Abstract
Objectives: To evaluate the 6-mo overall survival, safety and tolerability of lenalidomide in combination with standard gemcitabine as first-line treatment for patients with metastatic pancreatic cancer. Methods: Eligibility included: previously untreated metastatic adenocarcinoma of the pancreas with metastases incurable by surgery/radiation therapy; ECOG PS 0–2; adequate organ function; prophylactic anticoagulation for venous thromboembolic events (VTEs). Patients received lenalidomide 25 mg PO (days 1–21) and gemcitabine 1,000 mg/m2 IV (days 1, 8 and 15) each 28-day cycle, with response evaluations every eight weeks. Results: Between 5/2009–4/2010, 72 patients (median age 64 years; 68% male; 42% ECOG PS 0) were enrolled in this multicenter, community-based study. Six-month OS was 37% (95% CI 26–48%). Median PFS and OS were 2.3 (95% CI 1.9–3.5) and 4.7 (95% CI 3.4–5.7) months, respectively. Eight partial responses (11%) were documented. Thirty-nine patients (54%) experienced thrombocytopenia (2 patients, 3% grade 4). Hematologic toxicities resulted in dose modifications for the majority of patients. Twenty patients (28%) developed VTEs during treatment. Conclusions: The observed 6-month OS (37%) of lenalidomide with gemcitabine does not suggest improvement compared with historical results with gemcitabine alone. Toxicities and dose modifications likely limited dose intensity. Further development of this regimen in pancreas cancer is not recommended.
Keywords: pancreas cancer, lenalidomide, phase II, gemcitabine combination
Carcinoma of the pancreas is among the most lethal of human cancers. In the absence of effective early detection strategies, the majority of patients present with advanced disease that cannot be treated with surgery or radiation, and the five-year survival rate of these patients is only 4%.1 Furthermore, even those patients who are candidates for surgical resection experience high rates of recurrence, and the five-year survival rate following pancreaticoduodenectomy is only 8–20%.2-5
The definitive randomized trial of gemcitabine compared with 5-fluorouracil (5-FU) led to its FDA approval in 1996; the median survival for patients treated with gemcitabine was 5.65 mo, compared with 4.41 mo with 5-FU.6 For patients with advanced disease, gemcitabine is now considered a standard treatment, yet studies of gemcitabine combined with other cytotoxic chemotherapy agents have failed to improve survival compared with gemcitabine alone.7 Agents targeting molecular pathways have also been studied in combination with gemcitabine,7 yet erlotinib, which targets the epidermal growth factor receptor (EGFR), is the only agent to improve survival in combination with gemcitabine.8 While the improvement in survival is statistically significant compared with gemcitabine alone, the clinical benefit seen with the addition of erlotinib is modest.
Targeting the vascular endothelial growth factor (VEGF) receptor in combination with cytotoxic chemotherapy has been an effective strategy in both colorectal and non-small cell lung cancer (NSCLC).9,10 Though VEGF is overexpressed in pancreatic cancers,11 this strategy has had less success. In a randomized phase III study comparing gemcitabine with or without bevacizumab (an antibody to the VEGF ligand), the addition of bevacizumab did not improve survival in patients with advanced pancreas cancer.12 Similar results have been reported with axitinib,13 suggesting that in combination with gemcitabine, agents that solely inhibit the VEGF pathway will not be an effective strategy for patients with this disease.
Additional and more efficacious treatment strategies for patients with advanced carcinoma of the pancreas are urgently needed. Lenalidomide has known anti-angiogenic properties and enhanced immunomodulatory activity. With demonstrated inhibition of bFGF, VEGF and TNF-α-induced endothelial cell migration, lenalidomide has potentially improved potency with reduced toxicity, compared with its parent compound thalidomide. Indeed, lenalidomide reduces phosphorylated ERK expression in three pancreatic cell lines (PANC-1, MIA-PaCa-2 and BxPC-3). Furthermore, the combination of lenalidomide and gemcitabine reduced the IC50 of gemcitabine up to 40%, suggesting agents that reduced pERK activity may enhance gemcitabine efficacy.14
The purpose of this phase II study was to evaluate the anti-tumor activity and safety of the combination of lenalidomide and gemcitabine in patients with carcinoma of the pancreas.
Results
Patient characteristics and treatment received
Between May 2009 and April 2010, 72 patients initiated treatment with the combination of lenalidomide and gemcitabine. The median age was 64 years (range: 39–88 years), with nearly all patients having an ECOG performance status of 0 or 1. Patient characteristics are detailed in Table 1.
Table 1. Patient Characteristics (n = 72).
| Characteristic | Number of Patients (%) |
|---|---|
| Median age |
years (range) 64 (39 – 88) |
| Sex |
male 49 (68%) |
| female 23 (32%) | |
| ECOG performance status |
0: 30 (42%) |
| 1: 41 (57%) | |
| 2: 1 (1%) | |
| Prior radiation |
5 (7%) |
| Prior surgery |
15 (21%) |
| Prior systemic therapy (adjuvant) |
7 (10%) |
| Prophylactic anticoagulation |
aspirin 57 (79%) |
| warfarin* 9 (13%) | |
| LMWH 4 (5%) | |
| none 2 (3%) | |
| Prior history of venous thromboembolic events | 6 (8%) |
Pre-existing full-dose warfarin, four patients; prophylactic low-dose warfarin (1–2 mg), five patients
There were no DLTs reported among the 6 patients in the lead-in portion, and the study continued to full enrollment. Fifty-six patients (78%) completed two cycles (eight weeks) of treatment and were evaluable for response. The remaining 16 patients received less than eight weeks of treatment for the following reasons: intercurrent illness, six patients; symptomatic deterioration, four patients; death on study due to disease, three patients; patient requested to discontinue treatment, three patients.
The median number of cycles received was two (range: <1 to 24 cycles). Thirty-three patients had doses of lenalidomide held, and 27 patients required at least one dose reduction of lenalidomide, two of these to 10 mg (DL -3). The majority of modifications of lenalidomide dosing were due to hematologic toxicities (24 cycles held; 31 dose reductions). Modifications to gemcitabine dosing included 70 doses withheld among 37 patients (37 doses held due to hematologic toxicity) and 61 dose reductions among 33 patients (51 reductions due to hematologic toxicities), with 13 patients requiring reduction to DL -2 (Fig. 1).
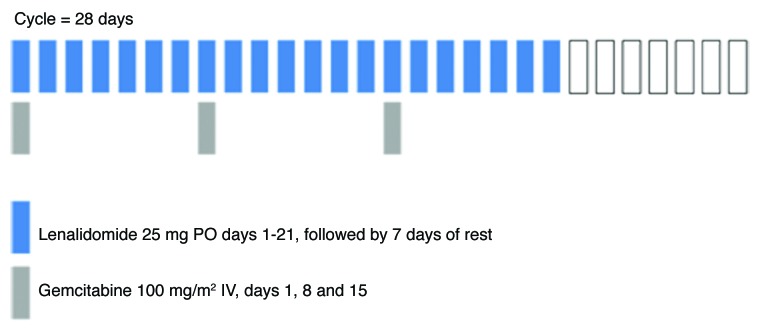
Figure 1. Treatment Schema
Efficacy
Eight patients achieved partial response (11%), with stable disease in an additional 26 patients (36%). Of the 26 patients with stable disease, 19 had measurable decreases in tumor size; however, only 28 patients (39%) remained on study for ≥4 cycles. Twenty-two patients (31%) had documented disease progression at the first evaluation. The remaining 16 patients did not complete 8 weeks of treatment and were unevaluable for response as previously described.
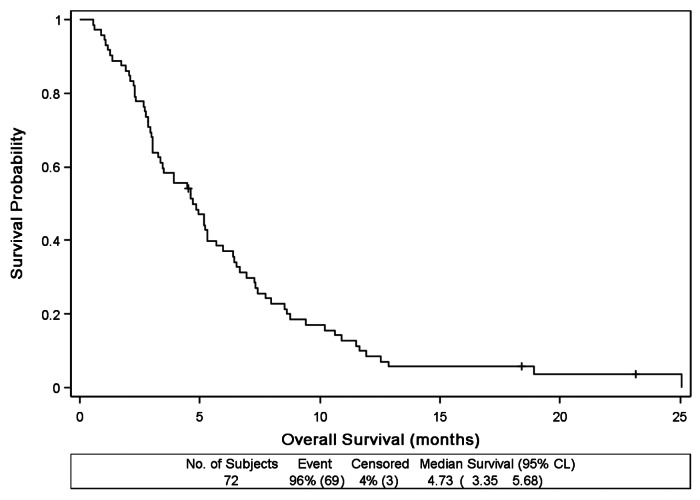
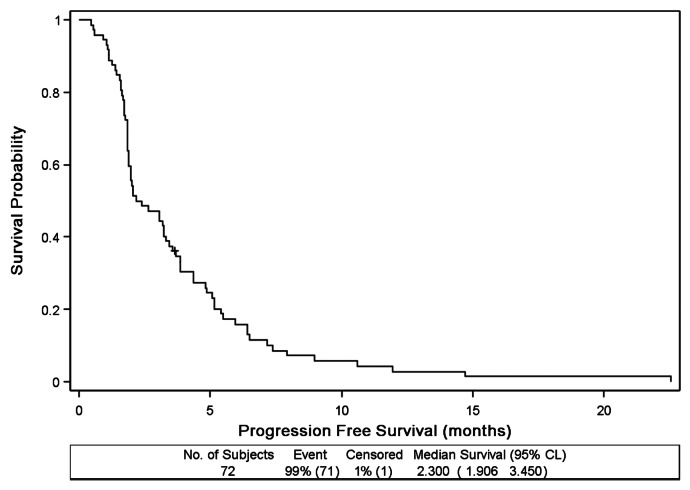
Progression-free survival is shown in Figure 2. The median PFS was 2.3 mo (95% CI: 1.9–3.5 mo). Median overall survival was 4.7 mo (95% CI: 3.4–5.7 mo) and is shown in Figure 3. At six months, overall survival probability was 37% (95% CI: 26–48%).
Figure 2. Progression-Free Survival (n = 72)
Figure 3. Overall Survival (n = 72)
Toxicity
Toxicities reported in >5% of patients are detailed in Table 2. Myelosuppression was common with thrombocytopenia or anemia reported in 54% and 49% (all grades), respectively. Although neutropenia was also fairly common (35% all grades), there was no febrile neutropenia reported. Among non-hematologic toxicities, fatigue (49%) and nausea (33%) were the most frequent, but primarily grade 1 or 2 (42% and 32%, respectively). Twenty patients (28%) experienced 24 venous thromboembolic events (VTE) of grade ≥2, yet this did not result in any treatment discontinuation. Fifty-six percent of these events occurred during the first eight weeks. Sixteen patients (22%) had unilateral deep vein thromboses (DVTs) of the extremities, three patients (4%) experienced DVT and pulmonary embolism and one patient (1%) developed bilateral DVTs. Two of these patients were not receiving protocol-specified anticoagulation at the initiation of treatment with lenalidomide and gemcitabine. Of the 17 treatment-related hospitalizations, four were due to VTEs.
Table 2. Toxicities Reported in > 5% of Patients, All Grades (n = 72), Number of Patients (%).
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total |
|---|---|---|---|---|---|
|
Hematologic | |||||
| Thrombocytopenia |
10 (14%) |
14 (19%) |
13 (18%) |
2 (3%) |
39 (54%) |
| Anemia |
9 (13%) |
21 (29%) |
4 (6%) |
1 (1%) |
35 (49%) |
| Neutropenia |
3 (4%) |
8 (11%) |
12 (17%) |
2 (3%) |
25 (35%) |
| Leukopenia |
4 (6%) |
8 (11%) |
8 (11%) |
1 (1%) |
21 (29%) |
|
Non-hematologic | |||||
| Fatigue |
11 (15%) |
19 (26%) |
5 (7%) |
0 |
35 (49%) |
| Nausea |
11 (15%) |
12 (17%) |
1 (1%) |
0 |
24 (33%) |
| Venous thromboembolism |
0 |
5 (7%) |
13 (18%) |
2 (3%) |
20 (28%) |
| Anorexia |
4 (6%) |
11 (15%) |
0 |
0 |
15 (21%) |
| Constipation |
5 (7%) |
9 (13%) |
0 |
0 |
14 (19%) |
| Rash/desquamation |
7 (10%) |
4 (6%) |
3 (4%) |
0 |
14 (19%) |
| Diarrhea |
8 (11%) |
3 (4%) |
2 (3%) |
0 |
13 (18%) |
| Vomiting |
3 (4%) |
6 (8%) |
1 (1%) |
0 |
12 (17%) |
| Dehydration |
5 (7%) |
3 (4%) 0 |
8 (11%) |
|
|
| ALT, SGPT (increased) |
3 (4%) |
2 (3%) |
2 (3%) |
0 |
7 (10%) |
| AST, SGOT (increased) |
2 (3%) |
3 (4%) |
1 (1%) |
0 |
6 (8%) |
| Dizziness |
5 (7%) |
1 (1%) |
0 |
0 |
6 (8%) |
| Chills |
4 (6%) |
1 (1%) |
0 |
0 |
5 (7%) |
| Edema, lower limbs |
1 (1%) |
4 (6%) |
0 |
0 |
5 (7%) |
| Confusion |
1 (1%) |
2 (3%) |
1 (1%) |
0 |
4 (6%) |
| Treatment-related hospitalizations |
17 |
||||
| Treatment-related deaths | 0 | ||||
Of the 13 remaining treatment-related hospitalizations, one was for myelosuppression (pancytopenia) and 12 were for various non-hematologic toxicities (weakness, two patients; dehydration, two patients; sepsis, two patients; one patient each: hypokalemia, failure to thrive, rectal hemorrhage, infection with normal ANC, nausea and atrial fibrillation). There were no treatment-related deaths.
Discussion
In this phase II trial, the addition of lenalidomide to gemcitabine did not improve survival over historical data as compared with gemcitabine alone in patients with untreated advanced carcinoma of the pancreas. Although 8 patients (11%) achieved objective partial responses and minor radiographic improvements were noted in additional patients, disease control was not durable. Unfortunately, these findings are consistent with those of other attempts to improve the efficacy of therapy by adding agents to gemcitabine. Since Burris et al.6 demonstrated that treatment with gemcitabine resulted in a median survival superior to fluorouracil, several cytotoxic and targeted agents have been studied in combination with gemcitabine with limited to no clinical benefit.7
This was the third clinical trial of an immunomodulatory compound like lenalidomide in combination with gemcitabine. The first was a phase II study of gemcitabine and thalidomide in 27 patients with metastatic pancreas cancer, which reported a median survival of approximately six months with partial responses or stable disease in 76% of the evaluable patients.17 Neutropenia was the most commonly reported severe hematologic toxicity reported here (11 of 27 patients), while only one patient experienced severe thrombocytopenia. The second trial was a phase I dose escalation study of gemcitabine and pomalidomide.16 In that study, both drugs were able to be given at full monotherapy doses in combination and three (15%) partial responses were observed among the 23 patients treated. Again, neutropenia was the DLT and most common grade 3/4 toxicity (38%). Interestingly, 22% of patients experienced grade 3/4 VTEs with the combination of gemcitabine and pomalidomide.
In the current trial, myelosuppression was also frequent; however, the incidence of severe neutropenia was decreased, while the rate of thrombocytopenia was increased with lenalidomide compared with thalidomide and pomalidomide.16,17 Additionally, more myelosuppression-related dose modifications were required in the present study, which limited consistent delivery of full doses of both drugs. The myelosuppression observed with this combination is likely attributed to overlapping toxicity, as each drug causes bone marrow suppression on its own. This study is limited by the absence of pharmacokinetics, as we are unable to confirm whether the exposures of the drugs change when given in combination. Our study suggests there is a lack of synergy when lenalidomide is given in combination with gemcitabine; however patients were unable to be maintained on full monotherapy doses for sustained period of time.
Despite protocol eligibility requirements for prophylactic anticoagulation, 20 (38%) of 72 patients experienced VTEs. Approximately 80% of the study patients were using aspirin as their required prophylactic anticoagulant. Two patients had a prior history of DVT, but were receiving stable doses of warfarin prior to the initiation of protocol therapy. No patients discontinued protocol therapy due to VTEs, and the majority of them received at least one cycle of treatment following the event onset. As compared with other tumor types, patients with pancreatic cancer have an increased risk for developing VTEs.18,19 Therapy with thalidomide, pomalidomide and lenalidomide16-18 has also been associated with the development of VTEs. Though this is a non-randomized, uncontrolled trial, the rate of observed VTEs in this trial is concerning and may limit development of these compounds in this patient population unless full-dose LMWH or warfarin is used prophylactically.
The results of this trial are disappointing and highlight the difficulty in improving outcomes for patients with metastatic pancreatic cancer. It was anticipated that the combination immunomodulatory and anti-angiogenesis activity seen with lenalidomide may differentiate it from the previously studied anti-VEGF therapies that have been unsuccessfully tested in combination with gemcitabine.7 The inability to consistently deliver both drugs in full doses and the high rate of VTEs may have contributed to the lack of efficacy observed in this clinical trial. Further development of this regimen for patients with advanced pancreas cancer is not recommended. New treatments for advanced pancreas cancer remain an unmet need.
Patient and Methods
This multicenter phase II study was initiated in May 2009 and was performed across 11 sites of the Sarah Cannon Oncology Research Consortium (Appendix). Prior to enrolling patients, the trial was approved by the institutional review boards of each site. The study was conducted in accordance with the Declaration of Helsinki. Prior to the initiation of treatment, all patients provided written informed consent.
Eligibility
To be eligible to enter this study, adult patients were required to have histologically or cytologically confirmed metastatic adenocarcinoma of the pancreas not amenable to curative surgery or definitive radiation. Patients were required to have measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1,15 and an Eastern Cooperative Oncology Group (ECOG) performance status ≤2 prior to starting treatment. Prophylactic anticoagulation with aspirin 81 or 325mg was required; warfarin or low molecular weight heparin was allowed in patients intolerant of aspirin. Additional inclusion criteria included: adequate bone marrow function (defined as absolute neutrophil count (ANC) ≥1,500/mm3 and platelet count ≥100,000/mm3); adequate renal and hepatic function (defined as serum creatinine ≤2.5 mg/dL, total bilirubin ≤2.0 mg/dL, AST/SGOT and ALT/SGPT ≤3.0× the institutional upper limit of normal (ULN) or ≤5× ULN for patients with hepatic metastases); registration into the RevAssist® program; negative serum or urine pregnancy test in women of child-bearing potential; agreement to use adequate contraceptive measures while on treatment; ability to swallow intact capsules.
Patients with prior systemic therapy for adenocarcinoma of the pancreas (except 5-fluorouracil or gemcitabine as a radiosensitizer in the adjuvant setting), known brain or leptomeningeal metastases, surgery or palliative radiation within 14 days of study entry or known chronic human immunodeficiency virus (HIV), hepatitis B and/or hepatitis C infections were excluded. Patients with history of or active venous thromboembolic events (VTE) were excluded unless therapeutically managed with stable dose anticoagulation. Additional exclusion criteria included: neuropathy grade ≥2; New York Heart Association classification III or IV; other cancers unless the patient has been free of disease for ≥1 year.
Pretreatment evaluation
Before beginning treatment, all patients underwent a complete medical history and physical examination, complete blood count (including differential and platelet counts), comprehensive metabolic profile (including AST/SGOT, ALT/SGPT, total bilirubin, total protein and albumin), CA 19–9, thyroid stimulating hormone (TSH) levels, electrocardiogram (ECG), serum or urine pregnancy test (in women of child-bearing potential) and assessment of ECOG performance status. Radiologic evaluation included CT (CT) scan or magnetic resonance imaging (MRI) of the chest/abdomen and pelvis, CT or MRI of the brain (if symptomatic) and bone scan in patients with prior positive bone scans or symptoms suggestive of bone metastases.
Treatment
The study began with a lead-in portion to confirm the tolerability of lenalidomide (25mg PO days 1–21) in combination with gemcitabine (1000mg/m2 IV days 1, 8 and 15). Six patients were treated in the lead-in portion; if more than 1 of these patients experienced a dose-limiting toxicity (DLT), the dose of lenalidomide would be reduced to 20 mg PO daily and six additional patients would be treated. DLTs were defined as: grade ≥3 non-hematologic drug-related toxicity (excluding alopecia, nausea and vomiting); febrile neutropenia; grade 4 neutropenia > 7 d’ duration; grade 4 thrombocytopenia; inability to complete cycle 1 or start cycle 2 within 7 d of scheduled date due to drug-related toxicity. Use of granulocyte colony-stimulating factors (G-CSFs) was prohibited during the lead-in phase, but was allowed at the discretion of the treating investigators or per American Society for Clinical Oncology (ASCO) guidelines during the remainder of the study. Toxicities were graded according to the National Cancer Institute (NCI) Common Toxicity Criteria for Adverse Events (CTCAE) version 3.0.
After completion of the lead-in phase, all subsequent patients received lenalidomide 25 mg PO on days 1–21 and gemcitabine 1000 mg/m2 IV days 1, 8 and 15 of 28-d treatment cycles. Patients were instructed to take lenalidomide at approximately the same time each morning. Patients were permitted to continue treatment until disease progression or intolerable toxicity occurred.
During treatment, patients were seen and examined every 28 d. On day 1 of each cycle, patients underwent interval medical history and physical examination, assessment of ECOG performance status, calculation of body-surface area (BSA) for gemcitabine dosing, evaluation of adverse events and concomitant medications and serum or urine pregnancy tests, in women of child-bearing potential. Complete blood counts were repeated days 1, 8,and 15 of each cycle; comprehensive metabolic profiles were repeated on days 1 and 15.
At the completion of 2 cycles (8 weeks) of protocol therapy and every 8 weeks thereafter, disease assessments were performed with repeat measurements of CA 19–9 and CT scans/MRI of the chest and abdomen for tumor measurements. TSH levels were repeated every 16 weeks (4 cycles). After patients discontinued treatment, they were followed for survival status and initiation of other anti-cancer therapies every 3 mo until 80% of patients were deceased.
Dose modifications
Dose modifications of lenalidomide and gemcitabine were mandated in patients who developed hematologic or non-hematologic toxicities related to treatment. Three dose reductions of lenalidomide (dose level -1, 20 mg PO days 1–21; dose level -2, 15 mg PO days 1–21; dose level -3, 10 mg PO days 1–21) and two reductions of gemcitabine (dose level -1, 750 mg/m2 IV days 1, 8 and 15; dose level -2, 500 mg/m2 IV days 1, 8 and 15) were allowed.
Dose modifications for hematologic toxicities were based on day 1, 8 and 15 complete blood counts. Complete dose modification recommendations were outlined in the study protocol. Doses of both agents were held for platelets < 50,000/mm3 or ANC < 750/mm3. If the platelet count was 50,000–75,000/mm3 or the ANC was 750–1,000/mm3, doses of both agents were reduced by 1 dose level. Neutropenic fever on any day of the cycle warranted withholding doses of both drugs; patients received a 1-level dose reduction upon ANC recovery to ≥1,000/mm3.
Doses were also modified based on non-hematologic toxicities. Patients who experienced grade 3 non-blistering rash or neuropathy had doses of both drugs held until recovery to grade < 2 and then resumed treatment at a 1-level dose reduction. Patients with grade 4 non-blistering rash or neuropathy discontinued lenalidomide treatment and held gemcitabine until the toxicity resolved. Any grade of venous thromboembolic event warranted held doses of both agents to initiate full anticoagulation; treatment could be resumed at the treating investigator’s discretion. For other non-hematologic toxicities of grade 3 or greater thought to be related to lenalidomide or gemcitabine, the offending agent was held until recovery to grade ≤2 and then resumed at a 1-level dose reduction.
Definition of response
Patients were evaluated for response to treatment after completing two cycles (eight weeks) of therapy. Response categories were assigned using RECIST version 1.1.14 Patients without evidence of disease progression or intolerable toxicity continued treatment with evaluations every eight weeks. The final response category assigned to the patients represents the best response achieved.
Statistical considerations
The primary efficacy endpoint of this study was the six-month survival rate. Gemcitabine alone produces an approximate six-month survival rate of 50% for patients with metastatic adenocarcinoma of the pancreas.6 An improvement to 65% survival at six months would warrant further testing of the combination of lenalidomide and gemcitabine in this group of patients. Treatment of 70 patients was needed to demonstrate an improvement in six-month survival to 65%, with 82% power and α of 0.05. All patients who received at least one dose of treatment were included in analyses of safety and efficacy.
Progression-free survival was calculated as the interval between study entry and the first observation of disease progression or death due to any cause. Overall survival was measured as the interval between study entry and death from any cause. Survival calculations included all patients who initiated treatment.
Appendix.
| Sarah Cannon Oncology Research Consortium | Participating Sites |
|---|---|
| Tennessee Oncology |
PLLC Nashville, TN |
| Florida Cancer Specialists |
Fort Myers, FL |
| Chattanooga Oncology Hematology Associates |
Chattanooga, TN |
| Watson Clinic for Cancer Research |
Lakeland, FL |
| South Carolina Oncology Associates |
Columbia, SC |
| Virginia Cancer Institute |
Richmond, VA |
| Oncology Hematology Care, Inc. |
Cincinnati, OH |
| Center for Cancer and Blood Disorders |
Bethesda, MD |
| Northeast Georgia Medical Center |
Gainesville, GA |
| Sletten Cancer Specialists |
Great Falls, MT |
| Norton Cancer Institute | Louisville, KY |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/23625
References
- 1.Freelove R, Walling AD. Pancreatic cancer: diagnosis and management. Am Fam Physician. 2006;73:485–92. [PubMed] [Google Scholar]
- 2.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68–72, discussion 72-3. doi: 10.1016/S0002-9610(05)80406-4. [DOI] [PubMed] [Google Scholar]
- 3.Regine W, Winter K, Abrams R. RTOG 9704 a phase III study of adjuvant pre and post chemoradiation (CRT) 5-FU vs. gemcitabine (G) for resected pancreatic adenocarcinoma. ASCO Meeting Abstracts 2006;24:4007. [Google Scholar]
- 4.Ries L, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2003. 2006.
- 5.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–79. doi: 10.1016/S1091-255X(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 6.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 7.Tabernero J, Macarulla T. Changing the paradigm in conducting randomized clinical studies in advanced pancreatic cancer: an opportunity for better clinical development. J Clin Oncol. 2009;27:5487–91. doi: 10.1200/JCO.2009.23.3098. [DOI] [PubMed] [Google Scholar]
- 8.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. National Cancer Institute of Canada Clinical Trials Group Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 9.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–5. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 10.Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–91. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 11.Saif MW. Anti-angiogenesis therapy in pancreatic carcinoma. JOP. 2006;7:163–73. [PubMed] [Google Scholar]
- 12.Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303) J Clin Oncol. 2010;28:3617–22. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kindler HL, Ioka T, Richel DJ, Bennouna J, Létourneau R, Okusaka T, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256–62. doi: 10.1016/S1470-2045(11)70004-3. [DOI] [PubMed] [Google Scholar]
- 14.Fryer RA, Barlett B, Galustian C, Dalgleish AG. Mechanisms underlying gemcitabine resistance in pancreatic cancer and sensitisation by the iMiD™ lenalidomide. Anticancer Res. 2011;31:3747–56. [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Infante JR, Jones SF, Bendell JC, Spigel DR, Yardley DA, Weekes CD, et al. A phase I, dose-escalation study of pomalidomide (CC-4047) in combination with gemcitabine in metastatic pancreas cancer. Eur J Cancer. 2011;47:199–205. doi: 10.1016/j.ejca.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Maples WJ, Stevenson J, Sumrall SV, et al. Advanced pancreatic cancer: a multi-institutional trial with gemcitabine and thalidomide. J Clin Oncol 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition): 22; 14s; 4082. [Google Scholar]
- 18.Shah MM, Saif MW. Pancreatic cancer and thrombosis. Highlights from the “2010 ASCO Annual Meeting”. Chicago, IL, USA. June 4-8, 2010. JOP. 2010;11:331–3. [PubMed] [Google Scholar]
- 19.Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. Lancet Oncol. 2004;5:655–63. doi: 10.1016/S1470-2045(04)01606-7. [DOI] [PubMed] [Google Scholar]