Abstract
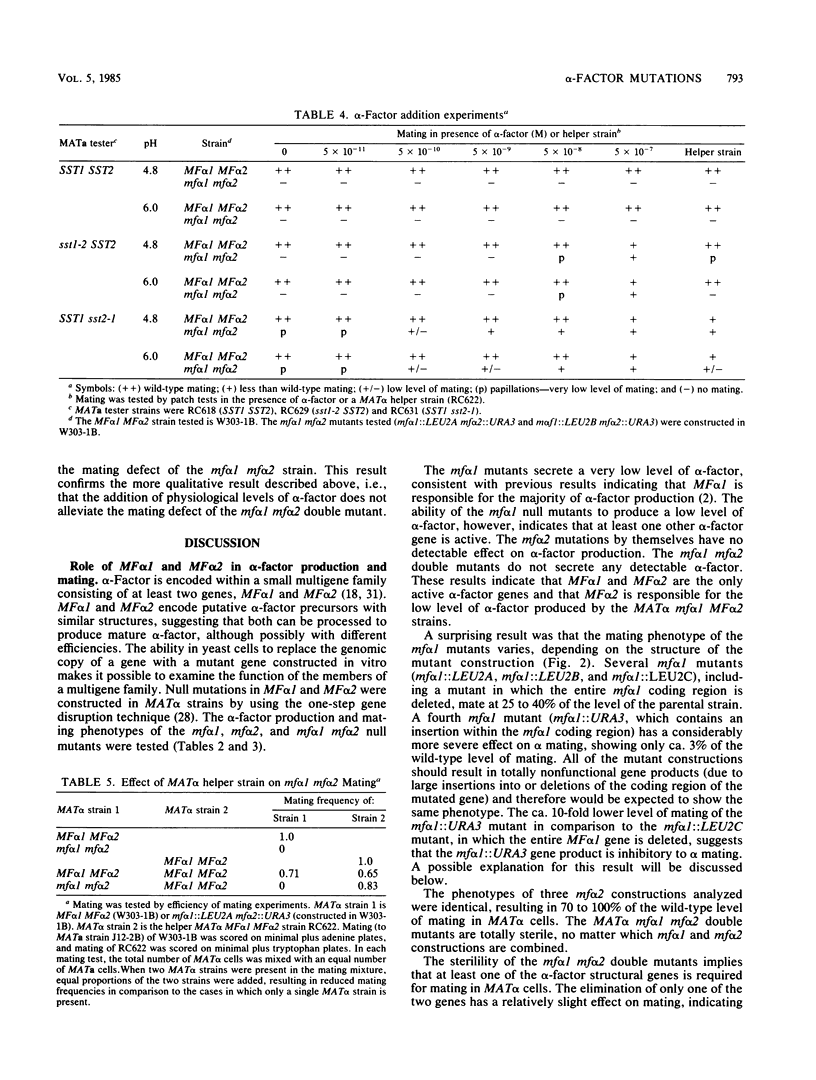
The role of alpha-factor structural genes MF alpha 1 and MF alpha 2 in alpha-factor production and mating has been investigated by the construction of mf alpha 1 and mf alpha 2 mutations that totally eliminate gene function. An mf alpha 1 mutant in which the entire coding region is deleted shows a considerable decrease in alpha-factor production and a 75% decrease in mating. Mutations in mf alpha 2 have little or no effect on alpha-factor production or mating. The mf alpha 1 mf alpha 2 double mutants are completely defective in mating and alpha-factor production. These results indicate that at least one alpha-factor structural gene product is required for mating in MAT alpha cells, that MF alpha 1 is responsible for the majority of alpha-factor production, and that MF alpha 1 and MF alpha 2 are the only active alpha-factor genes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brake A. J., Julius D. J., Thorner J. A functional prepro-alpha-factor gene in Saccharomyces yeasts can contain three, four, or five repeats of the mature pheromone sequence. Mol Cell Biol. 1983 Aug;3(8):1440–1450. doi: 10.1128/mcb.3.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Bücking-Throm E., Duntze W., Hartwell L. H., Manney T. R. Reversible arrest of haploid yeast cells in the initiation of DNA synthesis by a diffusible sex factor. Exp Cell Res. 1973 Jan;76(1):99–110. doi: 10.1016/0014-4827(73)90424-2. [DOI] [PubMed] [Google Scholar]
- Chan R. K., Melnick L. M., Blair L. C., Thorner J. Extracellular suppression allows mating by pheromone-deficient sterile mutants of Saccharomyces cerevisiae. J Bacteriol. 1983 Aug;155(2):903–906. doi: 10.1128/jb.155.2.903-906.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. K., Otte C. A. Isolation and genetic analysis of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and alpha factor pheromones. Mol Cell Biol. 1982 Jan;2(1):11–20. doi: 10.1128/mcb.2.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciejek E., Thorner J. Recovery of S. cerevisiae a cells from G1 arrest by alpha factor pheromone requires endopeptidase action. Cell. 1979 Nov;18(3):623–635. doi: 10.1016/0092-8674(79)90117-x. [DOI] [PubMed] [Google Scholar]
- Comb M., Seeburg P. H., Adelman J., Eiden L., Herbert E. Primary structure of the human Met- and Leu-enkephalin precursor and its mRNA. Nature. 1982 Feb 25;295(5851):663–666. doi: 10.1038/295663a0. [DOI] [PubMed] [Google Scholar]
- Duntze W., MacKay V., Manney T. R. Saccharomyces cerevisiae: a diffusible sex factor. Science. 1970 Jun 19;168(3938):1472–1473. doi: 10.1126/science.168.3938.1472. [DOI] [PubMed] [Google Scholar]
- Dutcher S. K., Hartwell L. H. The role of S. cerevisiae cell division cycle genes in nuclear fusion. Genetics. 1982 Feb;100(2):175–184. doi: 10.1093/genetics/100.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Mutants of Saccharomyces cerevisiae unresponsive to cell division control by polypeptide mating hormone. J Cell Biol. 1980 Jun;85(3):811–822. doi: 10.1083/jcb.85.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Synchronization of haploid yeast cell cycles, a prelude to conjugation. Exp Cell Res. 1973 Jan;76(1):111–117. doi: 10.1016/0014-4827(73)90425-4. [DOI] [PubMed] [Google Scholar]
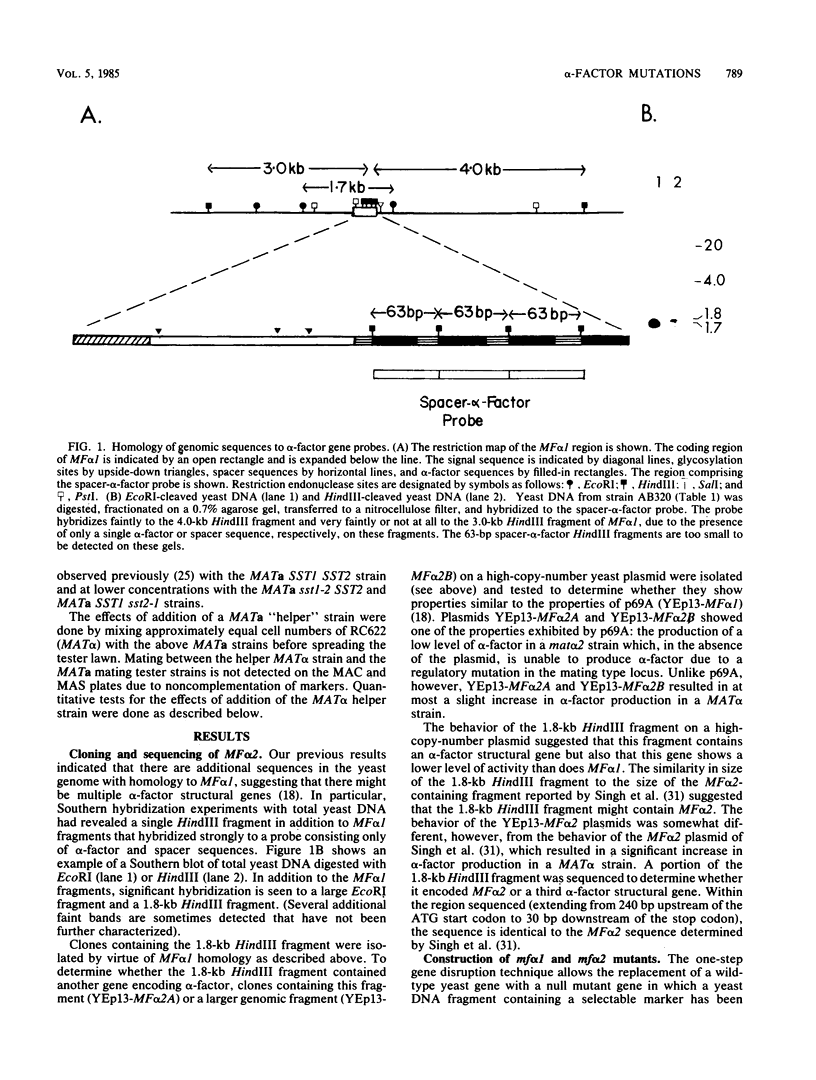
- Julius D., Blair L., Brake A., Sprague G., Thorner J. Yeast alpha factor is processed from a larger precursor polypeptide: the essential role of a membrane-bound dipeptidyl aminopeptidase. Cell. 1983 Mar;32(3):839–852. doi: 10.1016/0092-8674(83)90070-3. [DOI] [PubMed] [Google Scholar]
- Julius D., Brake A., Blair L., Kunisawa R., Thorner J. Isolation of the putative structural gene for the lysine-arginine-cleaving endopeptidase required for processing of yeast prepro-alpha-factor. Cell. 1984 Jul;37(3):1075–1089. doi: 10.1016/0092-8674(84)90442-2. [DOI] [PubMed] [Google Scholar]
- Julius D., Schekman R., Thorner J. Glycosylation and processing of prepro-alpha-factor through the yeast secretory pathway. Cell. 1984 Feb;36(2):309–318. doi: 10.1016/0092-8674(84)90224-1. [DOI] [PubMed] [Google Scholar]
- Kreil G. Transfer of proteins across membranes. Annu Rev Biochem. 1981;50:317–348. doi: 10.1146/annurev.bi.50.070181.001533. [DOI] [PubMed] [Google Scholar]
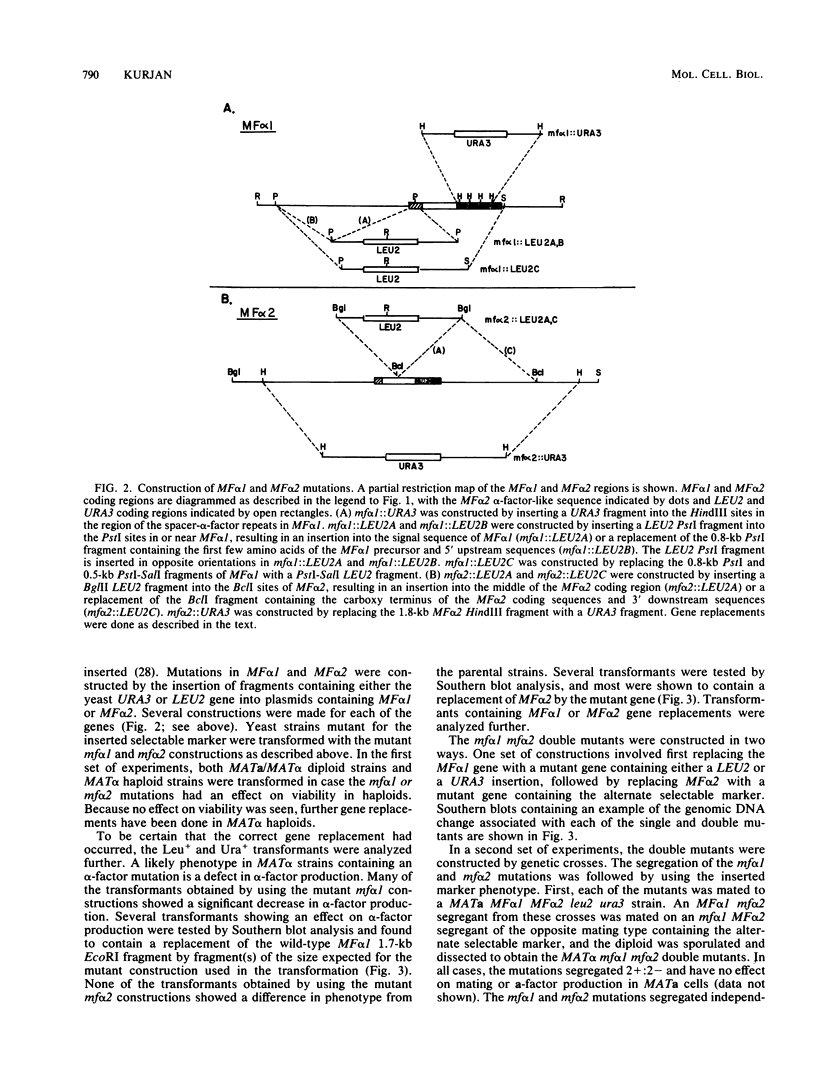
- Kurjan J., Hall B. D., Gillam S., Smith M. Mutations at the yeast SUP4 tRNATyr locus: DNA sequence changes in mutants lacking suppressor activity. Cell. 1980 Jul;20(3):701–709. doi: 10.1016/0092-8674(80)90316-5. [DOI] [PubMed] [Google Scholar]
- Kurjan J., Herskowitz I. Structure of a yeast pheromone gene (MF alpha): a putative alpha-factor precursor contains four tandem copies of mature alpha-factor. Cell. 1982 Oct;30(3):933–943. doi: 10.1016/0092-8674(82)90298-7. [DOI] [PubMed] [Google Scholar]
- Lund P. K., Goodman R. H., Dee P. C., Habener J. F. Pancreatic preproglucagon cDNA contains two glucagon-related coding sequences arranged in tandem. Proc Natl Acad Sci U S A. 1982 Jan;79(2):345–349. doi: 10.1073/pnas.79.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay V., Manney T. R. Mutations affecting sexual conjugation and related processes in Saccharomyces cerevisiae. I. Isolation and phenotypic characterization of nonmating mutants. Genetics. 1974 Feb;76(2):255–271. doi: 10.1093/genetics/76.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness P. F., Edelman G. M. Inactivation and chemical alteration of mating factor alpha by cells and spheroplasts of yeast. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1304–1308. doi: 10.1073/pnas.75.3.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manney T. R., Woods V. Mutants of Saccharomyces cerevisiae resistant to the alpha mating-type factor. Genetics. 1976 Apr;82(4):639–644. doi: 10.1093/genetics/82.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
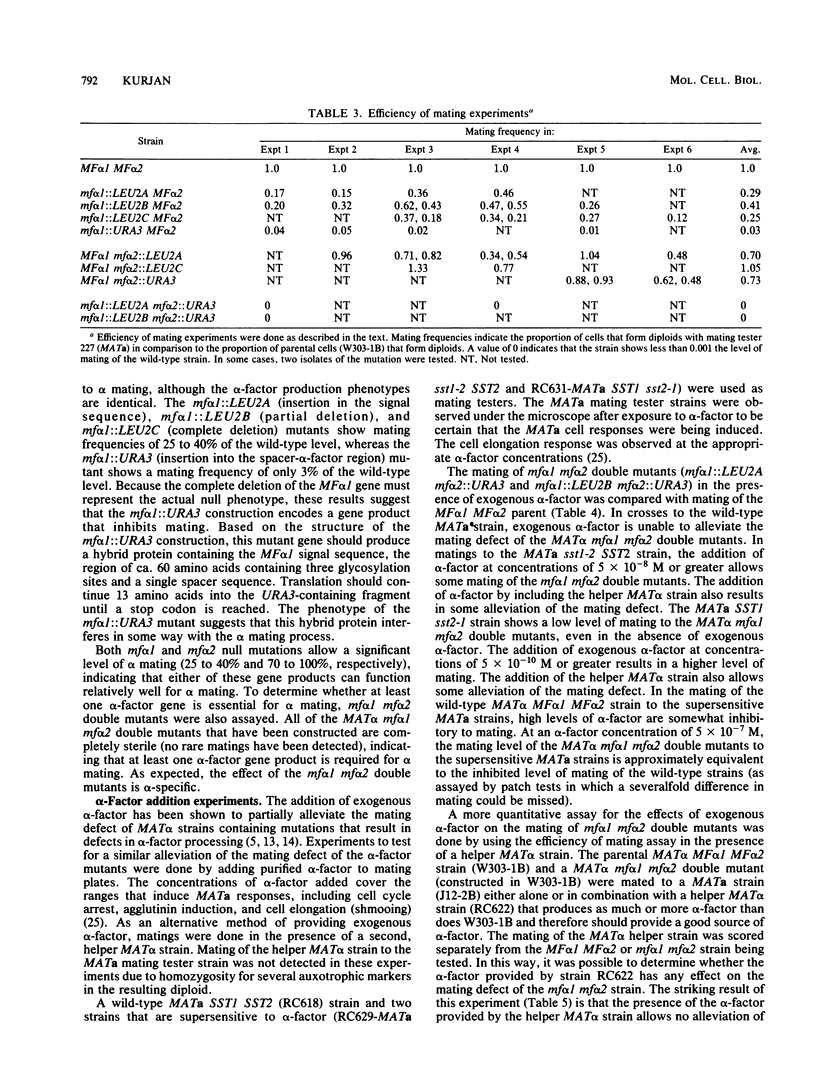
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Moore S. A. Comparison of dose-response curves for alpha factor-induced cell division arrest, agglutination, and projection formation of yeast cells. Implication for the mechanism of alpha factor action. J Biol Chem. 1983 Nov 25;258(22):13849–13856. [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A. C., Cohen S. N., Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979 Mar 29;278(5703):423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. A., Tatchell K. The structure of transposable yeast mating type loci. Cell. 1980 Mar;19(3):753–764. doi: 10.1016/s0092-8674(80)80051-1. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Chen E. Y., Lugovoy J. M., Chang C. N., Hitzeman R. A., Seeburg P. H. Saccharomyces cerevisiae contains two discrete genes coding for the alpha-factor pheromone. Nucleic Acids Res. 1983 Jun 25;11(12):4049–4063. doi: 10.1093/nar/11.12.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague G. F., Jr, Rine J., Herskowitz I. Control of yeast cell type by the mating type locus. II. Genetic interactions between MAT alpha and unlinked alpha-specific STE genes. J Mol Biol. 1981 Dec 5;153(2):323–335. doi: 10.1016/0022-2836(81)90281-3. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Quinn P. S., Chan S. J., Marsh J., Tager H. S. Processing mechanisms in the biosynthesis of proteins. Ann N Y Acad Sci. 1980;343:1–16. doi: 10.1111/j.1749-6632.1980.tb47238.x. [DOI] [PubMed] [Google Scholar]
- Stern A. S., Jones B. N., Shively J. E., Stein S., Undenfriend S. Two adrenal opioid polypeptides: proposed intermediates in the processing of proenkephalin. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1962–1966. doi: 10.1073/pnas.78.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stötzler D., Duntze W. Isolation and characterization of four related peptides exhibiting alpha factor activity from Saccharomyces cerevisiae. Eur J Biochem. 1976 May 17;65(1):257–262. doi: 10.1111/j.1432-1033.1976.tb10412.x. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Kita H. Degradation of mating factor by alpha-mating type cells of Saccharomyces cerevisiae. J Biochem. 1977 Dec;82(6):1689–1693. doi: 10.1093/oxfordjournals.jbchem.a131865. [DOI] [PubMed] [Google Scholar]