Abstract
Serotonin in blood plasma is primarily synthesized in the duodenum, as brain derived serotonin does not cross the blood-brain barrier. Because serotonin in the brain and retina is synthesized under the control of a circadian clock, we sought to determine if a circadian clock in the duodenum regulates serotonin synthesis and release in blood. We examined gene expression in the duodenum of chickens at different times of the day and found that the duodenum rhythmically expresses molecular circadian clock genes and genes controlling serotonin biosynthesis, specifically tryptophan hydroxylase, in a light dark cycle (LD). Analysis of the duodenum and blood plasma showed that the amount of serotonin in the duodenum varies across the day and that serotonin profiles in blood plasma are also rhythmic in LD, but were not rhythmic in constant darkness. Because serotonin in the gut affects duodenal nutrient absorption and gut motility, the control of serotonin production in the duodenum by LD cycles could provide an additional mechanism by which the external environment controls nutrient uptake and digestive function. The diurnal regulation of plasma serotonin may also serve as an additional biochemical signal in the blood encoding time and could be used by target tissues to indicate the status of nutrient absorption.
Introduction
All organisms synchronize their physiology and behavior to the abiotic cycles of their environment. The temporal control of these processes is regulated internally by biological clocks. In birds, circadian oscillators are found within the avian homolog of the suprachiasmatic nucleus, pineal gland, and retina. These clocks function together through a set of inhibitory interactions to form the avian circadian clock system, often described as a “Neuroendocrine Loop” [1]. Within this system, multiple sets of photoreceptors exist to enable entrainment of the system as a whole [1].
At the cellular level, circadian clocks function through the autoregulatory actions of interlocking positive and negative feedback loops of clock genes. The basic helixloophelixPAS transcription factors circadian locomotor output cycles kaput (clock) and brain and muscle ARNTlike protein (BMAL) heterodimerize and activate Ebox (5′CACGTG3′) mediated transcription of the negative elements of the loop, in particular Period (Per) and Cryptochrome (Cry). The protein products of per and cry translocate into the nucleus and repress BMAL and CLOCKmediated Ebox activation, thereby completing the cycle [for Review see 2, 3]. Genes that are controlled by molecular circadian clocks frequently have Eboxes in their promoter regions and are regulated at the level of transcription by CLOCK and BMAL activation in a rhythmic fashion [4].
In birds, several homologs of clock genes have been found in the retina, pineal, and SCN, including Per (Per2 and Per3), Cry (Cry1 and Cry2), Bmal (Bmal1 and Bmal2), and Clock [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]. Avian homologs of Bmal1, Bmal2, and Clock are rhythmically expressed in the pineal gland with levels highest later in the day. However, in the retina, only bmal1 expression is rhythmic. Similarly, Per2, Per3, Cry1, and Cry2 are all rhythmic in the pineal [15] whereas only Per3 and Cry1 are rhythmic in the retina [16]. In addition, molecular clocks have been described in heart, liver, ovary, and muscle [8], [17], [18]. The avian clock system therefore differs from that of mammals as indicated by the lack of an avian Per1 homolog and variations in the phasing of some of the clock gene constituents [7], [9], [19], [20]. In mice, clock genes are expressed in epithelial cells and neurons of the myenteric plexus of the colon and are suggested to play an important role in the circadian control of neurotransmitters associated with digestive function [21]. In essence, the gut possesses a time-keeping system that enables the synchronization of gut transit and nutrient absorption to the rhythmic environment and to help coordinate physiological events within the body [22].
Serotonin
Serotonin is a monoaminergic neurotransmitter derived from tryptophan that is well studied because of its association with mental disorders, most notably depression. Less well studied, however, is the peripheral regulation of serotonin. Most blood borne serotonin is synthesized in the duodenum and has widespread physiological functions beyond those typically associated with mood disorders [23]. Serotonin is unable to cross the bloodbrain barrier and, as a result, has varying functions throughout the body, depending on the location of its production and release.
Tryptophan hydroxylase 1 (Tph1) is the rate limiting enzyme involved in the synthesis of serotonin in the gut whereas tryptophan hydroxylase 2 (Tph2) is the predominant enzyme for serotonin synthesis in the brain and neural tissues [24]. Tph1 is activated by mucosal stimulation of the gut after meals [25] and is also regulated by lowdensity lipoprotein receptorrelated protein 5 (LRP5) [26]. Serotonin can then either diffuse into enteric nerve endings to promote the digestion and movement of food through the alimentary canal or it can enter circulation [23]. Serotonin is also a direct precursor to endogenous melatonin production and it has been suggested that the gut itself produces melatonin [27], [28]. Interestingly, serotonin and melatonin have opposing effects on gut physiology [29], [30]. Serotonin released into the blood may act as a hormone which, after binding to receptors found on target cells, could potentially provide timing cues to target tissues involving the digestive system and nutritional status. In some tissues, such as the eye and pineal, serotonin is produced under the control of the molecular circadian clock by activating transcription of Tryptophan Hydroxylase [31]. Because of these findings we hypothesized that a circadian clock could control serotonin production in the duodenum and thereby regulate both duodenal and plasma serotonin levels.
Materials and Methods
Duodenum collection
White leghorn laying hens (Gallus gallus; approximately 8 months old) were placed in a 16:8 photoperiod, a typical light-dark cycle for raising laying hens, at approximately 20°C, and fed ad libitum. Duodenum was harvested from hens that were cervically dislocated 3, 9, 15, and 21 hours after the lights came on, designated as Zeitgeber Time (n = 6 per timepoint). Duodenum was also harvested from birds that were placed in constant darkness for three days at 3, 9, 15, and 21 hours after the lights would normally have come on under their prior photoperiod and is designated as Circadian Time (n = 4 per timepoint). Tissues were frozen on dry ice and stored at −80°C. All animals were utilized in accordance with regulations from Pennsylvania State University's Animal Care and Use Committee (IACUC Protocol #29982).
RNA extraction
Messenger RNA (mRNA) was extracted from 0.1 g of tissue in 1 mL of Trizol by homogenizing with a rotostator for approximately 1 minute. Homogenized samples were centrifuged at 12,000×g at 4°C for 5 minutes and the mRNA was removed using an RNeasy kit (Qiagen; Valencia, CA). The supernatant was removed and added to 0.32 mL of chloroform (0.2 mL of chloroform per 1 mL of Trizol). The tubes were then shaken vigorously by hand for 15 seconds, incubated at room temperature for 3 minutes, and centrifuged at 12,000×g for 15 minutes at 4°C. The upper (aqueous) phase was carefully transferred to a fresh 2 mL tube. An equal quantity of 70% ethanol was added to the sample and vortexed. The sample (700 μL) was then transferred to the Qiagen RNeasy column and centrifuged for 30 seconds at 9,000×g. The liquid from the collection tube was discarded and the previous step repeated using the remaining sample. DNaseI solution (10 μL DNaseI and 70 μL RDD buffer) was added to the column and allowed to sit for 15 minutes at room temperature. RWI buffer (700 μL) was then added to the column. The column was centrifuged for 30 seconds at 9,000×g at room temperature, transferred to a new 1.5 mL tube, and centrifuged again at 15,000×g for 1 minute to remove any remaining ethanol. The RNA was eluted into a 1.5 mL collection tube using 35 μL RNase free water by centrifugation for 1 minute at 9,000×g. The sample was checked for concentration and quality of RNA with a spectrophotometer (Nanodrop; Waltham, MA) and then stored at −80°C until needed.
cDNA synthesis
Complementary DNA (cDNA) was synthesized by combining RNA (1 μg), 1 μL RNase Out (Invitrogen; Carlsbad, CA) and 1 μL random hexamers (New England Biolabs; Ipswich, MA) and heating at 70°C for 10 minutes in a thermocycler (BioRad; Hercules, CA) then immediately cooling on ice. To each tube, 2 μL 10× reaction buffer, 2 mM dNTP mix, and 1 μL MMLV Reverse Transcriptase (New England Biolabs) were added to make the total reaction volume 20 μL and incubated at 42°C for 60 minutes, followed by heating at 70°C for 15 minutes. After heating, 30 μL of nuclease free water was added to bring the volume to 50 μl and then stored at −80°C until use.
qRTPCR
Real time PCR (qRTPCR) was performed to determine the amount of mRNA of Per2, Per3, Clock, Bmal1, Bmal2, Cry1, Vip and the genes that encode for Tryptophan Hydroxylase, Tph1 and Tph2. A standard reaction volume of 20 μL was comprised of 10 μL SYBR Green Lo Rox Super Mix (Quanta), 6.3 μL water, 0.6 μL of forward primers (Invitrogen; Carlsbad, CA), 0.6 μL reverse primers (Invitrogen), and 2.5 μL of cDNA (50 ng/reaction). Control samples without template and samples without reverse transcriptase were included in each qRTPCR experiment. Each sample was added to a 96well plate on ice, covered, and briefly centrifuged at 200 rpm at 4°C. Samples (minus controls) were run in triplicate on the plates using an Opticon II thermocycler (BioRad). Cyclophilin was used as a reference to determine relative gene expression levels, as it is expressed constantly across the day. For each sample, a cycle threshold (Ct) was estimated from the number of cycles required for fluorescence to exceed the background level, and relative gene expression was determined using the formula: ΔΔC(t) = {C(t)target geneC(t)Cyclophilin}×time{C(t)target C(t)Cyclophilin [32]. Fold changes were normalized to levels at ZT3, which were arbitrarily set at 1. The following primer sets were utilized in our experiments: Cyclophilin (For:GCAAGCAGATCACCATTTCCA; Rev:CGGAATGTCAGGCGTTAAGAC); Per2 (For:CCCCAGTAGTTGGTGCTCACTT; Rev: GACTGGTGAGCGATACAACACTTT); Per3(For:CAGAATGGAAACGATCAGCCTAT; Rev: TCGGGAGAAAACAGGAAGCA); Bmal1 (For:TTCCCACAGCTTGCAGCTT; Rev: TTTTGGGCCGCCTTCTC); Bmal2 (For:GAAGCAGAAGTTCTGGAGACTTCAG; Rev: CCACCGAGGAGAGGCTCAT); Clock (For:CGTGTGGAGCGGTAATGGT; Rev: GGGCAGCAAAAGTGGGATAA); Cry1 (For:CCGGGAAACGCCCAAA; Rev: TGCTCTGCCGCTGGACTT); Vip (For:CGAAGCCAGGAAGAGTTAAATCCA; Rev: CGTGGCTCAGCAGTTCATCTACA); Tph1 (For:TGCAAGCAAGAGGGACAGCTTA; Rev: TGCAGGTGACCTTTGGATCA); and Tph2 (For:ACAGTGAGACCGGTTGCTGGAT; Rev: GATCCGAGCCGTGTCGTACATA).
HPLC
Highpressure liquid chromatography (HPLC) was used to determine concentrations of serotonin in the duodenum and plasma over the course of the day. Blood samples were collected from the brachial vein of laying hens 3, 9, 15, and 21 hours after lights on (n = 6 per timepoint) using capillary tubes coated with heparin sulfate (125 IU/mL) as an anticoagulant. Another group of birds were placed in constant darkness for three days and blood was collected from these birds 3, 9, 15, and 21 hours after the lights (n = 4 per timepoint) would have come on under their original photoperiod. Blood was placed in heparinized microcentrifuge tubes on ice, centrifuged at 300×g at 4°C for 20 minutes, and the plasma removed. Plasma, (200 μL) and 200 μL of extraction buffer (160 μL of 0.1 M HCLO4, 20 μL of 2.22 mM butylated hydroxytoluene and 20 μL of 0.07 mg/mL methylserotonin) were added to a microcentrifuge tube and vortexed. Methylserotonin is degraded in a similar manner as serotonin, but has a slightly longer elution time and was used as an internal control. Consequently, the extraction efficiencies and degradation of endogenous serotonin in the samples can be determined by ratios of remaining methylserotonin. The solution was then kept on ice for 1 hour to precipitate the protein from the mixture, centrifuged for 12000×g for 20 minutes at 4°C, and the supernatant was removed and filtered through a 0.22 μ SpinX filter (Corning; Corning, NY) at 2500×g for 5 minutes at 4°C. Duodenal samples (n = 6 per timepoint) were extracted in a similar manner except that 0.1 g of duodenal tissue was sonicated on ice for 1 minute in 1 ml of the extraction buffer.
Extracted samples (10 μl) were separated using a Hypersil Gold a Q column (150×4.6 mm, 5 μm particle size; Thermo Scientific; Waltham, MA) at a flow rate of 0.8 ml/minute. A Hypersil Gold a Q dropin guard cartridge (10×4.6 mm, 5 μm particle size; Thermo Scientific; Waltham, MA) was used as a prefilter. The amount of serotonin in the samples was detected using an electrochemical detector (Antec; Frement, CA) with a glass fiber electrode set at +400 mV. The mobile phase consisted of: 97% 20 mM KH2PO4 and 20 μM Na2EDTA with a pH of 3.0 and 3% MeOH. The column and electrochemical detector were kept at a constant temperature of 30°C. The electrochemical signal from each sample was compared to the signal from a known standard curve to determine the amount of serotonin in each sample. Each sample was run in triplicate.
Statistical Analysis
Differences among clock gene expression and serotonin content at different time points were evaluated using cosinor analysis to determine rhythmicity and One-way ANOVA with Tukey's post-hoc comparison to determine what time of day production of gene transcripts or serotonin content was different.
Results
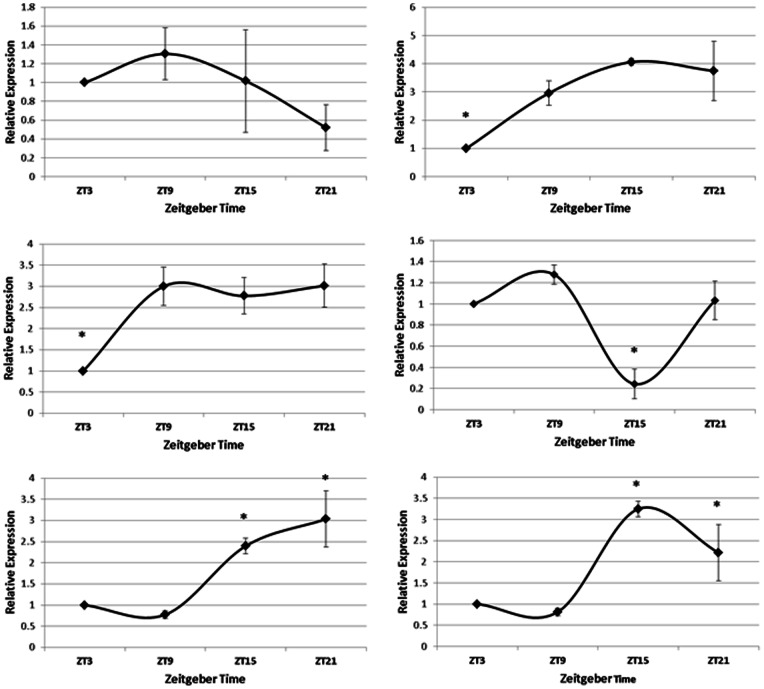
The expression profiles for the clock genes Per2, Per3, Clock, Bmal1, and Bmal2 were rhythmic over the course of the day in the duodenum, as determined by COSINOR analysis (Figure 1; p<0.05). The clock gene Cry1 was found to be not rhythmically expressed in duodenum. Clock was most highly expressed just prior to lights-off and levels of Clock remained high during lights out. Bmal1 expression was lowest at the beginning of the day, increased at mid-day and remained high throughout the night. Bmal2 expression was low during the day and increased right before lights off and stayed high during the night.
Figure 1. Gene Expression profiles of A) Cry1, B) Bmal1, C) Bmal2, D) Clock, E) Per2, and F) Per3 from duodenum of laying hens (n = 6).
Asterisks denote time of day when gene expression levels were significantly different from gene expression levels at other times of the day (p<0.05), as determined by One-way ANOVA with Tukey's post-hoc analysis.
Per2 levels greatly decreased in expression late during the day before lights out while remaining relatively constant throughout the rest of the 24-hour period. Per3expression fluctuated over the course of the day with the highest expression before the onset of darkness. Cry1expression did not exhibit much variation through the 24-hour period. Both genes encoding for Tryptophan Hydroxylase, Tph1 and Tph2, and Vip expression were rhythmic over the course of the day in the duodenum, as determined by COSINOR analysis (Figure 2; p<0.05). Vip expression was highest during the middle of the day and was reduced in expression immediately before lights off and remained low during the night. Levels of Tryptophan Hydroxylase 1 and 2 (Tph1 and Tph2) were highest right before darkness and lowest three hours after lights on.
Figure 2. Gene Expression profiles of A) Tryptophan Hydroxylase 1 (Tph1) B) Tryptophan Hydroxylase 2 (Tph2) and C) Vasoactive Intestinal Polypeptide (Vip) from laying hen duodenum (n = 6).
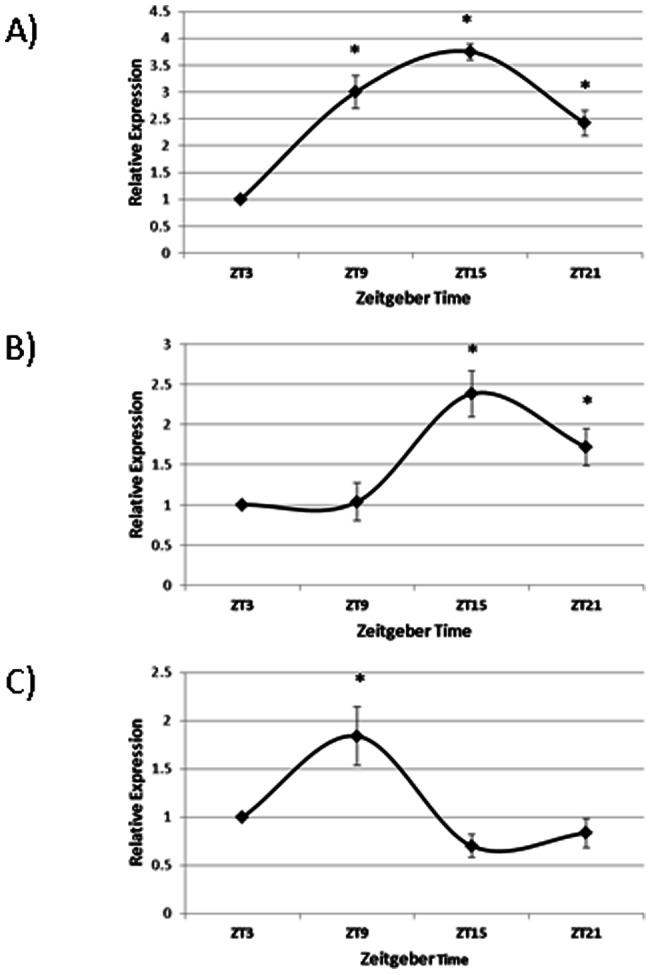
Asterisks denote time of day when gene expression levels were significantly different from gene expression levels at other times of the day (p<0.05), as determined by One-way ANOVA with Tukey's post-hoc analysis.
Serotonin concentrations in the duodenum and in plasma, as determined with HPLC, were similarly found to fluctuate over the course of the day under a 16:8 LD cycle (Duodenum: Figure 3; F(3,28) = 10.75, p<0.001); Plasma: Figure 4; F(3, 18) = 8.38, P<0.005). In the duodenum, levels of serotonin remained low during the day and increased during the night. In plasma, serotonin concentrations were similar in profile to duodenal serotonin content, being relatively constant throughout the day and peaking around the time of lights off. Under constant darkness, however, serotonin was not statistically rhythmic in either duodenum or in blood plasma. (Duodenum: Figure 5; F(3, 12) = 0.43, p = 0.739; Plasma: Figure 6; F(3, 11) = 1.83, p = 0.200). In plasma we did see a trend towards rhythmicity, but the data were highly variable, indicating that the rhythm had damped out after several days in constant darkness. The levels of serotonin during the subjective day in the duodenum were significantly higher under constant darkness than duodenal serotonin levels under the 16:8 LD cycle (p<0.05; Student's t-test).
Figure 3. Serotonin content of duodenum from laying hens (n = 6) peaks late in the day at ZT 21 in a 16:8 LD cycle.
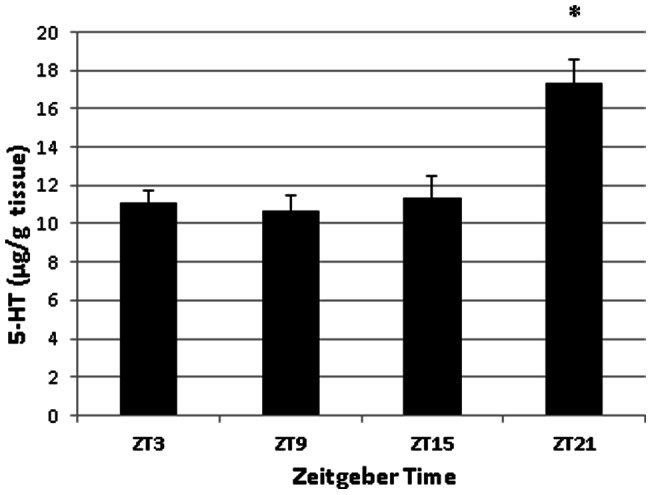
Asterisk denotes time of day when serotonin levels were significantly different from serotonin content at other times of the day (F(3,28) = 10.75, p<0.001), as determined by One-way ANOVA with Tukey's post-hoc analysis.
Figure 4. Serotonin content of blood plasma from laying hens (n = 6) in a 16:8 LD cycle.
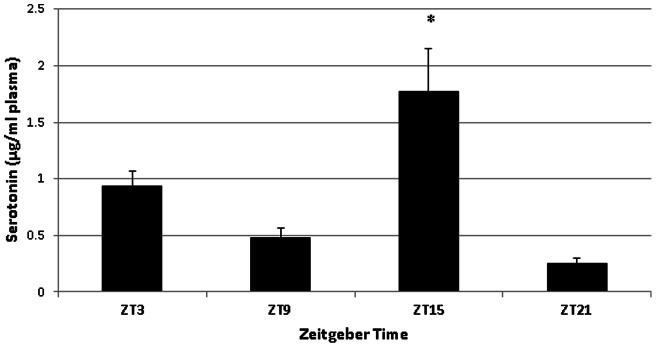
Asterisk denotes time of day when serotonin levels were significantly different from serotonin content at other times of the day (F(3, 18) = 8.38, P<0.005), as determined by One-way ANOVA with Tukey's post-hoc analysis.
Figure 5. Serotonin content of duodenum from laying hens (n = 4) in constant darkness.

Serotonin levels were not significantly different across the day (F(3, 12) = 0.43, p = 0.739), as determined by One-way ANOVA.
Figure 6. Serotonin content of blood plasma from laying hens (n = 4) in constant darkness.
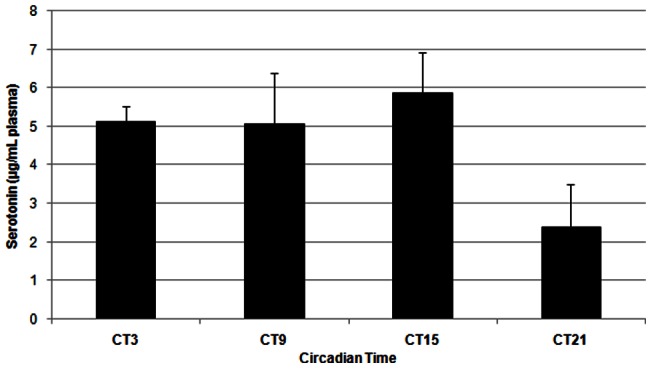
One sample collected at ZT 21 was lost during processing. Serotonin levels were not significantly different across the day (F(3, 11) = 1.83, p = 0.200), as determined by One-way ANOVA.
Discussion
The expression of clock gene products, except for Cry1, was rhythmic across the light/dark cycle in the duodenum. These data suggest that a molecular clock exists within the duodenum of chickens which could potentially drive the rhythmic expression of genes associated with feeding and digestion, at least under LD cycles. The ability of the duodenum to maintain time may allow it to anticipate the time of daily food intake, thereby optimizing processes required for efficient uptake of nutrients and the movement of alimentary canal contents in a caudal direction. Consequently, disruption of environmental cues, such as those which occur with phase shifts or changes in feeding schedules, may lead to a disruption of normal rhythmicity in intestinal function and possibly lead to the development of gastrointestinal disorders. Indeed, when birds were placed under constant darkness, the content of serotonin in both blood and duodenum became arrhythmic, suggesting that the duodenal clock is particularly sensitive to environmental input. It is possible that the lack of Cry1 rhythmicity could make the molecular clock in the duodenum less stable and therefore more dependent upon input from light/dark cycles.
Both Tryptophan Hydroxylase 1and 2 (Tph1 and Tph2) were rhythmically expressed in the duodenum. Because TPH1 and TPH2 are the enzymes involved in the rate-limiting step in serotonin biosynthesis, and because Tph1 and Tph2 transcription is under diurnal control, our findings suggest that the regulation of transcription by LD cycles could be responsible for the rhythmic expression of serotonin observed in the duodenum under LD. Duodenal serotonin content is comprised of two sources: those in enterochromaffin cells, which are synthesized by TPH1, and those in endocrine cells, which are synthesized by TPH2 [33], [34]. Our measurements of duodenal serotonin reflect the content of both origins and stores, although it has been reported that the amount of serotonin in both duodenum and blood which is derived from TPH1 activity is orders of magnitude greater than serotonin derived from TPH2 activity [35]. Based upon the rhythmic expression patterns of both TPH genes under LD and the lack of serotonin rhythmicity under constant darkness, it appears that serotonin stores from both enterochromaffin and enteric neurons are likely driven by the light/dark cycle. Interestingly, we observed plasma serotonin levels to peak earlier than the peak of serotonin levels in the duodenum under LD. This is most likely due to a reduction in serotonin release from duodenal tissues at night, thereby causing greater serotonin accumulation in the duodenum itself. The finding of higher levels of serotonin during the subjective day when birds are placed in constant darkness gives support to this hypothesis. It has been reported that melatonin, which is high at night in duodenum, can inhibit serotonin release from the gut [36], which could explain why serotonin levels peaked earlier in the day in plasma than serotonin levels in the duodenum. VIP, which we found to be rhythmically expressed in duodenum, can also inhibit the release of serotonin from duodenum [37]. This observation would support our findings too, but caution must be applied as those findings were observed in nocturnal animals and therefore the serotonergic system may be regulated differently than that of diurnal birds. Taken together, however, these works suggest a mechanism by which the high amounts of serotonin in duodenum at night could be regulated. Indeed, our findings of higher serotonin levels in the duodenum of birds during the subjective day than those of birds under LD cycles during the daytime would further suggest that light may promote the release of serotonin from the duodenum.
Our data demonstrate that a molecular clock exists within the duodenum that could regulate the transcriptional machinery required to produce peptides and hormones within the duodenum that could be used to control digestive function under LD cycles. Additionally, our findings suggest that the regulation of plasma serotonin by LD cycles could represent an additional biochemical signal in the blood encoding time and, as a result, serotonin could be used by target tissues throughout the body to integrate timing cues derived from the digestive system.
Acknowledgments
We would like to acknowledge the assistance of Dr. Mark Signs from the Pennsylvania State University for his assistance with HPLC.
Funding Statement
This project was supported in part by Competitive Grant no. 2010-65206-20779 from the USDA National Institute of Food and Agriculture. The authors also acknowledge funding from the Schreyer Honors College, the College of Agricultural Sciences, and the Department of Poultry Science of the Pennsylvania State University. The funders had no role in the design of the project or interpretation of the results.
References
- 1. Cassone V, Menaker M (1984) Is the Avian Circadian System a NeuroendocrineLoop? Journal of Experimental Zoology 232: 539–549. [DOI] [PubMed] [Google Scholar]
- 2. Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, et al. (2005) Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 6(7): 544–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herzog E, Tosini G (2001) The Mammalian Circadian Clock Shop. Cell and Developmental Biology 12: 295–303. [DOI] [PubMed] [Google Scholar]
- 4. Yan J, Wang H, Liu Y, Shao C (2008) Analysis of gene regulatory networks in the mammalian circadian rhythm. PLoS Comput Biol. 4(10): e1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chong NW, Bernard M, Klein DC (2002) Characterization of the chicken serotonin N-acetyltransferase gene: activation via clock gene heterodimer/E box interaction.”. J BiolChem 275: 32991–32998. [DOI] [PubMed] [Google Scholar]
- 6. Chong NW, Chaurasia SS, Haque R, Klein DC, Iuvone PM (2003) Temporal-spatial characterization of chicken clock genes: circadian expression in retina, pineal gland, and peripheral tissues. J Neurochem 85: 851–860. [DOI] [PubMed] [Google Scholar]
- 7. Yoshimura T, Yasuo S, Suzuki Y, Makino E, Yokota Y, et al. (2000) Molecular analysis of avian circadian clock genes. Mol Brain Res 78: 207–215. [DOI] [PubMed] [Google Scholar]
- 8. Yoshimura T, Suzuki Y, Makino E, Suzuki T, Kuroiwa A, et al. (2001) Identification of the suprachiasmatic nucleus in birds.”. Am J Physiol 280: R1185–R1189. [DOI] [PubMed] [Google Scholar]
- 9. Brandstätter R, Abraham U, Albrecht U (2001) Initial demonstration of rhythmic per gene expression in thehypothalamus of a nonmammalian vertebrate, the house sparrow.”. Neuroreport12: 1167–1170. [DOI] [PubMed] [Google Scholar]
- 10. Bailey MJ, Chong NW, Xiong J, Cassone VM (2002) Chicken's cry2: molecular analysis of an avian cryptochrome in retinal and pineal photoreceptors.” FEBS Lett. 513: 69–72. [DOI] [PubMed] [Google Scholar]
- 11. Fu Z, Inaba M, Noguchi T, Kato H (2002) Molecular cloning and circadian regulation of cryptochrome genes in Japanese quail (Coturnixcoturnix japonica). J Biol Rhythms 17: 14–27. [DOI] [PubMed] [Google Scholar]
- 12. Haque R, Chaurasia SS, Wessel JH, Iuvone PM (2002) Dual regulation of cryptochrome 1 mRNA expression in chicken retina by light and circadian oscillator. Neuroreport 13: 2247–2251. [DOI] [PubMed] [Google Scholar]
- 13. Yasuo S, Watanabe M, Okabayashi N, Ebihara S, Yoshimura T (2002) Circadian clock genes and photoperiodism: comprehensive analysis of clock gene expression in the mediobasal hypothalamus, the suprachiasmatic nucleus, and the pineal gland of Japanese quail under various light schedules. Endocrinology 144: 3742–3748. [DOI] [PubMed] [Google Scholar]
- 14. Yasuo S, Yoshimura T, Bartell PA, Iigo M, Makino E, et al. (2002) Effect of melatonin administration on qPer2, qPer3 and qClock gene expression in the suprachiasmatic nucleus of Japanese quail.”. Eur J Neurosci 16: 1541–1546. [DOI] [PubMed] [Google Scholar]
- 15. Bailey MJ, Beremand PD, Hammer R, Bell-Pedersen D, Thomas TL, et al. (2003) Transcriptional profiling of the chick pineal gland, a photoreceptive circadian oscillator and pacemaker. Mol. Endocrinol. 17: 2084–2095. [DOI] [PubMed] [Google Scholar]
- 16. Bailey MJ, Beremand PD, Hammer R, Reidel E, Thomas TL, et al. (2004) Transcriptional profiling of circadian patterns of mRNA expression in the chick retina. J. Biol. Chem. 279: 52247–52254. [DOI] [PubMed] [Google Scholar]
- 17. Karaganis SP, Bartell PA, Shende VR, Moore AF, Cassone VM (2009) Modulation of metabolic and clock gene mRNA rhythms by pineal and retinal circadian oscillators. General and Comparative Endocrinology 161: 179–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tischkau SA, Howell RE, Hickok JR, Krager SL, Bahr JM (2011) The luteinizing hormone surge regulates circadian clock gene expression in the chicken ovary. Chronobiol Int. 28(1): 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abraham U, Albrecht U, Gwinner E, Brandstätter R (2002) Spatial and temporal variation of Passer Per2 gene expression in two distinct cell groups of the suprachiasmatic hypothalamus in the house sparrow (Passer domesticus). Eur. J. Neurosci 16: 429–436. [DOI] [PubMed] [Google Scholar]
- 20. Abraham U, Albrecht U, Brandstätter R (2003) Hypothalamic Circadian Organization in Birds II. Clock Gene Expression. Chronobiology International 20: 657–669. [DOI] [PubMed] [Google Scholar]
- 21. Hoogerwerf WA, Hellmich HL, Cornélissen G, Halberg F, Shahinian VB, et al. (2007) Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen. Gastroenterology. 133(4): 1250–60. [DOI] [PubMed] [Google Scholar]
- 22. Hoogerwerf WA, Shahinian VB, Cornélissen G, Halberg F, Bostwick J, et al. (2010) Rhythmic changes in colonic motility are regulated by period genes. Am J Physiol Gastrointest Liver Physiol. 298(2): G143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosen CJ (2009) Breaking into bone biology: serotonin's secrets. Nat Med 15: 145–146. [DOI] [PubMed] [Google Scholar]
- 24. Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG (2004) Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 305(5681): 217. [DOI] [PubMed] [Google Scholar]
- 25. Chin A, Svejda B, Gustafsson BI, Granlund AB, Sandvik AK, et al. (2012) The role of mechanical forces and adenosine in the regulation of intestinal enterochromaffin cell serotonin secretion. Am J Physiol Gastrointest Liver Physiol. 302(3): G397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, et al. (2008) Lrp5 Controls Bone Formation by Inhibiting Serotonin Synthesis in the Duodenum. Cell 135: 825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van't Hof TJ, Gwinner E (1999) Influence of pinealectomy and pineal stalk deflection on circadian gastrointestinal tract melatonin rhythms in zebra finches (Taeniopygia guttata). J Biol Rhythms. 14(3): 185–9. [DOI] [PubMed] [Google Scholar]
- 28. Velarde E, Cerdá-Reverter JM, Alonso-Gómez AL, Sánchez E, Isorna E, et al. (2010) Melatonin-synthesizing enzymes in pineal, retina, liver, and gut of the goldfish (Carassius): mRNA expression pattern and regulation of daily rhythms by lighting conditions. Chronobiol Int. 27(6): 1178–201. [DOI] [PubMed] [Google Scholar]
- 29. Bubenik G (2002) Gastrointestinal Melatonin: Localization, Function, and Clinical Relevance.”. Digestive Diseases and Sciences 47: 2336–2348. [DOI] [PubMed] [Google Scholar]
- 30. Bubenik GA, Dhanvantari S (1989) Influence of serotonin and melatonin on some parameters of gastrointestinal activity. J Pineal Res. 7: 333–44. [DOI] [PubMed] [Google Scholar]
- 31. Thomas K, Iuvone P (1989) Tryptophan hydroxylase activity in chicken retina and pineal gland displays circadian rhythmicity.” (Abstract) Society. Neurosci. Abstr. 15: 1396. [Google Scholar]
- 32. Livak K J, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 33. Chen JJ, Wan S, Gershon MD (2004) Expression of two isoforms of tryptophan hydroxylase (TPH1 and TPH2) in human and mouse gut. Gastroenterology 126: A411. [Google Scholar]
- 34. Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, et al. (2011) Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 31(24): 8998–9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walther DJ, Peter JU, Bashammakh S, Hörtnagl H, Voits M, et al. (2003) Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 299(5603): 76. [DOI] [PubMed] [Google Scholar]
- 36. Kojima S, Tohei A, Ikeda M (2011) Melatonin inhibits tachykinin NK2 receptor-triggered 5-HT release from guinea pig isolated colonic mucosa. Br J Pharmacol. 162(5): 1179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fujimiya M, Yamamoto H, Kuwahara A (1998) Effect of VIP and PACAP on basal release of serotonin from isolated vascularly and luminally perfused rat duodenum. Am J Physiol. 275(4 Pt 1): G731–9. [DOI] [PubMed] [Google Scholar]