Abstract
High-risk neuroblastoma (NB) has a poor prognosis. Even with intensive myeloablative chemotherapy, relapse is common and almost uniformly fatal, and new treatments are needed. Translocator protein 18kDa (TSPO) ligands have been studied as potential new therapeutic agents in many cancers, but not in NB.
We studied the effects of TSPO ligands on cell proliferation, cell cycle progression and apoptosis using paired cell lines derived from the same patient at the time of initial surgery and again after development of progressive disease or relapse post-chemotherapy. We found that TSPO expression was significantly increased 2- to 10-fold in post-relapse cell lines compared with pre-treatment lines derived from the same individual. Subsequently, these cell lines were treated with the specific TSPO ligand 1-(2-chlorophenyl-N-methylpropyl)-3-isoquinolinecarboxamide (PK11195) (0–160µM) as a single agent, with cytotoxic chemotherapy agents alone (carboplatin, etoposide or melphalan), or with combinations of PK11195 and chemotherapy drugs. We found that PK11195 inhibited proliferation in a dose-dependent manner, induced apoptosis and caused G1/S cell cycle arrest in all tested NB cell lines at micromolar concentrations. In addition, PK11195 significantly decreased mRNA expression of the chemotherapy resistance efflux pumps ABCA3, ABCB1 and ABCC1 in two post-relapse NB cell lines. We also found that pre-treatment with PK11195 sensitized these cell lines to treatment with cytotoxic chemotherapy agents. These results suggest that PK11195 alone or in combination with standard chemotherapeutic drugs warrants further study for the treatment of neuroblastoma.
Keywords: neuroblastoma, TSPO, PK11195, apoptosis, cell cycle analysis, RT-PCR
Introduction
Neuroblastoma (NB), one of the most common childhood cancers, remains difficult to treat despite significant advances in treating other classes of pediatric tumors.1,2 Over half of all children with neuroblastoma will present with widespread, metastatic disease; of those children over 18 mo of age, only approximately 40% will be cured despite treatment with high-intensity, multi-modality therapy. Primary tumors usually respond to initial chemotherapy; however, upon relapse they are generally highly chemotherapy resistant.2,3 In addition, surviving children are at high risk for additional health problems related to long-term toxicities of aggressive cytotoxic treatment. Therefore, finding new therapeutic strategies to more effectively treat this disease while lowering long-term toxicities remains a critically important goal.
The translocator protein 18kDa (TSPO), is a transmembrane protein associated with the mitochondrial permeability pore (MPP).4 Previously called the peripheral benzodiazepine receptor,5 TSPO was thought to reside predominantly outside the central nervous system. Subsequent research showed that TSPO is abundant in glial cells in the brain as well as in peripheral tissues.6 A broad spectrum of actions has been suggested for TSPO, including regulation of steroid production,7-9 normal and abnormal, i.e., cancer, cell growth and differentiation10-12 and modulation of innate and acquired host defense response.13-16 However, its definitive function(s) remain a matter for debate. TSPO levels are increased in a wide variety of cancers, suggesting an important role of TSPO in cancer development. Specifically, increased TSPO expression is associated with advanced tumor stage and poor prognosis in human astrocytoma, colorectal cancers and breast cancer.17-21
Previous studies have demonstrated that TSPO ligands are capable of inducing apoptosis in numerous types of cancer cell lines.22 Mitochondrial transport plays a key role in the initiation of the apoptotic cascade and TSPO ligands at higher (>10 µM) concentrations promote apoptosis, presumably by modifying the channel function of the mitochondrial permeability pore (MPP).23 The specific TSPO ligand 1-(2-chlorophenyl-N-methylpropyl)-3-isoquinolinecarboxamide (PK11195) has been shown to facilitate the induction of,24 and to reverse the inhibition of apoptosis11,25 and to facilitate TNF-α induced necrosis.26 Therefore PK11195 appears to enhance several distinct pathways leading to cell death.27 Importantly, PK11195 has been shown to chemosensitize lymphoid leukemia cells in vitro despite expression of high levels of anti-apoptotic proteins including MCL-1, BCL-XL and BCL-2 by these cells.28 PK11195 is an easily manufactured small molecule that is stable in vivo, orally bioavailable and is well tolerated in healthy subjects.29-31
Here we report the effects of the TSPO ligand PK11195 on pre- and post-relapse neuroblastoma cell lines.
Results
TSPO expression is increased in post-relapse neuroblastoma cell lines
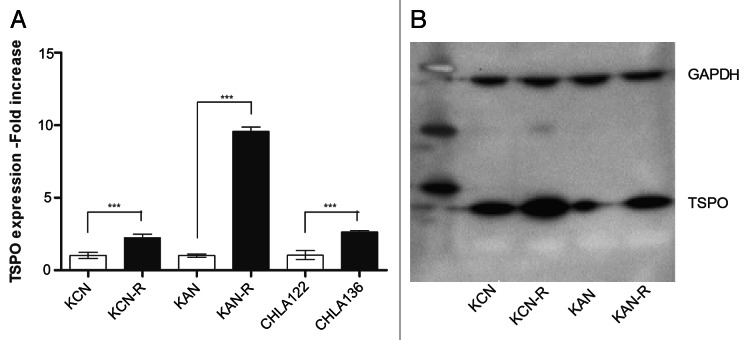
We measured baseline expression of TSPO in the paired neuroblastoma cell lines using real-time PCR and western blotting. For each pair of cell cultures, TSPO mRNA and protein expression was significantly increased in the relapsed cells compared with the pre-relapse cells. mRNA was approximately 10-fold higher in the KAN pair and twofold in the KCN and CHLA pairs (Fig. 1A). Western blot analysis showed a twofold increase in TSPO protein in the relapsed cells compared with the pre-relapse cells (Fig. 1B).
Figure 1. TSPO expression levels in pre-treatment and post-relapse NB cell lines. (A) Relative quantitation of baseline TSPO mRNA expression levels in paired pre- (open bars) and post- (black bars) relapse NB cell lines. TSPO mRNA expression was significantly increased from twofold in the SMS-KANR compared with the SMS-KAN cell line, to 10-fold in SMS-KCNR compared with SMS-KCN. These differences were highly significant (***p < 0.001). (B) Western Blot showing increased expression of TSPO protein in both the post-relapse cell lines SMS-KANR and SMS-KCNR, compared with their pre-treatment pair, consistent with the mRNA results. Computer densitometry analysis showed ~twofold increase in protein in the relapse lines compared with the pre-relapse cell lines.
PK11195 treatment inhibits NB cell proliferation
We next studied the effect of PK11195 on growth parameters in two pre- and post-relapse cell line pairs: SMS-KCN and KCNR and SMS-KAN and KANR. Cells were treated for 48 h with increasing doses of PK11195 and effects on growth measured by alamar blue assay. Dose-dependent decreases in proliferation with IC50’s ranging from 80−120 µM were observed in all four lines (Fig. 2). The results for the SMS-KAN and SMS-KANR cell lines showed no difference in sensitivity between the pre-treatment (SMS-KAN) and post-relapse (SMS-KANR) lines (Fig. 2A); this is in contrast however, to the SMS-KCN/KCNR pair where the post-relapse line was found to be less sensitive to the effects of PK11195 (Fig. 2B).
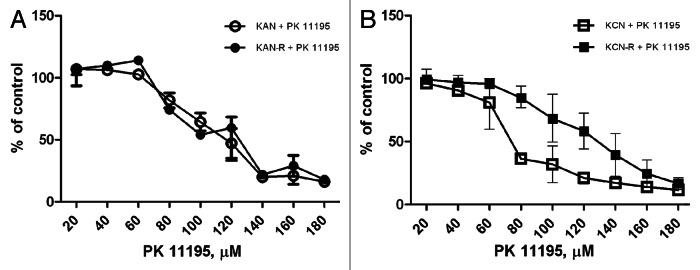
Figure 2. Effect of PK11195 treatment on NB cell proliferation. Four NB cell lines were grown for 24 h in 96 well plates prior to addition of increasing concentrations of PK11195. Cells were exposed to PK11195 for 48 h and proliferation assessed using the colorimetric Alamar blue assay. PK11195 caused dose-dependent decreased proliferation in all four cell lines. (A) No differences were seen in the concentrations required to cause decreased proliferation in the SMS-KAN/KANR paired lines; (B) The post-relapse line SMS-KCNR showed less inhibition of proliferation in the mid-range of doses (60−120 µM) compared with its pre-treatment pair SMS-KCN.
Treatment with PK11195 induces apoptosis in NB cell lines
As the TSPO protein is part of the mitochondrial membrane pore complex and appears to be involved in regulation and induction of apoptosis, we evaluated the ability of PK11195 to induce apoptosis in the KCNR cell line. We measured the level of cleaved caspase 3 in treated cells compared with untreated controls and found that 24-h treatment with 100µM PK11195 resulted in significantly higher levels of cleaved caspase-3 than control. PK11195 treatment also resulted in significantly increased cleavage of the caspase-3 substrate poly (ADP-ribose) polymerase (PARP), at both 50 µM and 100 µM doses, confirming the activation of caspase-3 by PK11195 (Fig. 3).
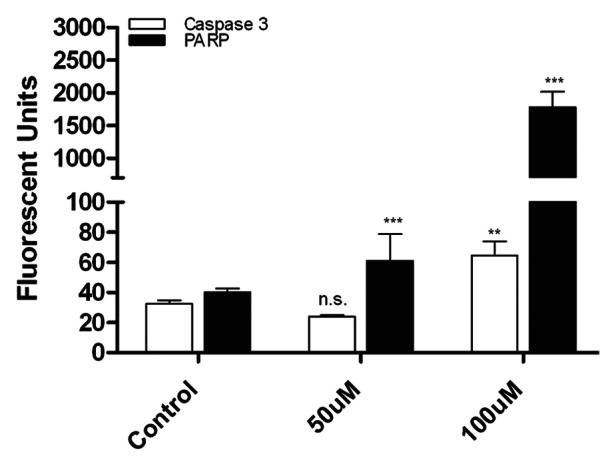
Figure 3. Treatment with PK11195 induces apoptosis in NB cell lines. After treatment with 50 µM PK11195 for 24 h, presence of cleaved caspase-3 and PARP was measured using a Luminex bead-based assay. Treatment with PK11195 significantly increased the levels of cleaved caspase-3 and PARP in treated cells compared with the untreated controls. (n.s. Not significant; **p < 0.01; ***p < 0.001)
PK11195 causes cell cycle arrest
To assess effects of TSPO on cell cycle progression, we treated cells with PK11195 and then performed cell cycle analysis using the propidium iodide method. We found that PK11195 treatment at a concentration of 60 µM for 18 h caused G1 cell cycle arrest in both the pre-treatment and post-relapse cell lines. This cell cycle arrest was most pronounced in the SMS-KAN/KANR pair, but was statistically significant in all four lines tested (Fig. 4).

Figure 4. Cell Cycle Analysis of NB cell lines after treatment with TSPO Ligands. Cells were treated with 60 µM PK11195 for 18 h and then were fixed and stained with propridium iodide and analyzed by flow cytometry. Treatment with PK11195 caused G1 cell cycle arrest in all cell lines tested. Representative flow cytometry histograms showing increase in G1 percentage from 63.1% before treatment (A), to 81.4% after PK11195 treatment (B) in the SMS-KAN cell line. S and G2 phase percentages fell from 32.5% to 16.7% for S phase and from 4.5% to 1.9% for cells in G2 phase. (C) Percentages of cell population in G1, S and G2 phases before and after treatment with PK11195. All four cell lines treated showed statistically significant increases in the percentage of cells in G1 phase compared with pre-treatment baseline; The cell cycle arrest was more pronounced in the SMS-KAN/KANR cell lines than in the SMS-KCN/KCNR cells.
PK11195 pre-treatment sensitizes NB cell lines to cytotoxic chemotherapy
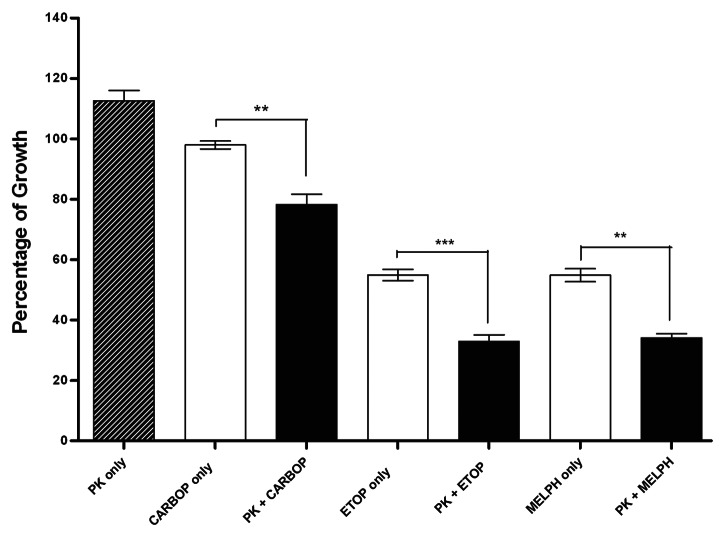
Having evaluated PK11195 as a single agent, we next investigated whether PK11195 could potentiate the action of established chemotherapeutic drugs in NB cell lines. Twenty-four hours after plating, SMS-KCNR cells were pre- treated with 50µM PK11195 for 48 h followed by the addition of chemotherapeutic drugs in current use (etoposide, melphalan or carboplatin) for an additional 72 h. Control cultures received chemotherapeutic drugs without PK11195 or with vehicle only. Cells pre-treated for 48h with PK11195 had significantly decreased viability compared with the chemotherapeutic drug or PK11195 alone (Fig. 5).
Figure 5. Effect of pretreatment of NB cell lines with PK11195. The SMS-KCNR cell line was treated with 50 µM PK11195 for 48 h prior to the addition of either 1ug/mL of carboplatin, 1 ug/mL of etoposide or 6 ug/mL of melphalan for an additional 72 h. In each case, cells pre-treated with PK11195 followed by the chemotherapy agent showed a statistically significant decreased proliferation compared with treatment with either drug alone. (**p < 0.01; ***p < 0.001)
PK11195 treatment decreases ABC transporter mRNA expression levels
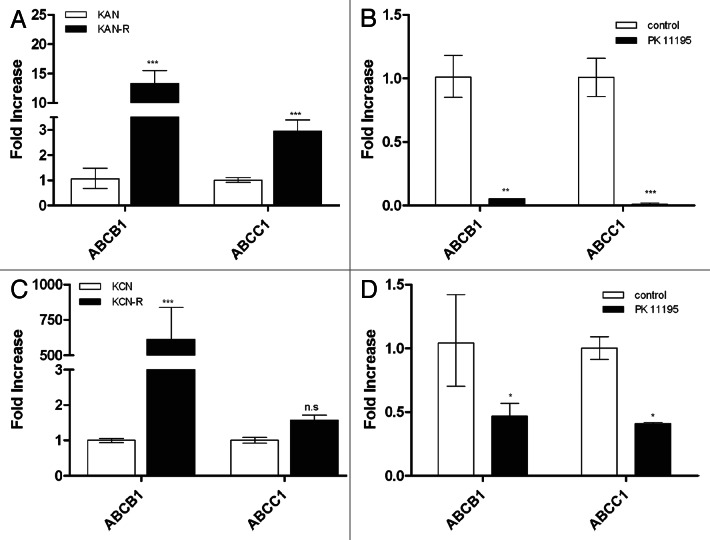
We measured mRNA expression of three ATP-binding cassette (ABC) transporter genes in both SMS-KAN/KANR and SMS-KCN/KCNR pairs (Fig. 6). mRNA expression levels of ABCA3, ABCB1 and ABCC1 were increased in both post- relapsed NB cell lines compared with its pre-treated pair, with the greatest difference found in the ABCC1 gene (Fig. 6A and C). Furthermore, we quantified transporter gene expression in relapsed cells treated with PK11195 (50 µM) for 48h (Fig. 6B and D). Our results show that treatment with PK11195 significantly decreased expression of the ABC transporter mRNA in SMS-KCNR and virtually eliminated ABCB1 and ABCC1 mRNA expression in SMS-KANR. The mRNA expression levels of ABCB1 and ABCC1 were already quite low at baseline in the pre-relapse lines SMS-KAN and SMS-KCN and treatment with PK11195 did not significantly change their expression levels. Treatment with PK11195 also did not appear to decrease expression of ABC transporter protein at these treatment durations and concentrations with no significant differences seen by western blotting (data not shown).
Figure 6. Expression of ABC transporter genes before and after treatment with PK11195. mRNA expression levels for the ABC transporter genes ABCB1 and ABCC1 were measured in the paired cell lines SMS-KAN/KANR and SMS-KCN/KCNR. (A) mRNA expression levels were significantly increased by 3- and 15-fold for ABCB1 and ABCC1 respectively in SMS-KANR compared with its pretreatment pair line SMS-KAN. (B) Treatment of SMS-KANR cells with PK11195 caused a dramatic decrease in expression of both ABC transporter genes. (C) SMS-KCNR showed very significantly increased (>500-fold) expression of ABCB1 at baseline compared with its pre-relapse paired line SMS-KCN. No significant difference in expression of ABCC1 between the two lines was seen. (D) Treatment of SMS-KCNR cells with PK11195 caused decreased expression of both ABCB1 and ABCC1 by ~50%. (*p < 0.05; **p < 0.01; ***p < 0.001)
Discussion
The specific goal of this study was to investigate the effect of the TSPO ligand PK11195 on NB cell growth, both alone and in combination with other cytotoxic chemotherapy agents commonly used in the treatment of NB. Using neuroblastoma cell lines, we found that PK11195 inhibited cell proliferation, induced apoptosis and cell cycle arrest and acted as a chemosensitizer to several common chemotherapy agents.
Since increased TSPO expression has been reported in multiple cancer types, we explored whether TSPO was expressed in our NB cell lines and found that TSPO was expressed in all lines, but that TSPO mRNA levels were significantly increased in the post-relapse cell lines compared with paired pre-relapse lines. Since these paired lines were established at diagnosis and then upon relapse after treatment in the same patient, this finding suggests that standard chemotherapy treatments may induce TSPO expression in NB and this increased expression may contribute to relapse and/or drug resistance.
TSPO is found in the outer mitochondrial membrane where it forms a molecular complex with the voltage-dependent anion channel and the adenine nucleotide translocase, two important regulators of apoptosis that in turn are regulated by proteins from the Bcl-2 family.32-34 These proteins participate in regulation of the mitochondrial permeability pore (MPP), which is critical in the initiation of apoptosis. PBR ligands such as PK11195 have been shown to block the ability of Bcl-2 and other related proteins to inhibit the MPP, thereby priming cells for apoptosis.35,36 TSPO is overexpressed in many cancers including colorectal cancers, breast cancer and prostate cancer and its expression levels correlate with tumor stage.18-20 These findings, combined with TSPO’s probable role in apoptosis regulation, have led to the hypothesis that increased TSPO expression may inhibit apoptosis induction in cancer cells. However, this hypothesis remains controversial, as several studies have suggested that the anti-cancer effects of TSPO ligands are independent of TSPO protein,24,37 while others have supported a direct TSPO-binding effect.11 Although the affinity of PK11195 for the TSPO protein is in the low nanomolar range, studies consistently report requiring 1000 times greater concentrations of the ligand to cause anti-proliferative and pro-apoptotic effects. We confirm in the present study that relatively high micromolar concentrations of PK11195 were necessary to cause such effects in NB cell lines. A diverse array of alternative mechanisms of action for PK11195 and other TSPO ligands including generation of reactive oxygen species,38 activation of c-Jun NH2 terminal kinase (JNK)39 or activation of the p38-mitogen-activated protein kinase (p38-MAPK) pathway40 have also been suggested. Recent reports have proposed that TSPO ligands may also inhibit various ABC transporter proteins including the multi-drug resistance (MDR) protein.41,42 Upregulation of ABC transporter proteins has been shown to correlate with disease stage and prognosis in NB.43 Our qRT-PCR data showed significant decreases in mRNA expression of the ABC transporters ABCB1 and ABCC1 (commonly known as MDR1 and MRP1), the two transporters most frequently associated with chemotherapy resistance, after PK11195 treatment. Inhibition of these drug efflux pumps may explain, at least in part, the mechanism of action of PK11195 in NB. Western blotting, however, did not show decreased protein expression of ABC transporters after treatment with PK11195, suggesting that ABC transport inhibition may not be the primary mechanism by which PK11195 treatment leads to chemosensitization. However, downregulation of mRNA concurrent with stable or upregulated protein expression may occur when protein half-life is increased due to stabilization through protein-protein interactions. Importantly, several other recent studies have also reported chemosensitization via inhibition of ABC transporter without decreases in ABC transporter protein levels.44,45 Further studies will be required to better elucidate these mechanisms.
It is well recognized that upon relapse, high-risk neuroblastoma is extremely drug-resistant. We found that PK11195 was able to inhibit growth and induce apoptosis in the drug-resistant post-relapse cell lines as well as in the drug-sensitive pre-treatment cell lines and that it significantly sensitized the cells to the action of several standard chemotherapy agents. These results are consistent with previously published results for other cancers including breast and colon cancers. Importantly, in the current study, PK11195 was active as a chemosensitizer in a post-relapse line, suggesting that PK11195 pre-treatment could help to overcome, the acquired chemotherapy resistance in NB.
PK11195 has been tested extensively as a potential radiolabeled ligand for neuroimaging in various neurological diseases including Alzheimer disease, Parkinson disease and schizophrenia.46-48 Additionally, several studies have used larger doses in humans with no toxicities seen.30,31 In the study by Ansseau, et al., ten psychiatric inpatients were treated for two weeks with doses of 200 or 400 mg orally without toxicities.31 In a detailed pharmacokinetic study by Ferry, et al., ten healthy volunteers were treated with both IV and oral doses of PK11195 and detailed pharmacokinetic testing was done. Those studies showed that a single oral dose of 400mg of PK11195 led to a serum drug concentration of 300 ng/ml, or ~1 uM. The elimination half-life was slightly greater than 24 h and they also reported no toxicities.30 It remains to be seen if serum drug levels in the range of 80−100 μM that are required for anti-proliferative effect in vitro can be safely achieved in humans; however, the current human pharmacokinetic and safety data suggest that additional animal studies are warranted. Additionally, pre- and post-treatment tumor imaging by [11C]-PK11195 PET could readily be performed, allowing pre-treatment identification of patients most likely to respond while permitting specific post-treatment response evaluation.
In conclusion, in this report we demonstrate for the first time in NB cells that the TSPO ligand PK11195 has in vitro anti-NB effects both as a single agent and chemosensitizing agent in post-relapse cell lines resistant to standard cytotoxic chemotherapy. Further studies exploring the use of TSPO ligands in the treatment of neuroblastoma are warranted.
Methods and Materials
Cell culture
Three paired pre- and post-relapse neuroblastoma cell lines were a gift from the laboratory of Dr C. Patrick Reynolds (Texas Tech University Health Sciences Center). These pairs are SMS-KCN and SMS-KCNR, SMS-KAN and SMS-KANR and CHLA-122 and CHLA-136. Each pair of cell lines was derived from the same patient, at the time of initial surgery and again at surgical interventions performed during progressive disease or relapse after chemotherapy. All cell lines were derived from original tumors and have not been otherwise modified. Cells were cultured in either RPMI 1640 or Iscove’s Modified Dulbecco's Medium supplemented with 10−20% fetal bovine serum and 10 µg/ml gentamicin at 37°C in 5% CO2 as previously described.3 All three pairs were used for TSPO gene expression analysis; SMS-KCN and SMS-KCNR, and SMS-KAN and SMS-KANR were used in subsequent experiments.
Cell proliferation assay
Cells were seeded into 96-well tissue culture plates at a density of 40,000 cells per well in culture medium (RPMI or IMDM) and incubated for 24 h at 37°C prior to drug treatment. Subsequently, the cells were treated with PK11195 (Sigma) in increasing concentrations (0−180 µM) for 48 h. For combination studies, cultures were treated with 50 µM PK11195 for 48 h prior to the addition of one of the following chemotherapeutic agents: Carboplatin 1 µg/mL (Sigma), etoposide 1 µg/mL (Sigma) or melphalan 6 µg/mL (Sigma) for an additional 72 h. DMSO was used as a vehicle control. All assays contained six replicates of each drug condition and all experiments were repeated a minimum of three times. Viabilities of proliferating cells exposed to control and treatment media were measured using the Alamar Blue assay according to manufacturer’s protocol (Trek Diagnostics Systems, Inc.). Briefly, Alamar Blue was diluted 1:10 in the cell culture media and absorbance was measured after 12 h. Colorimetric evaluation of cell proliferation was performed using a Multiskan Spectrum Microplate spectrophotometer with SkanIt software version 2.1 and using 570 nm as excitation wavelength and 600 nm as emission wavelength.
Real-time Reverse Transcription–Polymerase Chain Reaction (Real-time RT-PCR)
Relative gene expression levels of the TSPO, ABCA3, ABCB1 and ABCC1 genes were measured using real time PCR. 18S rRNA transcript level was used as an endogenous control. The specific primer and probe sets were designed from NCBI GenBank sequences using Primer Express, version 2.0 software (Applied Biosystems, Inc.), and synthesized by Integrated DNA Technologies (IDT). Total RNA was extracted using either the phenol guanidine isothiocyanate method (Molecular Research Center, Inc.) according to the manufacturer’s instructions, or the RNAeasy Kit (Qiagen). Gene expression was measured using a one-step real-time polymerase chain reaction in the ABI 7500 Real-Time PCR System (Applied Biosystems). Relative differences between samples were determined using the ΔΔCt method as outlined in the manufacturer’s instructions.49 Error bars represent positive and negative error of each calculated value.
Immunoblotting
Total protein was extracted from cultured NB cell lines (SMS-KCN, SMS-KCN-R, SMS-KAN and SMS-KAN-R with or without addition of 50 µM of PK11195 for 48 h) using M-PER protein lysis buffer (Invitrogen) with HaltTM Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific). Whole cell lysates were subjected to SDS-PAGE electrophoresis and proteins were transferred to a nitrocellulose membrane using the iBlot semi-dry system (Invitrogen) and incubated overnight at 4°C with an antibody against TSPO (Abcam). The membrane was subsequently incubated with an HRP-conjugated secondary antibody and stained with SuperSignal West Pico chemiluminescent substrate (Thermo Fisher Scientific). Chemiluminescent images were viewed and recorded by FluorChem (ProteinSimple). All membranes were stripped and re-blotted with GAPDH antibody used as protein loading control. The protein bands revealed by chemiluminescence were scanned to a computer and densitometric analysis was performed using NIH Image software (National Institutes of Health).
Cell cycle analysis
Cell cycle analysis was performed using propidium iodide on a BD LSR II flow cytometer (BD Biosciences). For each cell line tested, 10 million viable cells were grown in cell culture flasks for 24 h and were then treated with 50 uM PK11195 for 48 h. Control cells were treated with vehicle only (0.01% DMSO). Cells were then collected, washed twice in PBS and fixed in 70% cold ethanol at -20°C for two hours. After fixation, cells were again washed in PBS and permeabilized with 0.1%Triton X-100. The DNA was then stained for 15 min at 37°C with 10μg/ml propidium iodide (PI) (Sigma) in PBS containing 100μg/ml RNase (Sigma). Results were analyzed using Flow Cytometry Modeling Software (ModFit LT™) software (Verity Software House, Inc.) according to the manufacturer’s instructions.
Apoptosis analysis
We assessed the induction of apoptosis in PK11195-treated cells by measuring cleaved caspase-3 and PARP production with the Luminex® microbead assay (Invitrogen). Briefly, cells were plated in six well plates until confluence and then treated with DMSO only or PK11195 (0−100 uM). Cells were harvested using m-Per protein lysis buffer (Invitrogen) after 24 or 48 h of treatment according to the manufacturer’s protocol. Fluorescence levels of cleaved caspase-3 and PARP were measured in the cell lysates using a Luminex 100 bioassay detection system according to the manufacturer’s protocol, and differences in cleaved caspase-3 between untreated controls and PK11195 treated cells were calculated.
Statistical analysis
Differences between treatment groups in both cell proliferation analyses and in real-time PCR assays were evaluated using InStat software (GraphPad Software, Inc.) with one-way ANOVA followed by the Tukey-Kramer test to determine significance. A difference of p < 0.05 was considered significant.
Glossary
Abbreviations:
- NB
neuroblastoma
- TSPO
translocator protein 18kDa
- PK11195
1-(2-chlorophenyl-N-methylpropyl)-3-isoquinolinecarboxamide
- MPP
mitochondrial permeability pore
- PARP
poly (ADP-ribose) polymerase
- ABC
ATP-binding cassette
- JNK
c-Jun NH2 terminal kinase
- p38-MAPK
p38-mitogen-activated protein kinasel PCR, polymerase chain reaction
Disclosure of Potential Conflicts of Interest
The authors have no conflicts-of-interest or financial disclosures to make. The opinions expressed in this article are the opinions of the authors only and do not represent the views of the Department of Defense or the U.S. Government.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/23613
References
- 1.Brodeur G, Maris JM. Neuroblastoma. In: Pizzo PA PD, ed. Principles and Practice of Pediatric Oncology. Philadelphia: JB Lippincott, 2011:886-922. [Google Scholar]
- 2.Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–11. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keshelava N, Seeger RC, Groshen S, Reynolds CP. Drug resistance patterns of human neuroblastoma cell lines derived from patients at different phases of therapy. Cancer Res. 1998;58:5396–405. [PubMed] [Google Scholar]
- 4.Casellas P, Galiegue S, Basile AS. Peripheral benzodiazepine receptors and mitochondrial function. Neurochem Int. 2002;40:475–86. doi: 10.1016/S0197-0186(01)00118-8. [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, et al. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–9. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Veenman L, Levin E, Weisinger G, Leschiner S, Spanier I, Snyder SH, et al. Peripheral-type benzodiazepine receptor density and in vitro tumorigenicity of glioma cell lines. Biochem Pharmacol. 2004;68:689–98. doi: 10.1016/j.bcp.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Papadopoulos V, Amri H, Li H, Boujrad N, Vidic B, Garnier M. Targeted disruption of the peripheral-type benzodiazepine receptor gene inhibits steroidogenesis in the R2C Leydig tumor cell line. J Biol Chem. 1997;272:32129–35. doi: 10.1074/jbc.272.51.32129. [DOI] [PubMed] [Google Scholar]
- 8.Papadopoulos V, Mukhin AG, Costa E, Krueger KE. The peripheral-type benzodiazepine receptor is functionally linked to Leydig cell steroidogenesis. J Biol Chem. 1990;265:3772–9. [PubMed] [Google Scholar]
- 9.Papadopoulos V, Amri H, Boujrad N, Cascio C, Culty M, Garnier M, et al. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids. 1997;62:21–8. doi: 10.1016/S0039-128X(96)00154-7. [DOI] [PubMed] [Google Scholar]
- 10.Wang JK, Morgan JI, Spector S. Benzodiazepines that bind at peripheral sites inhibit cell proliferation. Proc Natl Acad Sci U S A. 1984;81:753–6. doi: 10.1073/pnas.81.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decaudin D, Castedo M, Nemati F, Beurdeley-Thomas A, De Pinieux G, Caron A, et al. Peripheral benzodiazepine receptor ligands reverse apoptosis resistance of cancer cells in vitro and in vivo. Cancer Res. 2002;62:1388–93. [PubMed] [Google Scholar]
- 12.Landau M, Weizman A, Zoref-Shani E, Beery E, Wasseman L, Landau O, et al. Antiproliferative and differentiating effects of benzodiazepine receptor ligands on B16 melanoma cells. Biochem Pharmacol. 1998;56:1029–34. doi: 10.1016/S0006-2952(98)00149-X. [DOI] [PubMed] [Google Scholar]
- 13.Ruff MR, Pert CB, Weber RJ, Wahl LM, Wahl SM, Paul SM. Benzodiazepine receptor-mediated chemotaxis of human monocytes. Science. 1985;229:1281–3. doi: 10.1126/science.2994216. [DOI] [PubMed] [Google Scholar]
- 14.Bessler H, Caspi B, Gavish M, Rehavi M, Hart J, Weizman R. Peripheral-type benzodiazepine receptor ligands modulate human natural killer cell activity. Int J Immunopharmacol. 1997;19:249–54. doi: 10.1016/S0192-0561(97)00013-1. [DOI] [PubMed] [Google Scholar]
- 15.Bessler H, Caspi B, Gavish M, Rehavi M, Weizman A. Significant inhibition of spontaneous IgA secretion by selective peripheral-type benzodiazepine receptor ligands. Clin Neuropharmacol. 1997;20:215–23. doi: 10.1097/00002826-199706000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Bessler H, Weizman R, Gavish M, Notti I, Djaldetti M. Immunomodulatory effect of peripheral benzodiazepine receptor ligands on human mononuclear cells. J Neuroimmunol. 1992;38:19–25. doi: 10.1016/0165-5728(92)90086-Z. [DOI] [PubMed] [Google Scholar]
- 17.Miettinen H, Kononen J, Haapasalo H, Helén P, Sallinen P, Harjuntausta T, et al. Expression of peripheral-type benzodiazepine receptor and diazepam binding inhibitor in human astrocytomas: relationship to cell proliferation. Cancer Res. 1995;55:2691–5. [PubMed] [Google Scholar]
- 18.Maaser K, Grabowski P, Oezdem Y, Krahn A, Heine B, Stein H, et al. Up-regulation of the peripheral benzodiazepine receptor during human colorectal carcinogenesis and tumor spread. Clin Cancer Res. 2005;11:1751–6. doi: 10.1158/1078-0432.CCR-04-1955. [DOI] [PubMed] [Google Scholar]
- 19.Han Z, Slack RS, Li W, Papadopoulos V. Expression of peripheral benzodiazepine receptor (PBR) in human tumors: relationship to breast, colorectal, and prostate tumor progression. J Recept Signal Transduct Res. 2003;23:225–38. doi: 10.1081/RRS-120025210. [DOI] [PubMed] [Google Scholar]
- 20.Galiègue S, Casellas P, Kramar A, Tinel N, Simony-Lafontaine J. Immunohistochemical assessment of the peripheral benzodiazepine receptor in breast cancer and its relationship with survival. Clin Cancer Res. 2004;10:2058–64. doi: 10.1158/1078-0432.CCR-03-0988. [DOI] [PubMed] [Google Scholar]
- 21.Vlodavsky E, Soustiel JF. Immunohistochemical expression of peripheral benzodiazepine receptors in human astrocytomas and its correlation with grade of malignancy, proliferation, apoptosis and survival. J Neurooncol. 2007;81:1–7. doi: 10.1007/s11060-006-9199-9. [DOI] [PubMed] [Google Scholar]
- 22.Santidrián AF, Cosialls AM, Coll-Mulet L, Iglesias-Serret D, de Frias M, González-Gironès DM, et al. The potential anticancer agent PK11195 induces apoptosis irrespective of p53 and ATM status in chronic lymphocytic leukemia cells. Haematologica. 2007;92:1631–8. doi: 10.3324/haematol.11194. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch T, Decaudin D, Susin SA, Marchetti P, Larochette N, Resche-Rigon M, et al. PK11195, a ligand of the mitochondrial benzodiazepine receptor, facilitates the induction of apoptosis and reverses Bcl-2-mediated cytoprotection. Exp Cell Res. 1998;241:426–34. doi: 10.1006/excr.1998.4084. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Polo RA, Carvalho G, Braun T, Decaudin D, Fabre C, Larochette N, et al. PK11195 potently sensitizes to apoptosis induction independently from the peripheral benzodiazepin receptor. Oncogene. 2005;24:7503–13. doi: 10.1038/sj.onc.1208907. [DOI] [PubMed] [Google Scholar]
- 25.Shoukrun R, Veenman L, Shandalov Y, Leschiner S, Spanier I, Karry R, et al. The 18-kDa translocator protein, formerly known as the peripheral-type benzodiazepine receptor, confers proapoptotic and antineoplastic effects in a human colorectal cancer cell line. Pharmacogenet Genomics. 2008;18:977–88. doi: 10.1097/FPC.0b013e3283117d52. [DOI] [PubMed] [Google Scholar]
- 26.Choi HB, Khoo C, Ryu JK, van Breemen E, Kim SU, McLarnon JG. Inhibition of lipopolysaccharide-induced cyclooxygenase-2, tumor necrosis factor-alpha and [Ca2+]i responses in human microglia by the peripheral benzodiazepine receptor ligand PK11195. J Neurochem. 2002;83:546–55. doi: 10.1046/j.1471-4159.2002.01122.x. [DOI] [PubMed] [Google Scholar]
- 27.Banker DE, Cooper JJ, Fennell DA, Willman CL, Appelbaum FR, Cotter FE. PK11195, a peripheral benzodiazepine receptor ligand, chemosensitizes acute myeloid leukemia cells to relevant therapeutic agents by more than one mechanism. Leuk Res. 2002;26:91–106. doi: 10.1016/S0145-2126(01)00112-6. [DOI] [PubMed] [Google Scholar]
- 28.Decaudin D, Castedo M, Nemati F, Beurdeley-Thomas A, De Pinieux G, Caron A, et al. Peripheral benzodiazepine receptor ligands reverse apoptosis resistance of cancer cells in vitro and in vivo. Cancer Res. 2002;62:1388–93. [PubMed] [Google Scholar]
- 29.Walter RB, Pirga JL, Cronk MR, Mayer S, Appelbaum FR, Banker DE. PK11195, a peripheral benzodiazepine receptor (pBR) ligand, broadly blocks drug efflux to chemosensitize leukemia and myeloma cells by a pBR-independent, direct transporter-modulating mechanism. Blood. 2005;106:3584–93. doi: 10.1182/blood-2005-02-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferry A, Jaillon P, Lecocq B, Lecocq V, Jozefczak C. Pharmacokinetics and effects on exercise heart rate of PK 11195 (52028 RP), an antagonist of peripheral benzodiazepine receptors, in healthy volunteers. Fundam Clin Pharmacol. 1989;3:383–92. doi: 10.1111/j.1472-8206.1989.tb00679.x. [DOI] [PubMed] [Google Scholar]
- 31.Ansseau M, von Frenckell R, Cerfontaine JL, Papart P. Pilot study of PK 11195, a selective ligand for the peripheral-type benzodiazepine binding sites, in inpatients with anxious or depressive symptomatology. Pharmacopsychiatry. 1991;24:8–12. doi: 10.1055/s-2007-1014425. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu S, Matsuoka Y, Shinohara Y, Yoneda Y, Tsujimoto Y. Essential role of voltage-dependent anion channel in various forms of apoptosis in mammalian cells. J Cell Biol. 2001;152:237–50. doi: 10.1083/jcb.152.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimizu S, Konishi A, Kodama T, Tsujimoto Y. BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc Natl Acad Sci U S A. 2000;97:3100–5. doi: 10.1073/pnas.97.7.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vieira HL, Haouzi D, El Hamel C, Jacotot E, Belzacq AS, Brenner C, et al. Permeabilization of the mitochondrial inner membrane during apoptosis: impact of the adenine nucleotide translocator. Cell Death Differ. 2000;7:1146–54. doi: 10.1038/sj.cdd.4400778. [DOI] [PubMed] [Google Scholar]
- 35.Hirsch T, Decaudin D, Susin SA, Marchetti P, Larochette N, Resche-Rigon M, et al. PK11195, a ligand of the mitochondrial benzodiazepine receptor, facilitates the induction of apoptosis and reverses Bcl-2-mediated cytoprotection. Exp Cell Res. 1998;241:426–34. doi: 10.1006/excr.1998.4084. [DOI] [PubMed] [Google Scholar]
- 36.Walter RB, Raden BW, Cronk MR, Bernstein ID, Appelbaum FR, Banker DE. The peripheral benzodiazepine receptor ligand PK11195 overcomes different resistance mechanisms to sensitize AML cells to gemtuzumab ozogamicin. Blood. 2004;103:4276–84. doi: 10.1182/blood-2003-11-3825. [DOI] [PubMed] [Google Scholar]
- 37.Hans G, Wislet-Gendebien S, Lallemend F, Robe P, Rogister B, Belachew S, et al. Peripheral benzodiazepine receptor (PBR) ligand cytotoxicity unrelated to PBR expression. Biochem Pharmacol. 2005;69:819–30. doi: 10.1016/j.bcp.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 38.Fennell DA, Corbo M, Pallaska A, Cotter FE. Bcl-2 resistant mitochondrial toxicity mediated by the isoquinoline carboxamide PK11195 involves de novo generation of reactive oxygen species. Br J Cancer. 2001;84:1397–404. doi: 10.1054/bjoc.2001.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chauhan D, Li G, Podar K, Hideshima T, Mitsiades C, Schlossman R, et al. Targeting mitochondria to overcome conventional and bortezomib/proteasome inhibitor PS-341 resistance in multiple myeloma (MM) cells. Blood. 2004;104:2458–66. doi: 10.1182/blood-2004-02-0547. [DOI] [PubMed] [Google Scholar]
- 40.Sutter AP, Maaser K, Barthel B, Scherübl H. Ligands of the peripheral benzodiazepine receptor induce apoptosis and cell cycle arrest in oesophageal cancer cells: involvement of the p38MAPK signalling pathway. Br J Cancer. 2003;89:564–72. doi: 10.1038/sj.bjc.6601125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jakubikova J, Duraj J, Hunakova L, Chorvath B, Sedlak J. PK11195, an isoquinoline carboxamide ligand of the mitochondrial benzodiazepine receptor, increased drug uptake and facilitated drug-induced apoptosis in human multidrug-resistant leukemia cells in vitro. Neoplasma. 2002;49:231–6. [PubMed] [Google Scholar]
- 42.Banker DE, Cooper JJ, Fennell DA, Willman CL, Appelbaum FR, Cotter FE. PK11195, a peripheral benzodiazepine receptor ligand, chemosensitizes acute myeloid leukemia cells to relevant therapeutic agents by more than one mechanism. Leuk Res. 2002;26:91–106. doi: 10.1016/S0145-2126(01)00112-6. [DOI] [PubMed] [Google Scholar]
- 43.Haber M, Bordow SB, Haber PS, Marshall GM, Stewart BW, Norris MD. The prognostic value of MDR1 gene expression in primary untreated neuroblastoma. Eur J Cancer. 1997;33:2031–6. doi: 10.1016/S0959-8049(97)00229-3. [DOI] [PubMed] [Google Scholar]
- 44.Mi YJ, Liang YJ, Huang HB, Zhao HY, Wu CP, Wang F, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 2010;70:7981–91. doi: 10.1158/0008-5472.CAN-10-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou WJ, Zhang X, Cheng C, Wang F, Wang XK, Liang YJ, et al. Crizotinib (PF-02341066) reverses multidrug resistance in cancer cells by inhibiting the function of P-glycoprotein. Br J Pharmacol. 2012;166:1669–83. doi: 10.1111/j.1476-5381.2012.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kannan S, Balakrishnan B, Muzik O, Romero R, Chugani D. Positron emission tomography imaging of neuroinflammation. J Child Neurol. 2009;24:1190–9. doi: 10.1177/0883073809338063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartels AL, Willemsen AT, Doorduin J, de Vries EF, Dierckx RA, Leenders KL. [11C]-PK11195 PET: quantification of neuroinflammation and a monitor of anti-inflammatory treatment in Parkinson’s disease? Parkinsonism Relat Disord. 2010;16:57–9. doi: 10.1016/j.parkreldis.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 48.van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry. 2008;64:820–2. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 49.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]