Abstract
Background
Bartonellae are fastidious bacteria causing persistent bacteremia in humans and a wide variety of animals. In recent years there is an increasing interest in mammalian bartonelloses in general and in rodent bartonelloses in particular. To date, no studies investigating the presence of Bartonella spp. in rodents and ectoparasites from Nigeria were carried out.
Methodology/Principal Findings
The aim of the current study was to investigate the presence of Bartonella spp. in commensal rodents and their ectoparasites in Nigeria. We report, for the first time, the molecular detection of Bartonella in 26% (46/177) of commensal rodents (Rattus rattus, R. norvegicus and Cricetomys gambianus) and 28% (9/32) of ectoparasite pools (Xenopsylla cheopis, Haemolaelaps spp., Ctenophthalmus spp., Hemimerus talpoides, and Rhipicephalus sanguineus) from Nigeria. Sequence analysis of the citrate synthase gene (gltA) revealed diversity of Bartonella spp. and genotypes in Nigerian rodents and their ectoparasites. Bartonella spp. identical or closely related to Bartonella elizabethae, Bartonella tribocorum and Bartonella grahamii were detected.
Conclusions/Significance
High prevalence of infection with Bartonella spp. was detected in commensal rodents and ectoparasites from Nigeria. The Bartonella spp. identified were previously associated with human diseases highlighting their importance to public health. Further studies need to be conducted to determine whether the identified Bartonella species could be responsible for human cases of febrile illness in Nigeria.
Author Summary
Bartonella species are zoonotic vector-borne bacteria that typically parasitize the erythrocytes of mammalian hosts, resulting in long lasting infections. They are responsible for a wide range of clinical manifestations in both immunocompetent and immunocompromised hosts.
Rodents and a wide range of small mammals serve as reservoirs of bartonellae, usually with no apparent clinical manifestations. Close association between rodents and humans especially in rural communities as well as in the overcrowded cities facilitates transmission of these bacteria.
There have been no studies investigating the presence of Bartonella spp. in rodents and ectoparasites from Nigeria. The aim of the current study was to investigate the presence of Bartonella spp. in commensal rodents and their ectoparasites in Nigeria and its public health implications. We report, for the first time, the molecular detection of Bartonella in 26% (46/177) of commensal rodents and 28% (9/32) of ectoparasite pools from Nigeria. Sequence analysis of the citrate synthase gene (gltA) revealed diversity of Bartonella spp. and genotypes in Nigerian commensal rodents and their ectoparasites. The Bartonella spp. detected in this study were identical or closely related to Bartonella elizabethae, Bartonella tribocorum and Bartonella grahamii previously associated with human diseases highlighting their importance to public health.
Introduction
Bartonellae are Gram-negative facultative intracellular alpha-proteobacteria belonging to the family Bartonellaceae. Many Bartonella species have been affecting human life for centuries [1]. Since the first Bartonella species discovery, namely Bartonella bacilliformis, in 1905 by Alberto Leonardo Barton Thompson, more than 30 species of Bartonella were identified [2], [3]. Bartonella species have been found in a variety of mammals, and the numbers of Bartonella species and their respective reservoir hosts are constantly growing [4]. They are pathogens of emerging and reemerging significance, causing a wide array of clinical syndromes in human and animal hosts [5].
These bacterial species are transmitted between the reservoir and the final mammal host by hematophagous arthropods and insects such as fleas, sand flies, mites, lice and possibly ticks, usually by their bites [6]–[8]. The range of vectors involved in the transmission of the different species of this genus has not been fully characterized [9]. Bacteria belonging to the genus Bartonella are slow growers in vitro, and the most used diagnostic methods are isolation, serology and polymerase chain reaction (PCR). The use of sequencing on PCR amplicons has been recommended in order to detect new species, especially when dealing with uncommon clinical presentations and settings [4].
Bartonella DNA has been detected in various hosts and possible vectors in many countries including, Israel [10], [11], Indonesia [12], Nepal [13], Thailand [6], [14], China [15], Taiwan [16], Korea [17], USA [18]–[20], UK [21], [22], Spain [23] and The Netherlands [24]. In Africa there are reports from Kenya [25], the Democratic Republic of Congo and Tanzania [26], Algeria [27], [28], Egypt [29], [30], Gabon [31] and South Africa [32], [33]. However, there is no report of molecular screening of humans or animals and their ectoparasites for Bartonella spp. in Nigeria.
Although there are no case estimates of fever of unknown origin (FUO) in Nigeria, the condition remains a challenging medical problem and unraveling the diagnosis could be a daunting task when investigating for common infective and non-infective causes. Moreover, since Bartonella spp. are difficult to diagnose and are seldom included in the differential diagnosis list in cases of FUO, specific Bartonella sp. treatment is rarely instituted to patients with FUO.
The objectives of this study were to determine the possible infection of commensal rodents and their ectoparasites from Nigeria with Bartonella spp., to investigate the presence of zoonotic Bartonella spp. in these rodents and ectoparasites and to evaluate genetic heterogeneity of circulating Bartonella strains in this country.
Materials and Methods
Ethics statement
The study protocol was read and approved by The National Veterinary Research Institute Vom Ethical Committee on Animal Use and Care. Permission to place the traps in the study area was granted by the residents. Animals were treated in a humane manner and in accordance with authorizations and guidelines for Ethical Conduct in the Care and Use of Nonhuman Animals in Research of the American Psychological Association (APA) for use by scientists working with nonhuman animals (American Psychological Association Committee on Animal Research and Ethics) in 2010.
Rodents and ectoparasites
Rodents were live trapped in domestic and peri- domestic areas in Vom (9°44′N/8°47′E) Nigeria between October–December 2011. A total of 177 rodents (48 Rattus rattus, 121 Rattus norvegicus, 6 Mus musculus and 2 Cricetomys gambianus) were captured. Trapping was done using wire cage traps baited with smoked fish and other food scraps set out in the evenings when rodents are known to leave their holes to scavenge in farmlands or nearby human habitations. Traps were checked for rodents early the next morning. Cages containing rodents were transported to the Parasitology Laboratory, National Veterinary Research Institute (NVRI) Vom Nigeria, where they were identified and classified by a zoologist. At the laboratory, the cages containing rodents were placed into a clear plastic bag, which was sealed at the opening. Halothane gas was applied into the bag and the activity of the rodents was monitored. Once the rodents were anaesthetized, they were removed from the cage and bled by cardiac puncture. Depending on the size, 0.5–3 ml of blood was drawn and aliquoted into an EDTA tube and labeled. Each rodent was checked for ectoparasites by brushing the fur with a tooth brush onto a white cardboard paper. Ectoparasites were placed in labeled vials containing absolute ethanol corresponding to the host from which they were removed and stored at −20°C. Both blood and ectoparasite samples were transported in a cool box to The Koret School of Veterinary Medicine, The Hebrew University of Jerusalem, Israel for analysis. The ectoparasites were morphologically identified by an entomologist (KYM) at the Department of Microbiology and Molecular Genetics in Jerusalem, Israel.
Bartonella culture
Two hundred microlitres of thawed whole blood sample was plated onto chocolate agar. The plate was incubated at 35°C and 5% CO2 and checked for growth of Bartonella species on alternate days for up to 30 days. Suspected colonies were randomly selected and separately sub-cultured onto different fresh agar plates to obtain pure colonies.
DNA extraction from rodent blood
DNA was extracted from blood using BiOstic Bacteremia DNA Isolation Kit (MO Bio Laboratories, Inc USA) according to manufacturer's instructions.
DNA extraction from ectoparasites
The ectoparasites collected from each rodent species were pooled (2–3 arthropods per pool) according to genus and/or species. DNA was extracted from each pool using Illustra tissue and cell genomic Prep miniSpin kit (GE Healthcare UK Limited) according to manufacturer's instructions.
DNA extraction from bacterial cultures
Pure cultured colonies of Bartonella sp. were aseptically scooped into microfuge tubes containing 50 µl of sterile Phosphate Buffered Saline (PBS). DNA was extracted from the bacterial colonies using Illustra tissue and cell genomic Prep miniSpin kit (GE Healthcare UK Limited) according to the manufacturer's instructions.
PCR assays for Bartonella sp. citrate synthase gene (gltA) from blood and ectoparasites
The oligonucleotide primers: forward BhCS871.p (5′ -GGGGACCAGCTCATGGTGG-3′) and reverse: BhCS1137.n (5′-AATGCAAAAAGAACAGTAAACA-3′) [34] were used for amplification of a 379 bp region of the Bartonella citrate synthase gene (gltA). Positive and negative controls were included in each PCR run. PCR was performed using reaction tubes, preloaded with a premier PCR master mix (Syntezza PCR-Ready High Specificity, Syntezza Bioscience, Israel). 50 µl total volume was used as follows: 3 µl of DNA template, 1 µl of 10 mM each primer, 1 µl MgCl2, 19 µl of ultra pure PCR water and 25 µl PCR master mix. Amplification was performed using a conventional thermocycler (Biometra, Goettingen, Germany) and the following program parameters: an initial denaturing at 95°C for five minutes, and 35 cycles of denaturation at 95°C for one minute, annealing at 56°C for one minute, and elongation at 72°C for one minute. Amplification was completed by holding the reaction mixture at 72°C for 10 minutes.
PCR products were tested for the presence of amplicons of the correct size by electrophoresis of 6 µl of the products on 1.5% agarose gels stained with ethidium bromide and checked under UV light for the size of amplified fragments by comparison to a 50 bp DNA molecular weight marker. Amplicons of the proper size were identified by comparison to the positive control lane on the gel.
Sequencing and analysis of DNA
Positive PCR products were purified using (EXO-SAP IT USB, Cleveland, Ohio, USA) and sequenced using the forward primer at the Center for Genomics Technologies, Hebrew University of Jerusalem, Israel. To avoid errors or misinterpretation of the sequencing results, we deleted primer sequences from the gltA sequences and removed all ambiguities in the sequences before sequence analysis was performed.
Phylogenetic analysis
Analysis of DNA sequences and phylogenetic relationships were done using MEGA 5.
Sequences were aligned by MUSCLE and the evolutionary history was inferred using the Maximum Likelihood method based on the Tamura-Nei model [35]. The bootstrap consensus tree inferred from 200 replicates was taken to represent the evolutionary history of the taxa analyzed. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test are shown next to the branches. All positions containing gaps and missing data were eliminated.
Results
Blood
A total of 177 cardiac blood samples from four rodent species were examined in this study: 68.4% (121/177) from Rattus norvegicus rats; 26% (48/177) from Rattus rattus rats; 1.1% (2/177) from Cricetomys gambianus rats, and 3.4% (6/177) from Mus musculus mice.
Ectoparasites
One hundred and seventy ectoparasites comprising of 85 ticks, Rhipicephalus sanguineus (79) and Haemaphysalis leachi (6); 13 fleas, Xenopsylla cheopis (8), Ctenophthalmus spp. (5) and 62 Haemolaelaps spp. (gamasid mites) were recovered from the rodents. Ten additional Hemimerus talpoides (earwig sp.) were removed from the 2 C. gambianus captured (Table 1).
Table 1. Number and percentage of Bartonella positive ectoparasites as determined by PCR targeting gltA fragment.
| S/no | Ectoparasite type | Number of pools tested | Number positive | Percent |
| 1 | Xenopsylla cheopis | 2 | 1 | 50.0 |
| 2 | Ctenophthalmus sp. | 3 | 2 | 66.7 |
| 3 | Haemolaelaps spp. | 14 | 3 | 21.4 |
| 4 | Rhipicephalus sanguineus | 10 | 1 | 10.0 |
| 5 | Hemimerus talpoides | 2 | 2 | 100.0 |
| 6 | Haemaphysalis leachi | 1 | 0 | 0 |
| Total | 32 | 9 | 28.1 |
Bartonella sp. culture
Due to contamination problems, bartonellae could be cultured from a small subset of 30 rodent blood samples only. Nine of the latter 30 blood samples produced typical bartonellae growth. The colonies were creamy white in color, small, moist with metallic sheen and tended to pit on the agar. Initial growth of Bartonella sp. cultures were seen after 5–7 days of incubation. Colonies were sub cultured onto new plates to obtain pure cultures, which were harvested and preserved in 10% glycerol at −80°C until molecularly analyzed.
Detection of citrate synthase gene (gltA) fragments in rodent blood and ectoparasites
Bartonella gltA gene fragments were detected in 46 of 177 (26%) rodent blood samples screened in this study. One of 2 C. gambianus (50%), 36 of 121 R. norvegicus (29.8%), and 9 of 48 R. rattus (18.8%) were positive for Bartonella sp. DNA. None of the 6 M. musculus examined was positive for Bartonella sp. gltA. Nine of 32 (28%) ectoparasite pools removed from 48/177 (27.1%) rodents were positive for Bartonella gltA DNA. All the ectoparasite species tested were positive for Bartonella sp. gltA except H. leachi (Table 1).
Forty six gltA sequences were obtained from blood, 3 from bacterial cultures and 9 from ectoparasite samples. Selected Bartonella sequences were deposited in GenBank under the following accession numbers: JX0265667–JX0265697 for blood, JX026972 for culture, and JX 026997–JX027006 for ectoparasites.
Comparison of retrieved Bartonella gltA sequences from rodent blood with GenBank deposits
Sequences obtained were compared with Bartonella sp. sequences deposited in GenBank for sequence similarity. Thirty six sequences were obtained from R. norvegicus blood, 26 of which had 98–100% identity with GenBank deposited B. elizabethae sequence (n = 2,100% identity; n = 23, 99%; n = 1, 98%). Nine of the sequences obtained from R. norvegicus blood had 97–98% identity with GenBank deposited Bartonella tribocorum sequence, while 1 sequence had 98% similarity with GenBank deposited Bartonella grahamii. Nine sequences were obtained from R. rattus blood, 7 of which had sequence identity of 98–100% with GenBank deposited B. elizabethae sequence (n = 3, 100% identity; n = 1, 99%; n = 3, 98%) (Table 2). The sequence retrieved from the blood of C. gambianus had 99% identity with GenBank deposited B. elizabethae.
Table 2. Genetic relationship between Bartonella species detected in this study and those from other geographic regions.
| Genotypes determined in this study with deposited accession number | Host in this study | First match identity with GenBank deposited sequence accession number. and similarity percentage | Host of related sequence | Country of host | Attributed to |
| RN 149-JX026969 | Rattus norvegicus | Bartonella sp. (EF213769.1); 99 | Rattus norvegicus | China | Bartonella elizabethae |
| RN4-JX026970 | Rattus norvegicus | Uncultured Bartonella sp. (FJ686050.1);99 | Acomys cahirinus | Israel | Bartonella elizabethae |
| RR28-JX026971 | Rattus rattus | Uncultured Bartonella sp. (FJ851115.1);99 | Small mammals | Equatorial Africa | Bartonella elizabethae |
| RN 128-JX026972 | Rattus norvegicus | Bartonella elizabethae (GQ225710.1);99 | Humans | Thailand | Bartonella elizabethae |
| CG169-JX026973 | Cricetomyces gambianus | Uncultured Bartonella sp. (FJ686050.1);99 | Wild rodents | Israel | Bartonella elizabethae |
| RN171-JX026976 | Rattus norvegicus | Bartonella elizabethae (GQ225710.1);98 | Humans | Thailand | Bartonella elizabethae |
| RN 132-JX026981 | Rattus norvegicus | Bartonella tribocorum (AM260525.1);97 | NA | NA | Bartonella tribocorum |
| RN 10-JX026990 | Rattus norvegicus | Uncultured Bartonella sp. (FJ851112.1);98 | Small mammal | Equatorial Africa | Bartonella grahamii |
| RN143-JX026977 | Rattus norvegicus | Uncultured Bartonella sp. (FJ686050.1);99 | Wild rodent | Israel | Bartonella elizabethae |
| RR159-JX026974 | Rattus rattus | Bartonella sp. FJ589062.1);99 | Rattus tanezumi flavipectus | China | Bartonella elizabethae |
| RN5-JX026978 | Rattus norvegicus | Uncultured Bartonella sp.(FJ851112.1);97 | Small mammal | Equatorial Africa | Bartonella elizabethae |
| RR164-JX026968 | Rattus rattus | Bartonella sp. (FJ589062.1);100 | Rattus tanezumi flavipectus | China | Bartonella elizabethae |
| RT20-JX026997 | Xenopsylla cheopis | Bartonella sp. (FJ589056.1);98 | Rattus tanezumi flavipectus | China | Bartonella elizabethae |
| RT22-JX027008 | Ctenophthalmus sp. | Uncultured Bartonella sp. (FJ851112.1); 97 | Small mammal | Equatorial Africa | Bartonella tribocorum |
| RT7-JX027005 | Rhipicephalus sanguineus | Bartonella elizabethae (GQ225710.1); 97 | Human | Thailand | Bartonella elizabethae |
| RT30-JX026999 | Hemimerus talpoides | Uncultured Bartonella sp.(FJ686050.1); 99 | Acomys cahirinus | Israel | Bartonella elizabethae |
Comparison of retrieved Bartonella gltA sequences from ectoparasites with GenBank deposits
Bartonella gltA sequences obtained from one pool each of X. cheopis, R. sanguineus, and 3 pools of Haemolaelaps sp. had 97–100% similarity to B. elizabethae deposited in GenBank while a sequence from Ctenophthalmus sp. pool had 97% identity with B. tribocorum sequence deposited in GenBank. Interestingly, Bartonella sp. DNA with 99% sequence identity to B. elizabethae deposited in the GenBank was detected from one pool of H. talpoides earwigs that were removed from C. gambianus rats.
Comparison of Bartonella DNA in ectoparasites and their hosting rodents
Bartonella spp. DNA was detected in 4 of 13 (30.8%) rodents from which the ectoparasites were removed. However, only one ectoparasite, H. talpoides removed from C. gambianus had the same percent sequence identity (100%) with that of the host. The DNA sequences from the ectoparasites had 97–99% identity with their first GenBank match (Table 3). The R. sanguineus pool that was positive for Bartonella spp. DNA was collected from R. norvegicus rat that was negative for Bartonella sp. DNA.
Table 3. Sequence similarity between Bartonella sp. DNA from ectoparasites and their hosting rodent.
| S/No | Ectoparasite type | GenBank first match, accession number and percentage similarity | |
| Ectoparasite | Host | ||
| 1 | Mesostigmata | Uncultured Bartonella sp. (FJ686050.1); 99 | Negative |
| 2 | Mesostigmata | Uncultured Bartonella sp.(FJ851115.1); 99 | Bartonella sp. (EF213769.1); 99 |
| 3 | Xenopsylla cheopis | Bartonella sp. (FJ589056.1)98% | Uncultured Bartonella sp. (FJ686050.1); 99 |
| 4 | Rhipicephalus sanguineus | Bartonella elizabethae (GQ225710.1) ; 97 | Negative |
| 5 | Ctenophthalmus sp. | Uncultured Bartonella sp. (FJ851112.1); 97 | Uncultured Bartonella sp. (FJ686050.1); 99 |
| 6 | Hemimerus talpoides | Uncultured Bartonella sp. (FJ686050.1); 99 | Uncultured Bartonella sp. (FJ686050.1); 99 |
Phylogenetic analysis of gltA sequences
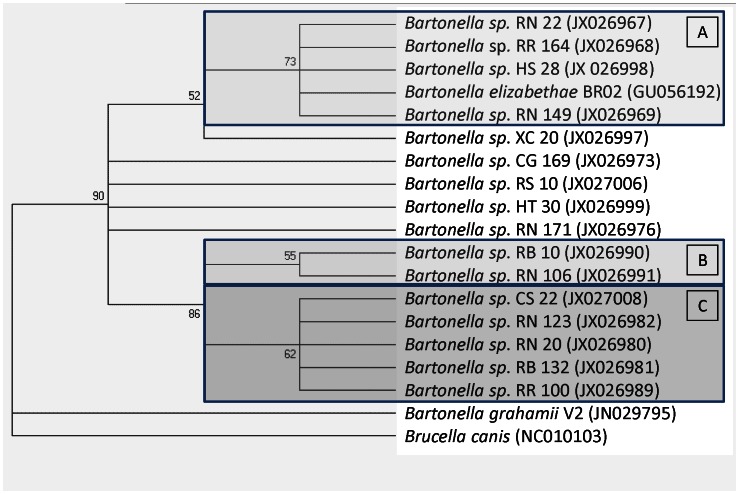
The phylogenetic relationship among the genotypes obtained in the present study and previously described Bartonella species is presented in Figure 1. Sequences of Bartonella sp. from this study formed 3 distinct clusters A-C along with B. elizabethae and B. grahamii (Fig. 1), but was distantly related to other sequences deposited in the GenBank. The first cluster (cluster A) consists of 4 sequences closely related to B. elizabethae. However, 5 other sequences that were 97–100% similar to B. elizabethae appear as single genotypes just below cluster A. Cluster B is made up of 2 sequences that were similar to B. grahamii deposited in the GenBank. The cluster C consists of 5 sequences that were 97–100% similar to B. tribocorum deposited in the genBank.
Figure 1. Phylogenic tree of Bartonella gltA sequences detected in this study showing three distinct clusters A–C.
Sequences were coded based on rodent or ectoparasites species from which they were detected, accession numbers are in parentheses; RR = Rattus rattus; RN = Rattus norvegicus; CG = Cricetomys gambianus; CS = Ctenophthalmus sp; HT = Hemimerus talpoides; RS = Rhipicephalus sanguineus, MS = Haemolaelaps spp.; XC = Xenopsylla cheopis.
Discussion
In this study, we report the molecular detection and genetic characterization of Bartonella species in rodents and ectoparasites from Nigeria, West Africa. Moreover, to the best of our knowledge, this is the first report of molecular investigation of Bartonella spp. in rodents and their ectoparasites in this country. The 26% prevalence of Bartonella DNA found in this study was higher than the 8.5% prevalence reported in small mammals from the Democratic Republic of Congo but lower than and 38% reported in Tanzania [26]. The differences between the findings in the latter studies and ours can be attributed to the fact that commensal rodents were screened in the current study while sylvatic rodents were screened in the DR Congo and Tanzania studies. Similarly, the 28% prevalence of Bartonella DNA by gltA PCR in ectoparasites in this study was slightly higher than the 21.5% reported in fleas from Algeria, targeting 3 genes and the inter-genic spacer (ITS) [28]. The high prevalence of detection of Bartonella spp. DNA in the ectoparasites attests to their role as vectors of these bacteria.
Several Bartonella spp. that were associated with human diseases were identified in this study, including B. elizabethae, B. grahamii and B. tribocorum. Bartonella elizabethae was found in patients with endocarditis [36]. Bartonella grahamii was associated with neuroretinitis or bilateral retinal artery branch occlusions [37]. A Bartonella genotype closely related (97%) to B. tribocorum was detected in the blood of human patient with fever from Thailand [25]. The finding of these zoonotic Bartonella spp. in commensal rodents from Nigeria demonstrates their importance as reservoirs for various zoonotic Bartonella species and warrants increased awareness of physicians and health care workers for these pathogens especially in unidentified febrile cases.
In this study, no DNA sequence similar to B. tribocorum was obtained from R. rattus rats. The detection of B. tribocorum only in R. norvegicus rats is in agreement with the earlier report of Márquez et al. [23] which supports the hypothesis that there is specificity of Bartonella spp. for their rodent hosts [15].
Although the role of R. sanguineus ticks in transmitting Bartonella spp. in nature is not proven [38] it is important to note that we detected Bartonella DNA in R. sanguineus ticks. Detection of Bartonella DNA in ticks was previously reported also by other authors [8], [17], [39]. The Bartonella spp. DNA detected from one R. sanguineus tick pool had 97 percent identity to B. elizabethae sequences deposited in GenBank. It is worthy to note that the host from which the R. sanguineus ticks were removed was negative for Bartonella spp. DNA. This suggests that the ticks might have acquired the bacteria during previous feeding on an infected host. The ability of the tick to transmit this organism to a susceptible host during the next feeding stage or to its progeny is worth further investigation.
Comparative analyses of the gltA sequences obtained from Bartonella spp. showed that commensal rodents in Nigeria harbor a diverse assemblage of Bartonella strains, some of which represent known Bartonella spp. and strains and others may represent distinct novel strains. Although only a portion of the citrate synthase gene (gltA) was used for phylogenetic analysis, this gene has been shown to be a reliable tool for distinguishing between closely related Bartonella genotypes [40]. By using this partial gene, it was possible to compare the variety of Bartonella genotypes isolated from rodents with homologous sequences of Bartonella strains found in other mammals, reported from other parts of the world. Finding considerable sequence diversity is typical for different species of Bartonella, although more characteristics are needed to describe novel Bartonella species [3].
In this study, the Bartonella genogroups identified in commensal rodents formed three separate clusters closely related to B. elizabethae but distantly related to other known Bartonella spp. Although BLAST searches shows some of the sequences had 97–100% similarity to B. tribocorum and B. grahamii sequences deposited in GenBank (Fig. 1). The findings of Bartonella sequences that were genetically distant from known GenBank deposited sequences requires further investigation in characterizing these genotypes and ascertaining whether they are pathogenic to animals and/or humans.
Pools of H. talpoides collected from C. gambianus in this study contained Bartonella DNA. Hemimerus talpoides (earwig sp.) are presumed to feed on the epidermis of their host or as a saprophytic on fungus from the skin of the host. The detection of Bartonella sp. DNA in this ectoparasite is interesting and requires further investigation [41].
In conclusion, this study has resulted in the identification and genetic characterization of Bartonella genotypes in commensal rodents and ectoparasites from Nigeria, West Africa. A high prevalence and diversity of Bartonella spp. and strains was detected in commensal rodents and their ectoparasites in this study. Several zoonotic Bartonella spp. including B. elizabethae, B. grahamii and B. tribocorum were identified for the first time in Nigeria highlighting their importance for public health in this country.
Acknowledgments
The authors acknowledge the technical assistance of Zohar Pasternak, Osnat Eyal and Ricardo Gutierrez (Faculty of Agriculture Food and Environment, The Hebrew University of Jerusalem, Rehovot Israel) and Kemza Sarah (NVRI Vom, Nigeria) for her assistance during sampling.
Funding Statement
The authors have indicated that no funding was received for this work.
References
- 1. Mogollon-Pasapera E, Otvos L Jr, Giordana A, Cassone M (2009) Bartonella: emerging pathogen or emerging awareness? Intern J Infect Dis 13: 3–8. [DOI] [PubMed] [Google Scholar]
- 2. Brouqui P, Raoult D (2006) New insight into the diagnosis of fastidious bacterial endocarditis. FEMS Immunol Med Microbiol 47: 1–13. [DOI] [PubMed] [Google Scholar]
- 3. Kosoy M, Hayman DT, Chan KS (2012) Bartonella bacteria in nature: Where does population variability end and a species start? Infect Genet and Evol 12: 894–904. [DOI] [PubMed] [Google Scholar]
- 4. Kaiser PO, Reiss T, O'Rourke F, Linke D, Kempf VA (2011) Bartonella spp.: Throwing light on uncommon human infections Mini review. Intern J Med Microbiol 301: 7–15. [DOI] [PubMed] [Google Scholar]
- 5. Dehio C (2004) Molecular and cellular basis of Bartonella pathogenesis. Annu Rev Microbiol 58: 365–390. [DOI] [PubMed] [Google Scholar]
- 6. Stevenson HL, Bai Y, Kosoy MY, Montenieri JA, Lowell JL, et al. (2003) Detection of novel Bartonella strains and Yersinia pestis in prairie dogs and their fleas (Siphonaptera: Ceratophyllidae and Pulicidae) using multiplex polymerase chain reaction. J Med Entomol 40: 329–337. [DOI] [PubMed] [Google Scholar]
- 7. Morway C, Kosoy M, Eisen R, Montenieri J, Sheff K, et al. (2008) A longitudinal study of Bartonella infection in populations of woodrats and their fleas. J Vector Ecol 33: 353–364. [DOI] [PubMed] [Google Scholar]
- 8. Kabeya H, Colborn JM, Bai Y, Lerdthusnee K, Richardson JH, et al. (2010) Detection of Bartonella tamiae DNA in ectoparasites from rodents in Thailand and their sequence similarity with bacterial cultures from Thai Patients. Vector-Borne Zoon Dis 10 5: 429–434. [DOI] [PubMed] [Google Scholar]
- 9. Liu Q, Eremeeva ME, Li D (2012) Bartonella and Bartonella infections in China: From the clinic to the Laboratory. Comp Immunol Microbiol and Infect Dis 35: 93–102. [DOI] [PubMed] [Google Scholar]
- 10. Morick D, Baneth G, Avidor B, Kosoy MY, Mumcuoglu KY, et al. (2009a) Detection of Bartonella spp. in wild rodents in Israel using HRM real-time PCR. Vet Microbiol 139: 293–297. [DOI] [PubMed] [Google Scholar]
- 11. Morick D, Krasnov BR, Khokhlova IS, Shenbrot GI, Kosoy MY, et al. (2010) Bartonella genotypes in fleas (Insecta: Siphonaptera) collected from rodents in the Negev Desert, Israel. Appl Environ Microbiol 76: 6864–6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marston EL, Finkel B, Regnery RL, Winoto IL, Graham RR, et al. (1999) Prevalence of Bartonella henselae and Bartonella clarridgeiae in an urban Indonesian cat population. Clin Diagn Lab Immunol 6 1: 41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gundi VAKB, Kosoy MY, Myint KSA, Shrestha SK, Shrestha MP, et al. (2010) Prevalence and genetic diversity of Bartonella species detected in different tissues of small mammals in Nepal. Appl Environ Microbiol 76: 8247–8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Castle KT, Kosoy M, Lerdthusnee K, Phelan L, Bai Y, et al. (2004) Prevalence and diversity of Bartonella in rodents of Northern Thailand: A comparison with Bartonella in rodents from Southern China. Am J Trop Med Hyg 70 4: 429–433. [PubMed] [Google Scholar]
- 15. Bai Y, Kosoy MY, Maupin GO, Tsuchiya KR, Gage KL (2002) Genetic and ecologic characteristics of Bartonella communities in rodents in southern China. Am J Trop Med 66 5: 622–7. [DOI] [PubMed] [Google Scholar]
- 16. Hsieh JW, Tung KC, Chen WC, Lin JW, Chien LJ, et al. (2010) Epidemiology of Bartonella infection in rodents and shrews in Taiwan. Zoon Pub Hlth 57: 439–446. [DOI] [PubMed] [Google Scholar]
- 17. Kim CM, Kim JY, Yi HY, Lee MJ, Cho MR, et al. (2005) Detection of Bartonella species from ticks, mites and small mammals in Korea. J Vet Sci 6 4: 327–334. [PubMed] [Google Scholar]
- 18. Kosoy M, Murray M, Gilmore RD Jr, Bai Y, Gage KL (2003) Bartonella strains from ground squirrels are identical to Bartonella washoensis isolated from a human patient. Journ Clin Microbiol 41 2: 645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bai Y, Kosoy M, Martin A, Ray C, Sheff K, et al. (2008) Characterization of Bartonella strains isolated from black-tailed prairie dogs (Cynomys ludovicianus). Vector-Borne Zoon Dis 8 1: 1–5. [DOI] [PubMed] [Google Scholar]
- 20. Bai Y, Cross PC, Malania L, Kosoy M (2011) Isolation of Bartonella capreoli from elk. Vet Microbiol 148: 329–332. [DOI] [PubMed] [Google Scholar]
- 21. Birtles RJ, Laycock G, Kenny MJ, Shaw SE, Day MJ (2002) Prevalence of Bartonella species causing bacteraemia in domesticated and companion animals in the United Kingdom. Vet Rec 24: 2002–225. [DOI] [PubMed] [Google Scholar]
- 22. Bown KJ, Bennett M, Begon M (2004) Flea-borne Bartonella grahamii and Bartonella taylorii in bank voles. Emerg Infect Dis www.cdc.gov/eid 10 4: 684–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Márquez FJ, Rodríguez-Liébana JJ, Pachón-Ibáñez ME, Docobo-Pérez F, Hidalgo-Fontiveros A, et al. (2008) Molecular screening of Bartonella species in rodents from South Western Spain. Vector-Borne Zoon Dis 8 5: 695–700. [DOI] [PubMed] [Google Scholar]
- 24. Morick D, Osinga N, Gruys E, Harrus S (2009b) Identification of a Bartonella species in the harbor seal (Phoca vitulina) and in seal lice (Echinophtirius horridus). Vector-Borne Zoon Dis 9 6: 751–753. [DOI] [PubMed] [Google Scholar]
- 25. Kosoy M, Bai Y, Sheff K, Morway C, Baggett H, et al. (2010) Identification of Bartonella infections in febrile human patients from Thailand and their potential animal reservoirs. Am J Trop Med Hyg 82 6: 1140–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gundi VA, Kosoy MY, Makundi RH, Laudisoit A (2012) Identification of diverse Bartonella genotypes among small mammals from Democratic Republic of Congo and Tanzania. Am J Trop Med Hyg 87 2: 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kernif T, Asisi M, Doumandji SE, Chomel BB, Raoult D, et al. (2010) Molecular evidence of Bartonella infection in domestic dogs from Algeria, North Africa, by polymerase chain reaction (PCR). Am J Trop Med Hyg 83 2: 298–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bitam I, Rolain JM, Nicolas V, Tsai YL, Parola P, et al. (2012) A multi-gene analysis of diversity of Bartonella detected in fleas from Algeria. Comp Immunol Microbiol Infect Dis 35: 71–76. [DOI] [PubMed] [Google Scholar]
- 29. Loftis AD, Reeves WK, Szumlas DE, Abbassy MM, Helmy IM, et al. (2006) Surveillance of Egyptian fleas for agents of public health significance: Anaplasma, Bartonella, Coxiella, Ehrlichia, Rickettsia, and Yersinia pestis . Am J Trop Med Hyg 183–8. [PubMed] [Google Scholar]
- 30. Reeves WK, Loftis AD, Szumlas DE, Abbassy MM, Helmy IM, et al. (2007) Rickettsial pathogens in the tropical rat mite Ornithonyssus bacoti (Acari: Macronyssidae) from Egyptian rats (Rattus spp.). Exp Appl Acarol 41: 101–107. [DOI] [PubMed] [Google Scholar]
- 31. Gundi VAKB, Bourry O, Davoust B, Raoult D, La Scola B (2004) Bartonella clarridgeiae and B. henselae in dogs, Gabon. Emerg Infect Dis www.cdc.gov/eid 10 12: 2261–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kelly PJ, Matthewman LA, Hayter D, Downey S, Wray K, et al. (1996) Bartonella (Rochalimaea) henselae in Southern Africa – evidence for infections in domestic cats and implications for veterinarians. Journ South Afri Vet Assoc 67: 182–187. [PubMed] [Google Scholar]
- 33. Pretorius AM, Beati L, Birtles RJ (2004) Diversity of bartonellae associated with small mammals inhabiting Free State province, South Africa. Int J Sys Evol Microbiol 54: 1959–1967. [DOI] [PubMed] [Google Scholar]
- 34. Norman AF, Regnery R, Jameson P, Greene C, Krause DC (1995) Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J Clin Microbiol 33: 1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Daly JS, Worthington MG, Brenner DJ, Moss CW, Hollis DG, et al. (1993) Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol 31: 872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Serratrice J, Rolain JM, Granel B, Ene N, Conrath J, et al. (2003) Bilateral retinal artery branch occlusions revealing Bartonella grahamii infection. Rev Med Intern 24: 629–630. [DOI] [PubMed] [Google Scholar]
- 38. Telford SR III, Wormser GP (2010) Bartonella spp. transmission by ticks not established. Emerg Infect Dis www.cdc.gov/eid 16 3: 379–384 DOI:10.3201/eid1603.090443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tsai YL, Chuang ST, Chang CC, Kass PH, Chomel BB (2010) Bartonella species in small mammals and their ectoparasites in Taiwan. Am J Trop Med Hyg 83 4: 917–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. La Scola B, Zeaiter Z, Khamis A, Raoult D (2003) Gene-sequence-based criteria for species definition in bacteriology: the Bartonella paradigm. Trends Microbiol 11: 318–21 DOI:10.1016/S0966-842X(03)00143-4. [DOI] [PubMed] [Google Scholar]
- 41. Rehn JA, Rehn JWH (1936) A study of the genus hemimerus (Dermater hemimerina Hemimeridae). Proc Acad Nat Sciences, Philadelphia 87: 457–508. [Google Scholar]