-9. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rivin C. J., Fangman W. L. Replication fork rate and origin activation during the S phase of Saccharomyces cerevisiae. J Cell Biol. 1980 Apr;85(1):108–115. doi: 10.1083/jcb.85.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcomb D. T., Struhl K., Davis R. W. Isolation and characterisation of a yeast chromosomal replicator. Nature. 1979 Nov 1;282(5734):39–43. doi: 10.1038/282039a0. [DOI] [PubMed] [Google Scholar]
- Stinchcomb D. T., Thomas M., Kelly J., Selker E., Davis R. W. Eukaryotic DNA segments capable of autonomous replication in yeast. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4559–4563. doi: 10.1073/pnas.77.8.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Blackburn E. H. Cloning yeast telomeres on linear plasmid vectors. Cell. 1982 May;29(1):245–255. doi: 10.1016/0092-8674(82)90109-x. [DOI] [PubMed] [Google Scholar]
- Zannis-Hadjopoulos M., Chepelinsky A. B., Martin R. G. Mapping of the 3'-end positions of simian virus 40 nascent strands. J Mol Biol. 1983 Apr 25;165(4):599–607. doi: 10.1016/s0022-2836(83)80269-1. [DOI] [PubMed] [Google Scholar]
- Zannis-Hadjopoulos M., Kaufmann G., Martin R. G. Mammalian DNA enriched for replication origins is enriched for snap-back sequences. J Mol Biol. 1984 Nov 15;179(4):577–586. doi: 10.1016/0022-2836(84)90156-6. [DOI] [PubMed] [Google Scholar]
- Zannis-Hadjopoulos M., Persico M., Martin R. G. The remarkable instability of replication loops provides a general method for the isolation of origins of DNA replication. Cell. 1981 Nov;27(1 Pt 2):155–163. doi: 10.1016/0092-8674(81)90369-x. [DOI] [PubMed] [Google Scholar]