Abstract
Among the different types of tests used for cancer diagnosis, molecular tests have been increrasingly incorporated because of their ability to detect either expression or functional changes in the molecules associated with the disease. Mammaglobin is a protein found in mammary tissue and can be detected in serum. This protein has been proposed as a biomarker to diagnose breast cancer, given that patients exhibit an increased amount of the protein in serum and tumor tissue, in comparison to healthy individuals. The ELISA test was used in the present study to detect mammaglobin in blood samples from 51 breast cancer patients and 51 control individuals. Antibodies against mamaglobin were generated in rabbits by using the following synthetic peptides: A (amino acids 13 to 21), B (amino acids 31 to 39), C (amino acids 56 to 64) and a D peptide, corresponding to the protein isoform without three amino acids (59, 60 and 61 amino acids) from peptide C. All peptides were immunogenic and allowed generation of antibodies that were able to discriminate patients from controls. The best results were obtained for antiserum B, achieving the best sensitivity (86.3%) and specificity (96%).
Keywords: ELISA, biomarker, breast cancer, diagnosis, human mammaglobin
Introduction
The National Cancer Institute (NCI) indicated an almost 13% increase in the number of cancer cases in 2004 for Colombia, breast cancer constituting the second most common malignancy affeccting the Colombian female population.1
Mammography is the main test used to detect malignant breast lesions; however, its sensitivity ranges from 68 to 90%.2 Other options include a physical examination or ultrasound and, more recently, molecular tests that can detect alterations in gene sequences and expression regarding a particular molecule. Such markers can directly associate such changes with neoplastic processes, making them useful biomarkers in cancer detection. These molecular tests represent a remarkable contribution toward detecting, diagnosing and treating cancer patients. Several studies have, thus, suggested that the mammaglobin glycoprotein might be a useful cellular marker to diagnose and monitor breast cancer. Human mammaglobin is specifically expressed in mammary glands and overexpressed in most primary and metastatic breast tumors.3 cDNA was first isolated from human primary breast cancer tumor ARNm.3 Mammaglobin is quite a small 93 amino acid (aa) long protein, having 8.48 kDa molecular weight; it may be N-glycosylated at Asn35 and Asn50 residues and contains a 20 amino acid-long secretory signal peptide. Mammaglobin is a member of the secretoglobin protein family, which also contains lypophilin B (for a review see refs. 4–6); it forms complexes with the latter through disulphide bridges between residues Cys 4, 47 and 72 and Cys3, 44 and 67, respectively. The complex’s function has not been fully established, but it is believed that it might be related to steroid metabolism regulation and some immune functions.
Mammaglobin displays a small helical globular domain and a hydrophobic pocket in its structure; thereby, facilitating binding to steroid and biphenyl-like molecules.5 A 90 aa protein isoform produced by the loss of 9 bp through alternative splicing at the second gene exon has been reported in the NCBI database (accession number: 28932885).
The mammaglobin concentration detected in serum of breast cancer patients ranged from 0.07 to 9.6 ng/ml compared with 0 to 0.07 ng/ml in healthy individuals.7 If an association between this protein’s concentration in serum and the presence of breast cancer can be confirmed, its seric detection could well become a promising, non-invasive tool that would likely make a real contribution in diagnosing this disease. Data obtained from ELISA tests on women enduring different stages of breast cancer and from healthy individuals revealed that mammaglobin expression was not dependent on disease stage or degree of development.8 An ROC curve was calculated where mammaglobin concentration cutoff was established at 1.71 ng/ml; the test was considered positive above said value.8 Patients enduring disease stages I to III had 0.9 to 1.4 ng/ml concentrations and stage IV patients had 2.3 ng/ml. A strong positive association between mammaglobin level and tumor size was found; patients with bigger tumors have higher mammaglobin levels. Mean mammaglobin concentration in serum for a group of women with metastatic breast cancer was 9.38 ng/ml (7.9 ng/ml control group); an 8.8 ng/ml cutoff value was established above which individuals were considered positive for the test. Sensitivity reached 68% and specificity 88.8% in that study.9 The difference between the last two studies mentioned above lies in the antibodies used; the first used a monoclonal antibody generated by taking a native mammaglobin complex,8 while the second used polyclonal antibodies generated against the EVFMQLIYDSSLCDLF C-terminal peptide.
Mammaglobin protein concentration was determined in the present study via ELISA test on serum samples; four polyclonal antisera were obtained from rabbits immunised with four synthetic peptides. Better discrimination between patients and controls was observed with antiserum B, which was obtained when the CGDDNATTNAIGC peptide was used as immunogen. This peptide covered the protein’s 31−39 aa; ELISA reactions with this serum revealed 96% specificity and 86.3% sensitivity. This antibody could, thus, be used to discriminate breast cancer patients from controls.
Results
Sample description
Patients who had received no prior treatment against breast cancer, such as chemotherapy or radiotherapy, were suffering from clinically and pathologically confirmed breast cancer. Control subjects were suffering from no type of cancer or mammary disease. Specific relevant characteristics regarding the subjects participating in the study are shown in Table 1.
Table 1. Characteristics of subjects participating in the study.
| Features a | Patients | Controls |
|---|---|---|
|
Age |
27.0 – 85.9 y |
28.1 – 85 y |
|
BMI |
17.3 – 40.0 |
19.1 – 35.1 |
|
Brassiere cup |
A: 7 (14%) B: 42 (82%) C: 2 (4%) |
A: 7 (14%) B: 42 (82%) C: 2 (4%) |
|
Age of menarche |
9 – 20 y |
11–16 y |
|
Pregnancy |
Yes: 46 (90.2%) No: 5 (9.8%) |
Yes: 46 (90.2%) No: 5 (9.8%) |
|
Age of first Pregnancy |
16 – 38 y |
15 – 36 y |
|
Oral contraceptives |
Yes: 14 (27.4%) No: 37 (72.6%) |
Yes: 15 (29.4%) No: 36 (70.6%) |
|
Hormonal therapyb |
Yes: 2 (3.9%) No: 49 (96.1%) |
Yes: 1 (2.0%) No: 50 (98.0%) |
|
Menopause |
Yes: 39 (76.5%) No: 12 (23.5%) |
Yes: 41 (80.4%) No: 10 (19.6%) |
|
Age of menopause |
39 – 59 y |
34 – 55 y |
| Family member with cancer | Yes: 29 (56.9%) No: 22 (43.1%) |
Yes: 28 (54.9%) No: 23 (45.1%) |
a Variables that have been reported associated with breast cancer development were analyzed in patients and controls (BMI: Body mass index).
b Individuals who use or used hormone replacement therapy after menopause
Table 2 describes tumor characteristics; the most frequently occurring cancer was invasive ductal carcinoma (32−67.4%) followed by infiltrating canalicular carcinoma (7–13.7%); 43.1% of the patients were positive for progesterone receptors and 49% for estrogen. Only 10% of the patients were positive for HER2 expression. The most frequent clinical stage was IIIA (35.3%) followed by stages IIB (23.5%) and IIIB (17.6%). Information regarding tumor tissue characteristics, estrogen receptor status and HER2 gene expression was obtained from the patients’ medical histories.
Table 2. Clinical and pathological characteristics of tumor tissue.
| Characteristic | Description |
|---|---|
|
Type of Cancer |
Ductal in situ: 2 (3.9%) Infiltrating Lobular: 2 (3.9%) Infiltrating Ductal: 32 (67.4%) Infiltrating Canalicular: 7 (13.7%) Paget’s disease: 1 (1.9%) Adenocarcinoma: 1 (1.9%) Apocrine Carcinoma: 1 (1.9%) ND: 5 (9.8%) |
|
Progesterone Receptor |
Positive: 22 (43.1%) Negative: 11 (21.6%) ND: 18 (35.3%) |
|
Estrogen Receptor |
Positive: 25 (49%) Negative: 9 (17.7%) ND: 17 (33.3%) |
|
HER2 |
Positive: 10 (19.6%) Negative: 15 (29.4%) ND: 26 (51%) |
| Stage | I: 2 (3.9%) IIa: 5 (9.8%) IIb: 12 (23.5%) IIIa: 18 (35.3%) IIIb: 9 (17.6%) IV: 5 (9.8%) |
Information regarding characteristics of the tumor tissue and the status of estrogen receptors and HER2 gene was obtained from the medical records of patients.
Rabbit sera specificity and activity
Antisera displaying the highest OD values and specificity regarding their corresponding peptides were selected for further tests with sera from women participating in the study. A useful criterion to select a serum was its low cross-reactivity with other peptides. Sera having cross-reactivity were incubated with the interfering peptide until such interference was eliminated. Peptide A was best recognized by antiserum 54, peptide B by antiserum 55, peptide C by antiserum 62 and peptide D by antiserum 78.
Recognition of human recombinant mammaglobin by rabbit antisera peptide
A standard curve using different recombinant human mammaglobin concentrations was constructed before determining mammaglobin presence in sera from participating subjects. Ten human mammaglobin protein (1:2) serial dilutions were prepared (Ray Biotech, Inc. Catalog N° 228–11074). Antisera 54, 55 and 62 had a similar pattern regarding the recombinant protein; whereas, antiserum D did not recognize the protein. R2 values were 0.9931 for sera 54, 0.9914 for sera 55 and 0.9856 for sera 62; these three antibodies, thus, recognized the recombinant mammaglobin in a dose-dependent manner.
Determining serum mammaglobin concentration
Antisera 54 and 55 best differentiated breast cancer patients from the healthy control group, showing the higest OD values (Fig. 1). Control group OD values were more homogeneous than those from the breast cancer patient group. Antisera 55 performed best in discriminating patients from the control group.
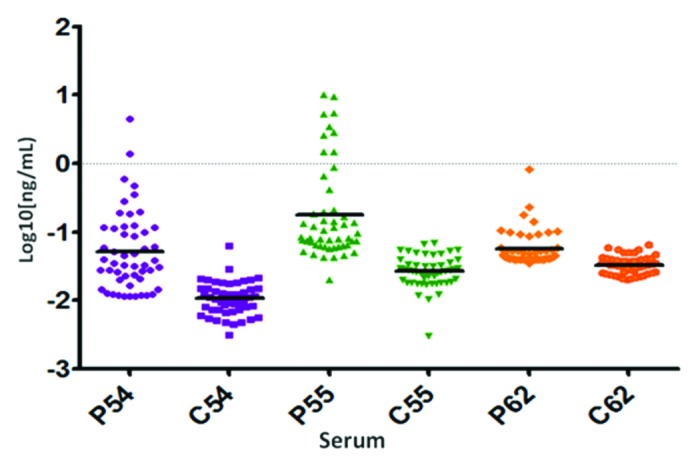
Figure 1. Mammaglobin concentration is shown (log 10 scale, ng/ml) for patient (P) and control (C) groups, determined by using polyclonal antibodies 54, 55 and 62.
Statistical analysis
Both cases and control groups were matched 1:1 to compare mammaglobin concentration in serum. This distribution considered variables like age, pregnancy or menopause so that significant differences between patients and controls in mammaglobin levels might be associated to the presence of the disease. Table 3 shows each antiserum’s minimum and maximum values, means and standard deviations for mammaglobin concentration (ng/ml). The Kolmogorov-Smirnov test was used to analyze this data (p < 0.05 for all antisera). The results indicated that the antiserum could discriminate between breast cancer patients and healthy controls.
Table 3. Analysis of Mammaglobin concentration in sera.
| Sera | Group | n | Minimum value | Maximum value | Mean | SD | Kolmogorov-Smirnov Test | |
|---|---|---|---|---|---|---|---|---|
| |
|
|
|
|
|
|
Statistical Value |
P-Value |
| 54 (peptide A) |
Cases |
51 |
0.011 |
9.7 |
0.381 |
1.481 |
3.466 |
0 |
| Controls |
51 |
0.003 |
0.006 |
0.012 |
0.008 |
|||
| 55 (peptide B) |
Cases |
51 |
0.002 |
10.022 |
0.928 |
2.164 |
4.159 |
0 |
| Controls |
51 |
0.003 |
0.069 |
0.03 |
0.014 |
|||
| 62 (peptide C) | Cases |
51 |
0.035 |
0.818 |
0.074 |
0.112 |
3.466 | 0 |
| Controls | 51 | 0.02 | 0.064 | 0.034 | 0.01 | |||
The minimum and maximum values, mean and standard deviation for mammaglobin concentration are shown. These data were analyzed with the Kolmogorov-Smirnov test, finding p < 0.05 with all antisera.
No association could be observed between menopause, progesterone and estrogen receptor positivity for HER2 expression or mammaglobin concentration serum in breast cancer patients (data not shown). No significant differences were noted between the disease’s clinical stage and mammaglobin serum concentration in breast cancer patients when antisera 54, 55 and 62 were used (data not shown). No statistical analysis was performed to correlate relapse or metastasis with mammaglobin abundance because a few cases had these characteristics (n = 4 for relapse, n = 8 for metastasis).
No correlation was found when analyzing the relationship between patient age and mammaglobin concentration. The hypothesis test involving Pearson’s correlation coefficient led to considering correlation between age and antisera 54, 55 and 62 as zero (p = 0.796, p = 0.558 and p = 0.472, respectively).
Linear regression analysis was conducted to observe the effect of pairing variables such as brassiere cup size, oral contraceptive use, hormone replacement therapy and body mass index (BMI) on mammaglobin concentration using antisera 54, 55 and 62. Breast cancer was the only variable having direct relationship with mammaglobin concentration (a slight effect). Brassier cup size C and hormone therapy were considered symbolically because there were very few cases. SPSS 20 software was used for statistical analysis.
Detectability index and ROC curve
A detectability index was calculated and an ROC curve was constructed for the three antisera used. Antiserum 55 turned out to be the best candidate for a possible screening test (Table 4). Figure 2 shows the ROC curve for antiserum 55; the threshold point best fitting the screening test yielded 86.3% sensitivity and 96% specificity. The high specificity was due to very high values in the cases group, as described when explaining the protein concentration pattern regarding cases and controls. The values observed here agreed with similar values already observed in the control group; the antiserum screening test, therefore, had more specificity than sensitivity.
Table 4. Detectability index for each of the anti-peptide sera.
| Variable | Detectability index | ROC area |
|---|---|---|
|
Antiserum A (54) |
1.8 |
0.897 |
|
Antiserum B (55) |
3.0a |
0.961b |
| Antiserum C (62) | 2.5 | 0.849 |
a Antiserum B presents the highest detectability index.
b Antiserum B shows the area under the curve closest to 1.
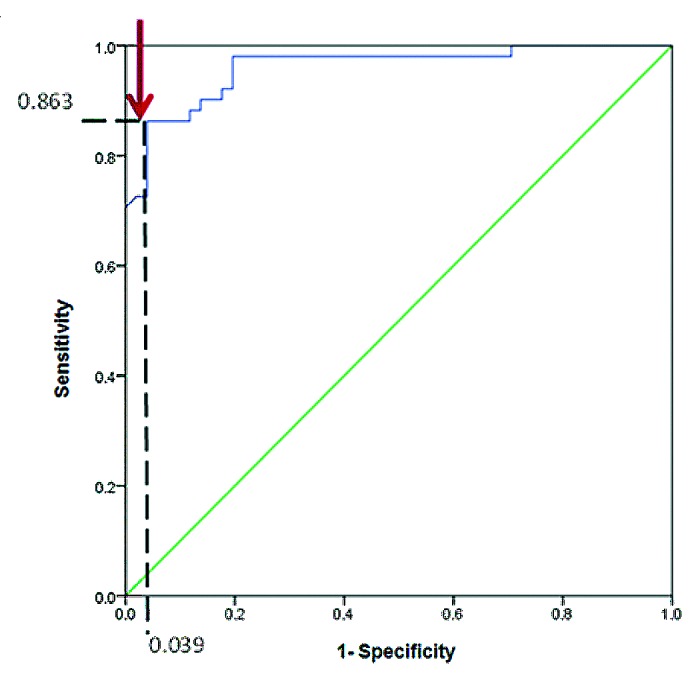
Figure 2. ROC curve for assays performed with antiserum (55) showed 96% specificity and 86.3% sensitivity in ELISA tests with antiserum 55, as indicated by the arrow.
Discussion
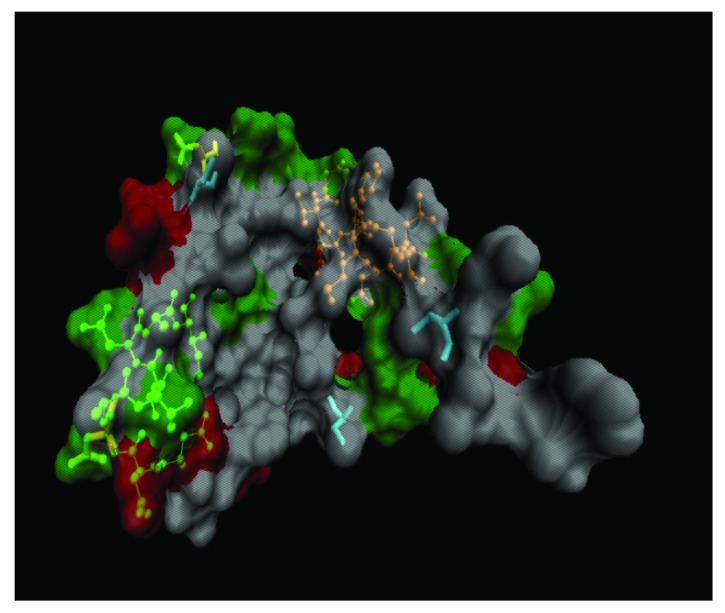
Mammaglobin has been described as a breast and breast tumor specific protein.10 However, mRNA has also been detected in primary carcinoma tumor tissue in the biliary tract and in lymph nodes with micrometastases.11 Higher mammaglobin mRNA levels have been detected in breast cancer patients’ peripheral blood samples in studies made in our laboratory, compared with those from healthy women and the present study was aimed at assessing four rabbit polyclonal antisera’s ability to detect serum mammaglobin concentration in similar groups. Antibodies were obtained from rabbits immunised with synthetic peptides A, B, C and D. Peptide C lay within the mammaglobin dimerization domain (where lipophylin B binds). Peptide A was in the protein’s N-terminal region, while peptide C formed part of the C-terminal region containing the dimerization domain (Fig. 3). Taking into account that the recombinant mammaglobin is expressed alone and is, thus, unable to form dimers, then the peptide C region could be more easily recognized by the corresponding antiserum.
Figure 3. The green structure indicates the location of peptide B (left), the orange structure indicates the location of peptide C (right) and the three turquoise structures show Cys 4, 47 and 72. Polar residues are represented in green, acid residues in red and non-polar residues in white. Note that non-polar residues are part of the nucleus or “core,” which at the time of the complex formation with Lypophilin B through disulfide bridges between the cysteines will allow binding of steroid molecules for transport (For this graphic representation, the Visual Molecular Dynamics, Univ. Illinois software was used).
The anti-peptide B antibody was the best candidate for detecting mammaglobin protein concentration in serum samples via ELISA test; this peptide is localized in the protein’s central region, which seems to be exposed, even though mammaglobin forms a complex with lipophylin B (Fig. 3). Using anti-peptide B led to obtaining higher OD values and the best detectability index, as well as an area below the ROC curve very close to 1. This is the first study reporting the successful use of this region in obtaining antibodies that can detect mammaglobin serum concentration via ELISA test.
Peptide D had a sequence similar to that of peptide C; three amino acids being excluded from the central region, which are not present in the mammaglobin isoform due to alternate splicing in exon two. Immunisation with peptide D was aimed at generating antibodies specifically recognizing this mammaglobin isoform; these antibodies did not recognize the recombinant mammaglobin protein and detected the seric protein in only one breast cancer patient. This breast cancer patient did not show specific clinical or pathological characteristics or any significant feature that could have established a remarkable difference regarding other members of the patients’ group. Although the OD values were low compared with assays with other antibodies, they remained higher than the target. All the antibodies used in this study led to discriminating the cases from the control group; mammaglobin serum concentration was higher in breast cancer patients than in the control group, as previously reported (for a review see refs6,7,9). Patient mammaglobin concentration detected here ranged from 0.391 to 0.928 ng/ml (0.012 to 0.03 ng/ml in controls).
Antiserum generated with peptide B had 86.3% sensitivity and 96% specificity, indicating that this screening test was more specific than sensitive. Sensitivity and specificity values obtained in the present study were higher than previously reported values (68.8 and 88.8%, respectively)9 probably due to different experimental conditions; a 16-amino acid peptide from the C-terminal end of the protein was used to generate rabbit antibodies, close to where peptide C in the study is located. A monoclonal antibody has recently been obtained by immunising mice with recombinant mammaglobin protein.12 The immunogen covered amino acid 8 to 93; thus, including the four peptides synthesized here (unfortunately, no sensitivity or specificity data or protein serum concentration were reported).
Mammaglobin protein serum levels in the present study were not associated with hormonal factors like estrogen or progesterone receptor expression, and no association was found with menopausal age, estrogen replacement therapy use, oral contraceptive use, the disease’s clinical stage or individuals’ age. The present study led to determining protein concentration in all the disease’s clinical stages; furthermore, no significant differences in the determined concentrations were found regarding clinical stages, even though less samples were assayed for the former, compared with more advanced stages. This differed from other studies where the protein was detected only in stages IIa and IV, concentration being higher in stage IV.8
Prior studies have found a significant correlation between high mammaglobin expression and the presence of progesterone and estrogen receptors; this factor could have been associated to less aggressive tumors and a better response to endocrine therapy. Such divergent results might have been attributed to the methodology and sample source as they determined mRNA expression by RT-PCR in tumor specimens.13
Thus, mammaglobin has been shown to be a stable biomarker and determining its serum concentration via ELISA test is useful for identifying patients suffering from breast cancer. Further studies involving more patients are required to establish the cut-off values.
Materials and Methods
Serum sample collection
Blood (5 ml) was collected from 51 patients who had been clinically diagnosed as suffering from breast cancer (confirmed via histology). All patients who had received prior treatment or were currently being treated were excluded. The control group was comprised of 51 healthy women. Serum was separated and stored at -80°C until required. Samples were only taken when individuals had filled in and signed an informed consent form. Both groups were contacted in the Centro de Investigaciones Oncológicas Clínica San Diego (CIOSAD) and the Mayor-Méderi teaching hospital.
Peptide synthesis
BepiPred software (version 1.0) was used to predict potential B-cell epitopes present in mammaglobin protein (http://www.cbs.dtu.dk/services/BepiPred/). Three sequences were chosen (Table 1) and peptides were synthesized at Fundación Instituto de Inmunología de Colombia (FIDIC) by using the t-boc method; the fourth peptide (D) was synthesized to determine the presence of the mammaglobin protein isoform (Table 5).
Table 5. Amino acid sequence of synthetic mammaglobin peptides.
| Peptide | Peptide sequencea | Rabbit c |
|---|---|---|
|
A |
GCINPQVSKTECG |
53, 54 |
|
B |
GCDDNATTNAICG |
55, 57 |
|
C |
GCNVEVFMQLICG |
62, 75 |
| D b | GCSNVE QLIYDCG | 78, 80 |
a Mammaglobin peptides A, B and C amino acid sequences, these sequences were predicted as antigenic when analyzed with the BepiPred software.
b Peptide D corresponds to the exon 2 isoform sequence without the three amino acids that differentiate it from the native protein.
c Number that identifies the rabbits immunized with each peptide.
Anti-mammaglobin sera
A serum sample was taken before rabbits were immunised and used as pre-immune control. Eight rabbits (two rabbits per peptide) were immunised by inoculating them with three doses of each synthetic peptide; peptide dose administration was spaced out by 20 d. The first peptide dose was emulsified with Freund’s complete adjuvant (Sigma-Aldrich Co, Catalog N° F5506).14 Subsequent doses were combined with Freund’s incomplete adjuvant (Sigma-Aldrich Co, Catalog N° F5881). Twenty days after the last inoculation, and before they were used in ELISA reactions, each rabbit serum was collected and successively pre-absorbed on CNBr-activated Sepharose 4B (Amershan Biosciences) columns coupled with M. smegmatis and E. coli lysates and the Spf66 synthetic peptide.
Antisera activity assessment
The antiserum’s ability to recognize the recombinant mammaglobin protein and each synthesized peptide was evaluated as follows. Ten dillutions of human mammaglobin protein (Ray Biotech, Inc. Catalog N° 228–11074) were prepared (ranging between 25 and 0.048 ng/ml) and ELISA tests were performed.
Mammaglobin quantification
The mammaglobin concentration test was performed in duplicate; sera were tested by ELISA with antiserum A, B, C and D to determine mammaglobin concentration. Patient and control undiluted sera groups were fixed to ELISA plates and independently incubated with the rabbit antiserum. A standard concentration curve was constructed by using the recombinant protein; it was used to quantify the mammaglobin serum concentration. Another group consisting of 15 men was also analyzed (values were close to expected/target values, data not shown).
Statistical analysis
Paired patient/control sampling compared mammaglobin serum concentrations. Age and being pregnant or undergoing menopause were the variables selected for pairing. Each variable was described in terms of means, standard deviations and percentiles. Kolmogorov-Smirnov tests were used to analyze the significance of differences between groups. Linear regression models were adjusted to evaluate the effect of different variables, which could have influenced mammaglobin serum levels. A Pearson correlation test was conducted to determine the relationship between patients’ age and mammaglobin concentration; the detectability index was also determined.
Ethical aspects
This study was approved by the ethics committee at Universidad del Rosario. The study posed minimum risk to the health of all individuals involved (according to Colombian Ministry of Health criteria established for research involving humans in resolution 008430/1993). Patients received suitable information regarding this project’s objectives, methods, benefits and the possible discomfort involved, as well as their freedom to participate and their option to withdraw at any time they might consider it appropriate.
Particular emphasis was placed on patients’ privacy and integrity and patients or their legal representatives provided informed written consent to participate in the study. They were informed that this study’s results would be confidential and that any benefit to the scientific community arising from them would contribute to knowledge regarding the disease’s etiology.
Rabbits were handled in line with Colombian bioethics’ standards for experimental animal handling (Colombian legislation 84/1989).
Acknowledgments
This work was funded by a grant from Fondo de Investigaciones Universidad del Rosario (FIUR) awarded to S.R.C. and supported by the Fundación Instituto de Inmunología de Colombia (FIDIC).
Glossary
Keywords:
- breast cancer
human mammaglobin, ELISA, biomarker, diagnosis
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/23614
References
- 1.http://www.cancer.gov.co, accesed January 2012. Instituto Nacional de Cancerología. Anuario Estadístico 2007.
- 2.Díaz S, Piñeros M, Sánchez O. Detección temprana del cáncer de mama: aspectos críticos para un programa de tamizaje organizado en Colombia. Revista Colombiana de Cancerología. 2005;9:93–105. [Google Scholar]
- 3.Fleming TP, Watson MA. Mammaglobin, a breast-specific gene, and its utility as a marker for breast cancer. Ann N Y Acad Sci. 2000;923:78–89. doi: 10.1111/j.1749-6632.2000.tb05521.x. [DOI] [PubMed] [Google Scholar]
- 4.Watson MA, Fleming TP. Mammaglobin, a mammary-specific member of the uteroglobin gene family, is overexpressed in human breast cancer. Cancer Res. 1996;56:860–5. [PubMed] [Google Scholar]
- 5.Carter D, Douglass JF, Cornellison CD, Retter MW, Johnson JC, Bennington AA, et al. Purification and characterization of the mammaglobin/lipophilin B complex, a promising diagnostic marker for breast cancer. Biochemistry. 2002;41:6714–22. doi: 10.1021/bi0159884. [DOI] [PubMed] [Google Scholar]
- 6.Zehentner BK, Carter D. Mammaglobin: a candidate diagnostic marker for breast cancer. Clin Biochem. 2004;37:249–57. doi: 10.1016/j.clinbiochem.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Fanger GR, Houghton RL, Retter MW, Hendrickson RC, Babcook J, Dillon DC, et al. Detection of mammaglobin in the sera of patients with breast cancer. Tumour Biol. 2002;23:212–21. doi: 10.1159/000067251. [DOI] [PubMed] [Google Scholar]
- 8.Zehentner BK, Persing DH, Deme A, Toure P, Hawes SE, Brooks L, et al. Mammaglobin as a novel breast cancer biomarker: multigene reverse transcription-PCR assay and sandwich ELISA. Clin Chem. 2004;50:2069–76. doi: 10.1373/clinchem.2004.038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernstein JL, Godbold JH, Raptis G, Watson MA, Levinson B, Aaronson SA, et al. Identification of mammaglobin as a novel serum marker for breast cancer. Clin Cancer Res. 2005;11:6528–35. doi: 10.1158/1078-0432.CCR-05-0415. [DOI] [PubMed] [Google Scholar]
- 10.Watson MA, Dintzis S, Darrow CM, Voss LE, DiPersio J, Jensen R, et al. Mammaglobin expression in primary, metastatic, and occult breast cancer. Cancer Res. 1999;59:3028–31. [PubMed] [Google Scholar]
- 11.Okami J, Dohno K, Sakon M, Iwao K, Yamada T, Yamamoto H, et al. Genetic detection for micrometastasis in lymph node of biliary tract carcinoma. Clin Cancer Res. 2000;6:2326–32. [PubMed] [Google Scholar]
- 12.Huang Y, Zhang HQ, Wang J, Song XG, Wang GH, Guan Q, et al. Cloning expression, monoclonal antibody preparation and serologic study of mammaglobin in breast cancer. Neoplasma. 2011;58:436–40. doi: 10.4149/neo_2011_05_436. [DOI] [PubMed] [Google Scholar]
- 13.Núñez-Villar MJ, Martínez-Arribas F, Pollán M, Lucas AR, Sánchez J, Tejerina A, et al. Elevated mammaglobin (h-MAM) expression in breast cancer is associated with clinical and biological features defining a less aggressive tumour phenotype. Breast Cancer Res. 2003;5:R65–70. doi: 10.1186/bcr587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogel FR, Hem SL. Immunologic Adjuvants. In: Plotkin SA, Orenstein WA eds. Vaccines. 4th edn. Filadelfia: Elsevier Inc. 2004;69-79. [Google Scholar]