Abstract
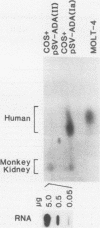
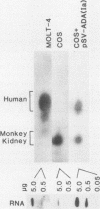
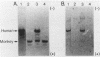
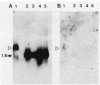
Human adenosine deaminase (ADA) is an important purine catabolic enzyme which irreversibly deaminates adenosine and deoxyadenosine. Severe genetic deficiency of ADA leads to an immunological deficiency state in which T-lymphoid cells are selectively destroyed by the accumulation of toxic levels of deoxyadenosine and deoxy-ATP. In preparation for transfer of ADA sequences into a variety of cell types, we explored expression of ADA cDNAs transfected into cultured cells within a simian virus 40-based expression vector. After transfection into monkey kidney (COS) cells, ADA cDNA encompassing the entire coding region of the protein generated human ADA activity. An unexpected finding, however, was the identification of a cDNA clone that failed to produce either human enzyme activity or immunoreactive ADA protein. As this pattern is typical of many naturally occurring mutant ADA alleles, we characterized the molecular defect in this clone. DNA sequence analysis revealed a single nucleotide substitution in amino acid position 50 (glycine-valine). Northern blotting with a unique 17-mer oligonucleotide demonstrated the absence of the mutant sequence in the mRNA from which the cDNA library giving rise to the mutant cDNA was constructed. Therefore, the substitution in the variant cDNA was created during cloning. These data define one critical region of the human ADA protein molecule and suggest a convenient strategy for characterization of the phenotypes associated with naturally occurring mutant alleles.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian G. S., Hutton J. J. Adenosine deaminase messenger RNAs in lymphoblast cell lines derived from leukemic patients and patients with hereditary adenosine deaminase deficiency. J Clin Invest. 1983 Jun;71(6):1649–1660. doi: 10.1172/JCI110920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian G. S., Wiginton D. A., Hutton J. J. Structure of adenosine deaminase mRNAs from normal and adenosine deaminase-deficient human cell lines. Mol Cell Biol. 1984 Sep;4(9):1712–1717. doi: 10.1128/mcb.4.9.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkowsky W., Gershon A. A., Shenkman L., Hirschhorn R. Adenosine deaminase deficiency without immunodeficiency: clinical and metabolic studies. Pediatr Res. 1980 Jul;14(7):885–889. doi: 10.1203/00006450-198007000-00009. [DOI] [PubMed] [Google Scholar]
- Chen S. H., Ochs H. D., Scott C. R., Giblett E. R., Tingle A. J. Adenosine deaminase deficiency: disappearance of adenine deoxynucleotides from a patient's erythrocytes after successful marrow transplantation. J Clin Invest. 1978 Dec;62(6):1386–1389. doi: 10.1172/JCI109259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daddona P. E., Frohman M. A., Kelley W. N. Human adenosine deaminase and its binding protein in normal and adenosine deaminase-deficient fibroblast cell strains. J Biol Chem. 1980 Jun 25;255(12):5681–5687. [PubMed] [Google Scholar]
- Daddona P. E., Kelley W. N. Human adenosine deaminase. Purification and subunit structure. J Biol Chem. 1977 Jan 10;252(1):110–115. [PubMed] [Google Scholar]
- Daddona P. E., Mitchell B. S., Meuwissen H. J., Davidson B. L., Wilson J. M., Koller C. A. Adenosine deaminase deficiency with normal immune function. An acidic enzyme mutation. J Clin Invest. 1983 Aug;72(2):483–492. doi: 10.1172/JCI110996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daddona P. E., Shewach D. S., Kelley W. N., Argos P., Markham A. F., Orkin S. H. Human adenosine deaminase. cDNA and complete primary amino acid sequence. J Biol Chem. 1984 Oct 10;259(19):12101–12106. [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Martiniuk F., Roegner-Maniscalco V., Ellenbogen A., Perignon J. L., Jenkins T. Genetic heterogeneity in partial adenosine deaminase deficiency. J Clin Invest. 1983 Jun;71(6):1887–1892. doi: 10.1172/JCI110944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin D. W., Jr, Gelfand E. W. Biochemistry of diseases of immunodevelopment. Annu Rev Biochem. 1981;50:845–877. doi: 10.1146/annurev.bi.50.070181.004213. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McDevitt M. A., Imperiale M. J., Ali H., Nevins J. R. Requirement of a downstream sequence for generation of a poly(A) addition site. Cell. 1984 Jul;37(3):993–999. doi: 10.1016/0092-8674(84)90433-1. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Howard B. H., Berg P. Synthesis of rabbit beta-globin in cultured monkey kidney cells following infection with a SV40 beta-globin recombinant genome. Nature. 1979 Jan 11;277(5692):108–114. doi: 10.1038/277108a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Daddona P. E., Shewach D. S., Markham A. F., Bruns G. A., Goff S. C., Kelley W. N. Molecular cloning of human adenosine deaminase gene sequences. J Biol Chem. 1983 Nov 10;258(21):12753–12756. [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr, Antonarakis S. E., Ostrer H., Goff S. C., Sexton J. P. Abnormal RNA processing due to the exon mutation of beta E-globin gene. Nature. 1982 Dec 23;300(5894):768–769. doi: 10.1038/300768a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Markham A. F., Kazazian H. H., Jr Direct detection of the common Mediterranean beta-thalassemia gene with synthetic DNA probes. An alternative approach for prenatal diagnosis. J Clin Invest. 1983 Mar;71(3):775–779. doi: 10.1172/JCI110826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkman R., Gelfand E. W., Rosen F. S., Sanderson A., Hirschhorn R. Severe combined immunodeficiency and adenosine deaminase deficiency. N Engl J Med. 1975 Apr 3;292(14):714–719. doi: 10.1056/NEJM197504032921402. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. The end of the message and beyond. Nature. 1984 Feb 2;307(5950):412–413. doi: 10.1038/307412a0. [DOI] [PubMed] [Google Scholar]
- Subramani S., Southern P. J. Analysis of gene expression using simian virus 40 vectors. Anal Biochem. 1983 Nov;135(1):1–15. doi: 10.1016/0003-2697(83)90723-6. [DOI] [PubMed] [Google Scholar]
- Thompson L. F., Seegmiller J. E. Adenosine deaminase deficiency and severe combined immunodeficiency disease. Adv Enzymol Relat Areas Mol Biol. 1980;51:167–210. doi: 10.1002/9780470122969.ch4. [DOI] [PubMed] [Google Scholar]
- Treisman R., Orkin S. H., Maniatis T. Specific transcription and RNA splicing defects in five cloned beta-thalassaemia genes. Nature. 1983 Apr 14;302(5909):591–596. doi: 10.1038/302591a0. [DOI] [PubMed] [Google Scholar]
- Valerio D., Duyvesteyn M. G., Meera Khan P., Geurts van Kessel A., de Waard A., van der Eb A. J. Isolation of cDNA clones for human adenosine deaminase. Gene. 1983 Nov;25(2-3):231–240. doi: 10.1016/0378-1119(83)90227-5. [DOI] [PubMed] [Google Scholar]
- Valerio D., Duyvesteyn M. G., van Ormondt H., Meera Khan P., van der Eb A. J. Adenosine deaminase (ADA) deficiency in cells derived from humans with severe combined immunodeficiency is due to an aberration of the ADA protein. Nucleic Acids Res. 1984 Jan 25;12(2):1015–1024. doi: 10.1093/nar/12.2.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Weyden M. B., Kelley W. N. Human adenosine deaminase. Distribution and properties. J Biol Chem. 1976 Sep 25;251(18):5448–5456. [PubMed] [Google Scholar]
- Wiginton D. A., Adrian G. S., Friedman R. L., Suttle D. P., Hutton J. J. Cloning of cDNA sequences of human adenosine deaminase. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7481–7485. doi: 10.1073/pnas.80.24.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiginton D. A., Adrian G. S., Hutton J. J. Sequence of human adenosine deaminase cDNA including the coding region and a small intron. Nucleic Acids Res. 1984 Mar 12;12(5):2439–2446. doi: 10.1093/nar/12.5.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiginton D. A., Hutton J. J. Immunoreactive protein in adenosine deaminase deficient human lymphoblast cell lines. J Biol Chem. 1982 Mar 25;257(6):3211–3217. [PubMed] [Google Scholar]
- Woychik R. P., Lyons R. H., Post L., Rottman F. M. Requirement for the 3' flanking region of the bovine growth hormone gene for accurate polyadenylylation. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3944–3948. doi: 10.1073/pnas.81.13.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]