Abstract
The pore-forming subunit of voltage-gated calcium channels is associated to auxiliary subunits among which the cytoplasmic β subunit. The different isoforms of this subunit control both the plasma membrane targeting and the biophysical properties of the channel moiety. In a recent study, we demonstrated that the Cacnb4 (β4) isoform is at the center of a new signaling pathway that connects neuronal excitability and gene transcription. This mechanism relies on nuclear targeting of β4 triggered by neuronal electrical stimulation. This re-localization of β4 is promoted by its interaction with Ppp2r5d a regulatory subunit of PP2A in complex with PP2A itself. The formation, as well as the nuclear translocation, of the β4/ Ppp2r5d/ PP2A complex is totally impaired by the premature R482X stops mutation of β4 that has been previously associated with juvenile epilepsy. Taking as a case study the tyrosine hydroxylase gene that is strongly upregulated in brain of lethargic mice, deficient for β4 expression, we deciphered the molecular steps presiding to this signaling pathway. Here we show that expression of wild-type β4 in HEK293 cells results in the regulation of several genes, while expression of the mutated β4 (β1–481) produces a different set of gene regulation. Several genes regulated by β4 in HEK293 cells were also regulated upon neuronal differentiation of NG108-15 cells that induces nuclear translocation of β4 suggesting a link between β4 nuclear targeting and gene regulation.
Keywords: HP1γ, gene transcription regulation, phosphatase 2A, thyroid receptor alpha, tyrosine hydroxylase, voltage-gated calcium channel, β4 subunit
Calcium entering neurons in response to the spontaneous or electrically-evoked activation of voltage-gated calcium channels (VGCC) is the first step that switches on a number of intracellular pathways leading to specific physiological responses.1 Several cellular events that control neurons development, such as cell migration or axon path-finding, and neuronal functions, such as transmitter release, have been shown to rely on the activation of VGCC.2 Calcium involvement in such pathways was originally associated to the direct control of the activity of several enzymes, ATPases, kinases or phosphatases, and/or calcium binding proteins, allowing a rapid response of the cell to electrical stimulation. More recently, results have accumulated that demonstrate the link between neuronal activity and regulation of gene expression.3 In this context, the exact role of the VGCC has been intensively investigated.4 Three different signaling pathways linking VGCC and gene expression can be described.5 The two firsts have their source in the entry of calcium through VGCC and lead either to the activation of cytoplasmic calcium binding proteins that propagate the information to the nucleus and modify gene transcription machinery, or to the direct diffusion of calcium to the nucleus where it interacts with proteins involved in gene transcription. In striking contrast to these two pathways, the third route is based on a totally new aspect of VGCC function. Indeed, it was demonstrated that domains of the VGCC, either a fragment of the pore-forming α1 subunit6 or a short isoform of the cytoplasmic β4 subunit,7 β4C, relocate under certain circumstances in the nucleus and directly participate to gene regulation. The VGCC fragment corresponding to the C-terminus domain of α1 plays the role of transcription factor.
Recently, we characterized an entirely new signaling pathway linking the activation of neuronal VGCC and gene transcription.8 We highlighted the presence of the cytoplasmic Cacnb4 (β4) auxiliary subunit of VGCC within the nucleus of differentiated neurons. Nuclear translocation of β4 increases along neurons differentiation and relies on the neuronal electrical activity as evidenced by the strong inhibition of this translocation under TTX treatment. Using a combination of methods, including yeast two-hybrid screening, immunoprecipitation, sub-cellular fractionation and confocal imaging approaches, we identified the protein phosphatase 2A Ppp2r5d (B56δ) subunit as the cytoplasmic β4 partner that allows β4 nuclear targeting. An important finding was that a human mutation within CACNB4, the β4 encoding gene, which has been associated to juvenile myoclonic epilepsy,9 prevents both the nuclear translocation of β4 and its association with B56δ. This R482X stop mutation leads to the truncation of 38 amino acids residues within the C-terminus of β4, producing the β1–481 variant. Our search for the identification of molecular determinants of β4 that govern its sub-cellular localization highlighted the imperious necessity for the preservation of β4 structural integrity. In particular, our data demonstrated that an intact interaction between SH3 and GK domains of β4 is required for β4 nuclear targeting. Point mutations of one or the other of these two domains (SH3 L125P or GK P225R), known to preclude their interaction,10,11 strongly impair β4/B56δ interaction and β4 nuclear localization. These results point out the previously unrecognized role of the C-terminus domain, whose structure is still unknown, in modulating the global folding of β4 and as a consequence its functionality. Similarly, the human mutation R482X results in a change in β4 structural conformation that precludes the SH3/GK interaction and the association to B56δ. As a result, this mutated form of β4 hardly travels to the nucleus. In addition to B56δ, β4 was also found in complex with the catalytic subunit of PP2A. The existence of the β4/B56δ/PP2A complex in adult mice brain was demonstrated by its precipitation using antibodies directed against either β4 or B56δ. Similar experiments using brain obtained from lethargic mice (lh mice), defective for β4 expression,12 or B56δ−/− mice13 did not allow immunoprecipitation of the β4/B56δ/PP2A complex. Again the β4 human mutation, by preventing the association with B56δ, also prevents the interaction with PP2A.
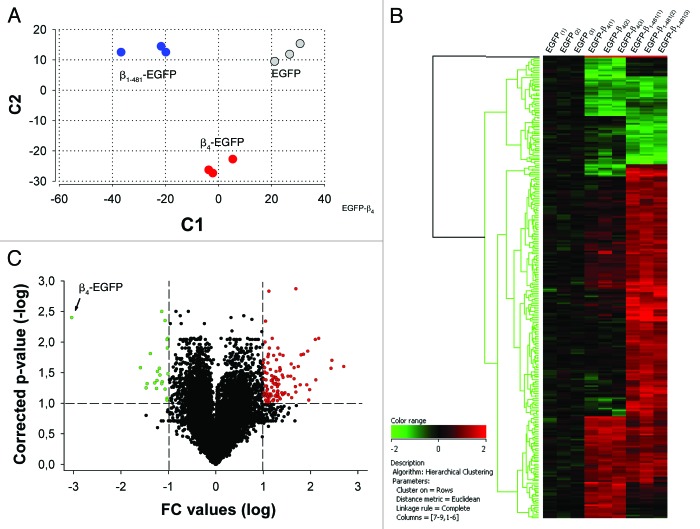
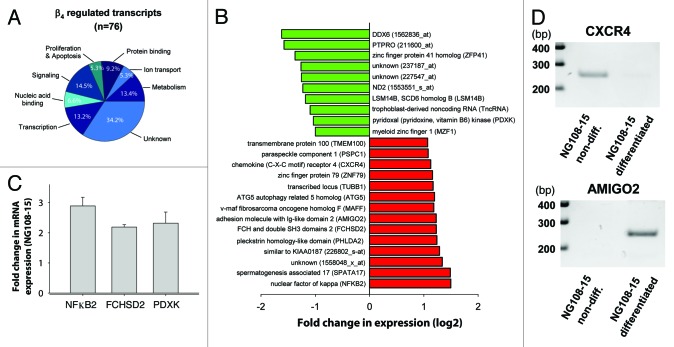
In order to reveal general aspects of β4 in transcriptional regulation, we analyze here the modification of gene expression profile of HEK293 cells expressing either β4-EGFP, β1–481-EGFP (carrying the human R482X mutation) or EGFP alone (Fig. 1). Total RNA were analyzed with Affymetrix GeneChip Human Genome U133 Plus 2.0 Arrays. The data gathered from total RNA of EGFP-transfected cells were used as reference. It was obvious that distinct expression patterns were generated from the expression of EGFP, β4-EGFP or β1–481-EGFP (Fig. 1A). This was further exemplified by hierarchical clustering display using 268 significantly regulated probe sets in β4-EGFP and β1–481-EGFP compared with EGFP control (Fig. 1B). There were obvious gene clusters that indicate that the 38 amino acids deletion of β4 leads to a differential expression profiles. A volcano plot representing the distribution of all 55,000 probe sets according to fold-change and p-value for statistical comparison between β4-EGFP and EGFP, β1–481-EGFP and EGFP, β1–481-EGFP and β4-EGFP indicates that the number of genes regulated by the expression of β4-EGFP and β1–481-EGFP were in fact quite restricted (Fig. 1C). Taken alone, the expression of β4-EGFP results in up- and downregulation of 49 and 27 genes respectively. Based on gene ontology annotations, clustering of regulated transcripts in functional groups illustrates that many encode proteins with functions related to transcription and signaling (Fig. 2A). Among the genes modified by β4-EGFP expression, 52% are no longer regulated by β1–481-EGFP expression, some of which, the most representatives, are shown in Figure 2B. These results demonstrate that overexpression of β4 modifies the expression of a set of genes among which a large numbers are differently regulated when β4 is replaced by the variant carrying the premature stop mutation associated to juvenile epilepsy. To assess the relevance of these genes regulation with regard to nuclear translocation of β4 we used the hybrid mouse neuroblastoma × rat glioma NG108–15 cell line. In these cells, as in cultured neurons, nuclear translocation of β4 coincides with differentiation.8 Primers recognizing both rat and mouse sequences could only be designed for eight transcripts which allowed us to test their expression in NG108–15 cells. qRT-PCR experiments show that NFκB2, FCHSD2 and PDXK expression levels were significantly increased by NG108–15 differentiation (Fig. 2C). Two others, which could not be quantified by the Light Cycler, were either downregulated (CXCR4) or upregulated (AMIGO2) as shown on gel electrophoresis (Fig. 2D). Three other transcripts (DDX6, MZF1 and PSPC1) showed no change (data not shown). For PDXK and CXCR4, the direction of the changes in expression levels in differentiated NG108–15 cells was opposite to the changes observed in HEK293 cells. The fact that a gene may be differently regulated by expression of β4 depending on the cell lines likely reflects the versatility of the protein composition of the β4 platform in these cell lines. Indeed, β4 acting through the recruitment of transcription factors, we surmise that these differences reflect the recruitment of different transcription factors in both cell systems. Although these results show that β4 dependent regulation of several genes coincides with β4 nuclear targeting, it is clear that other neuronal differentiation factors may also be involved in change of gene expression during differentiation. Interestingly, many genes of this group are directly related to essential neuronal functions. Indeed, NFκB2 is a transcription factor involved in neuronal plasticity and is activated in several regions of brain during neurogenesis.14,15 NFκB2 has been linked to neurodegenerative disorders.16 Similarly CXCR4 is a G protein-coupled receptor for chemokines that are essential attractants during brain development. Abnormal cerebellar and hippocampal dentate gyrus development were observed in the absence of CXCR4.17,18 Finally, pyridoxal kinase (PDXK) is an enzyme that converts vitamin B6 derivates in pyridoxal phosphate, a cofactor in the synthesis of various neurotransmitters. Decrease brain level of this cofactor has been reported to cause epilepsy.19 However, the role of β4 in the regulation of these genes in brain remains to be established.
Figure 1.
Differential transcriptional activity evoked by expression of β4-EGFP or β1–481-EGFP. (A) Principal component analysis of global expression profiles from HEK293 transfected cells reveals distinct expression patterns in cells expressing wild-type β4-EGFP, β1–481-EGFP mutant and EGFP control using two parameters. The high quality of microarray hybridizations was determined using independent internal standards, the housekeeping β-actin and GADPH mRNAs. (B) Hierarchical clustering using 268 significantly regulated probe sets in β4-EGFP and β1–481-EGFP compared with EGFP control shows gene clusters with differential expression profiles linked to the 38 amino acids deletion. (C) Volcano Plot representing the distribution of all 55,000 probe sets according to fold-change and p-value from statistical comparison between β4-EGFP and EGFP, β1–481-EGFP and EGFP, β1–481-EGFP and β4-EGFP expressed in HEK293 cells. Probe sets with adjusted p-value < 0.1 and fold-change > 2 are in red while probe sets with fold-change < - 2 are in green.
Figure 2.
Transcriptional activity induced by the expression of β4-EGFP. (A) Pie charts showing functional classification of regulated probe sets by β4-EGFP expression in HEK293 cells. Functional groups have been established using annotations from the Gene Ontology Consortium (http://www.geneontology.org/). The data gathered from total RNA of EGFP-transfected cells were used as a reference. (B) Histogram showing the genes upregulated (in red) and downregulated (in green) with a fold-change > 2 detected on probe sets from wild-type β4-EGFP but absent on probe sets from β1–481-EGFP. (C) RT-PCR experiments showing upregulation of NFkB2, FCHSD2 and PDXK upon neuronal differentiation of NG108–15 cells. (D) Agarose gel electrophoresis data illustrating the disappearance of the CXCR4 transcript and appearance of AMIGO2 transcript upon NG108–15 differentiation.
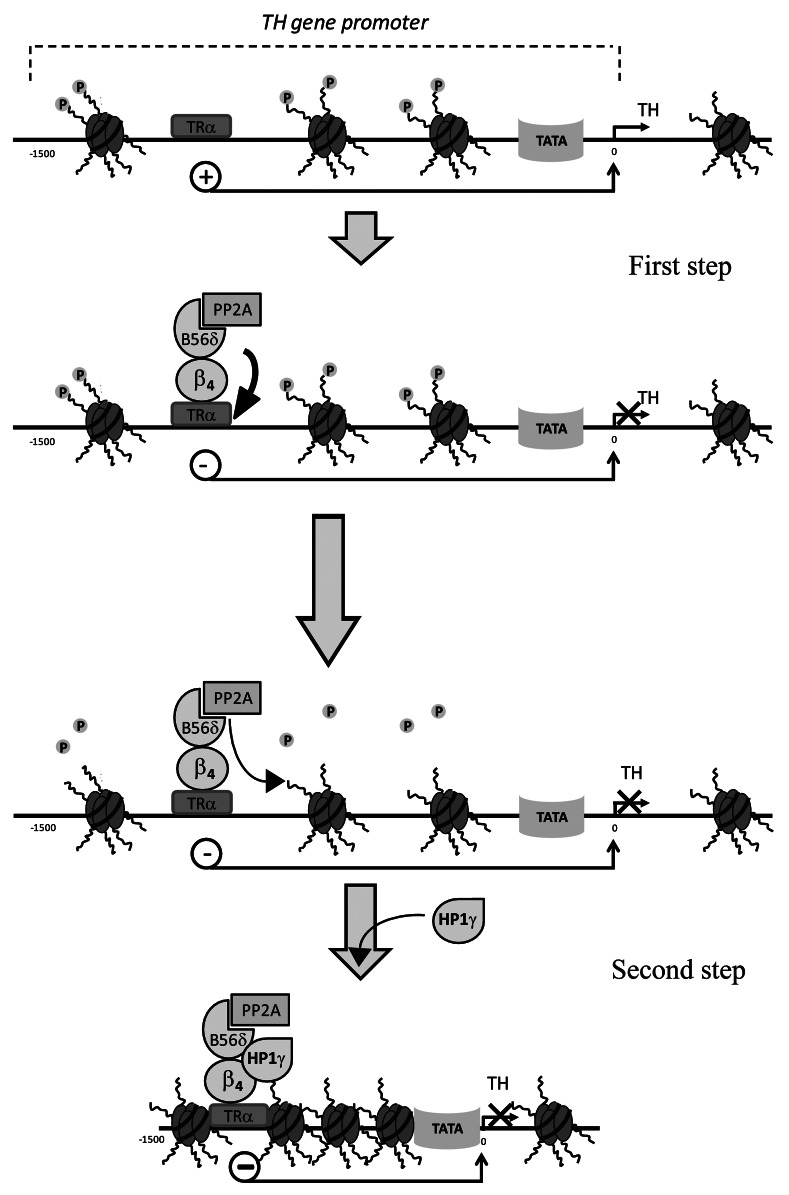
From the analysis of publicly available microarray data set for gene expression in lh vs. wt mice cerebellum we identified 94 genes whose expression is significantly modified in lh vs. wt mice.8 The gene encoding tyrosine hydroxylase (TH) shows the strongest upregulation in lh mice brain, indicating that β4 acts as a strong repressor of TH expression. The interaction of β4 with TH gene promoter region was confirmed by chromatin immunoprecipitation of this gene, using antibodies directed against β4. No immune precipitation of TH gene was observed in the same conditions when using brain extract obtained from lh mice. By using yeast two hybrid screening we also identified various transcription factors that may interact with β4 (data not shown). One of these transcription factors is the thyroid hormone nuclear receptor α (TRα). We thus demonstrated that targeting of the β4/B56δ/PP2A complex to TH promoter via TRα initiates the recruitment of several proteins on the nuclear β4 platform. This nuclear macromolecular complex permits two levels of regulation of TH gene transcription that are schematized on Figure 3. The first level of repression corresponds to the effect of β4 on TRα itself. Using luciferase as a reporter of TH promoter activity, we showed that, in the presence of β4, TRα behaves as a potent inhibitor of TH promoter. The second level of repression induced by β4 relies on two major properties of the β4/B56δ/PP2A complex. First, owing to the phosphatase activity carried by PP2A, the immunoprecipitated β4/B56δ/PP2A complex is able to dephosphorylate a peptide corresponding to the N-terminus domain of the histone H3 carrying a phosphorylation on Ser10. Second, the β4/B56δ complex recruits the heterochromatin protein 1 gamma (HP1γ). HP1γ is a nuclear protein involved in gene silencing and transcription regulation. Previous studies had evidenced the interaction of HP1γ with a short splice variant of β4, but not with the full length β4, although the HP1γ binding motif is present on both proteins.7,20 Our results demonstrate that B56δ is a key protein to control the fate of β4 and its involvement in this gene regulation signaling pathway. We thus proposed that B56δ induces a structural modification of β4 that directs it to the nucleus and controls its ability to interact with essential nuclear protein players. Again, as a consequence, our results demonstrate that the human epileptic mutation of β4, by precluding B56δ/β4 interaction, strongly modifies β4 fate. HP1γ was previously shown to preferentially associated with histone H3 carrying a dephosphorylated Ser10. This result lends support to the hypothesis that β4 by targeting PP2A phosphatase activity to specific nucleosomes favors HP1γ association to them and the long-term silencing of the associated gene possibly by promoting chromatin remodeling. Association of β4/B56δ/PP2A to the nucleosome was demonstrated by co-immunoprecipitation of histones H3, H4 and H2B using antibodies β4 in brain extract as well as in HEK293 cells expressing β4 and B56δ. Interestingly, these two levels of regulation by β4 might intervene to precisely fine-tune the time scale of gene silencing. The direct effect of β4 on TRα would likely allow short-term inhibition of TH transcription while the dephosphorylation of histone H3 together with the recruitment of HP1γ would lead to a long-term transcription arrest.
Figure 3.
Schematic illustration of the nuclear steps of TH gene regulation by the β4. First, the β4/B56δ/PP2A complex interacts with the transcription factor TRα that becomes inhibitor of the TH gene transcription. Second, β4/B56δ/PP2A complex promotes the dephosphorylation of the histone H3 Ser10 and recruits HP1γ. This second step may lead long-term inhibition of TH gene transcription possibly by chromatin remodeling.
Based on all these results, we propose that the β4 subunit of VGCC closely links neuronal activity to the gene transcription machinery. Under membrane depolarization β4 dissociates from the channel moiety and undertakes a new journey by associating to the B56δ regulatory subunit within the cytoplasm as well as to the associated catalytic subunit of PP2A. This complex thus migrates to the nucleus where it serves as a recruitment platform in order to modulate transcription of several genes. Therefore, the first event of this signaling pathway is represented by the dissociation of β4 from the VGCC. Dissociation of β subunit from the VGCC has previously been shown to occur in different situations, such as spontaneously,21 in growth cones of outgrowing neurons,22 under G protein regulation23 or in competition with other β subunit isoforms.24 It is however the first time that depolarization is demonstrated to trigger this dissociation. The main site of interaction of the β4 subunit with the VGCC has been located within the I-II cytoplasmic loop of the α1 subunit.25 However other interactions within the VGCC complex have been described, in particular between the C-terminus domain of β4 and the C-terminal domain of Cav2.1 (α1).26 It will be important to address the respective role of each of these sites in the β4 dissociation from α1 under depolarization. The β4 human mutation associated with juvenile myoclonic epilepsy, that only mildly affects calcium current carried by VGCC,9 induces the early disruption of the signaling pathway. This effect is due to the alteration of a structural modification of β4 required for interaction with B56δ. It is at this time unclear whether this β4 conformational change is triggered by the depolarization and concomitant to the dissociation of β4 from the VGCC or if it results from the association of β4 with other protein partners after dissociation from the VGCC. Here again, it will be important to study the effect of this mutation on β dissociation. This point is even more important to take into account since several known α1 mutations, found in association with important neuronal disorders,27 could possibly modify the parameters of β4 dissociation from the VGCC. As mentioned above, β4 mutations that prevent the interaction between the SH3 and GK domains also preclude the interaction of β4 with B56δ, an important step on the route of β4 to nucleus. Whether or not these mutations also affect the interaction of β4 with protein partners other than B56δ remain to be established. Curiously, electrical stimulation has also been reported to promote nuclear export of expressed β4 in neurons.28 Although the factors and mechanisms responsible for this nuclear export of β4 remain to be deciphered, these results suggest that the presence of β4 within the nucleus is strongly and precisely controlled by several factors.
Transcriptomic analysis of cells expressing β1–481 vs. wt β4 as well as of brains obtained from lh vs. wt mice strongly suggest that β4 is enrolled in a set of signaling routes possibly involving different protein partners and ending in the regulation of different genes. Indeed the absence of β4 in lh mice brain results in activation (56 genes) as well as inactivation (38 genes) of gene expression. Expression of the epileptic β1–481 mutant results in the change of expression of a number of genes although its nuclear localization is strongly disfavored. According to the signaling pathway that we described, DNA targeting of β4 is controlled by its association with a transcription factor. It is likely that β4 may interact with different transcription factors depending on the cell type and/or its association with other protein partners. Similarly, the effect of β4 on gene transcription strongly depends on the proteins it will recruit within the cytoplasm and/or the nucleus. Our yeast two hybrid screening of a mouse brain cDNA library using β4 as bait gave rise to 62 positive clones. Among these, several represent proteins involved in histone post transcriptional modification and/or in transcription regulation. Others might guide β4 to different routes also ending within the nucleus. Although our recent study focused on a signaling pathway that is abolished when β4 carries the human mutation associated to juvenile epilepsy, it is clear that other signaling pathway involving β4 exist that remain to be discovered. We also show that, among the different isoforms of β subunit, β3 is also able to interact with B56δ here again suggesting the existence of redundant pathways involving β subunits. We are currently analyzing these interactions in order to better identify the different complexes involving β4 as well as their sub-cellular localization.
In conclusion, our data demonstrate that, besides its role in VGCC targeting and regulation of their biophysical properties, β4 achieves a completely novel function as a nuclear protein. By this newly uncovered function, β4 has become a key player in a novel signaling pathway that directly links the neuronal excitability to gene transcription. We also demonstrate that an early step of this pathway is interrupted by a β4 mutation that has been associated with juvenile epilepsy. The interruption of this pathway in lh mice, deficient for β4, leads to a strong reactivation of TH gene transcription in some regions of the brain. This appends predominantly in the cerebellum where β4 expression normally dominates other β subunits. In brain regions, such as hippocampus, where β3 expression matches the expression level of β4, TH expression remains unchanged, suggesting the existence of redundant pathways. Interestingly, lh mice are considered as an animal model of absence epilepsy. It is therefore tempting to propose that disruption of this new signaling pathway may be in part responsible for the epileptic phenotype. Therefore, these results open new avenues of research not only on the mechanisms linking neuronal electrical activity and gene transcription but also on the molecular genetic events leading to epilepsy.
Materials and Methods
Microarray experiments and statistical analysis
Microarray experiments were performed commercially through PartnerChip (Evry-France), using the Affymetrix GeneChip® Human Genome U133 Plus 2.0 Arrays. Approximately, 5 µg of total RNA extracted from HEK293 cells, collected by fluorescence-activated cell sorting on the basis of EGFP tag fluorescence, were used for each array. Preparation of cRNA was performed according to protocols of the manufacturer (Affymetrix). Finally, 20 µg of cRNA were chemically fragmented in less than 200 nucleotides fragments before hybridization overnight on Affymetrix HG-U133 Plus 2.0 array. Washes and streptavidin-phycoerythrin (SAPE) staining procedures were performed using Affymetrix Fluidics Station 450 and arrays were finally scanned into Affymetrix GeneChip Scanner 3000. Hybridization quality check and data analysis were performed using Bioconductor packages (http://www.bioconductor.org). Preprocessing and normalization were assessed through GC-RMA algorithm. Principal component analysis (PCA) was used to visualize biological and technical variability between samples and student test was applied on data. To sort statistically significant regulated transcripts, 2 filter parameters were used: a regulation factor greater than 2 and statistical significance with a p-value under 0.1, after p value adjustment using Benjamini-Hochberg procedure29 to control false discovery rate. PCA and Volcano Plot graphs were generated using Bioconductor packages and hierarchical clustering analysis through ArrayAssist software (Stratagene).
NG108–15 cell differentiation
NG108.15 cells were differentiated two days after plating by decreasing fetal calf serum to 1% and addition of 1 mM dibutyryl cAMP.
Quantitative RT-PCR
Total RNAs were extracted using the RNeasy kit, supplemented with DNase I (Qiagen). cDNAs were synthesized from 1 µg total RNA using random hexamer primers (Promega) and Superscript II (Invitrogen). Primers, recognizing sequences from both mouse and rat genes of interest, were designed with the Primer 3 software (http://frodo.wi.mit.edu/). The cycling protocol was 10 min at 95°C followed by 40 cycles of 3 steps each (10 sec at 95°C, 5 sec at 60°C, and 10 sec at 72°C). The specificity of the amplification was checked by realizing a melting curve ranging from 65 to 95°C. Multiple normalization of gene expression30 was performed using 3 housekeeping genes (glyceraldehyde 3-phosphate dehydrogenase, hypoxanthine phosphoribosyl transferase 1, and peptidylprolyl isomerase A).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- VGCC
voltage-gated calcium channels
- PP2A
protein phosphatase 2A
- TRα
thyroid hormone receptor alpha
- TH
tyrosine hydroxylase
- HP1γ
heterochromatin protein 1 gamma
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/23895
References
- 1.Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–47. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- 2.Tam T, Mathews E, Snutch TP, Schafer WR. Voltage-gated calcium channels direct neuronal migration in Caenorhabditis elegans. Dev Biol. 2000;226:104–17. doi: 10.1006/dbio.2000.9854. [DOI] [PubMed] [Google Scholar]
- 3.Hardingham GE, Chawla S, Johnson CM, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature. 1997;385:260–5. doi: 10.1038/385260a0. [DOI] [PubMed] [Google Scholar]
- 4.Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–60. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Barbado M, Fablet K, Ronjat M, De Waard M. Gene regulation by voltage-dependent calcium channels. Biochim Biophys Acta. 2009;1793:1096–104. doi: 10.1016/j.bbamcr.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel Ca(V)1.2 encodes a transcription factor. Cell. 2006;127:591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hibino H, Pironkova R, Onwumere O, Rousset M, Charnet P, Hudspeth AJ, et al. Direct interaction with a nuclear protein and regulation of gene silencing by a variant of the Ca2+-channel beta 4 subunit. Proc Natl Acad Sci U S A. 2003;100:307–12. doi: 10.1073/pnas.0136791100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tadmouri A, Kiyonaka S, Barbado M, Rousset M, Fablet K, Sawamura S, et al. Cacnb4 directly couples electrical activity to gene expression, a process defective in juvenile epilepsy. EMBO J. 2012;31:3730–44. doi: 10.1038/emboj.2012.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escayg A, De Waard M, Lee DD, Bichet D, Wolf P, Mayer T, et al. Coding and noncoding variation of the human calcium-channel beta4-subunit gene CACNB4 in patients with idiopathic generalized epilepsy and episodic ataxia. Am J Hum Genet. 2000;66:1531–9. doi: 10.1086/302909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGee AW, Nunziato DA, Maltez JM, Prehoda KE, Pitt GS, Bredt DS. Calcium channel function regulated by the SH3-GK module in beta subunits. Neuron. 2004;42:89–99. doi: 10.1016/S0896-6273(04)00149-7. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi SX, Miriyala J, Tay LH, Yue DT, Colecraft HM. A CaVbeta SH3/guanylate kinase domain interaction regulates multiple properties of voltage-gated Ca2+ channels. J Gen Physiol. 2005;126:365–77. doi: 10.1085/jgp.200509354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burgess DL, Jones JM, Meisler MH, Noebels JL. Mutation of the Ca2+ channel beta subunit gene Cchb4 is associated with ataxia and seizures in the lethargic (lh) mouse. Cell. 1997;88:385–92. doi: 10.1016/S0092-8674(00)81877-2. [DOI] [PubMed] [Google Scholar]
- 13.Louis JV, Martens E, Borghgraef P, Lambrecht C, Sents W, Longin S, et al. Mice lacking phosphatase PP2A subunit PR61/B’delta (Ppp2r5d) develop spatially restricted tauopathy by deregulation of CDK5 and GSK3beta. Proc Natl Acad Sci U S A. 2011;108:6957–62. doi: 10.1073/pnas.1018777108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–60. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- 15.O’Neill LA, Kaltschmidt C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20:252–8. doi: 10.1016/S0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- 16.Lilienbaum A, Israël A. From calcium to NF-kappa B signaling pathways in neurons. Mol Cell Biol. 2003;23:2680–98. doi: 10.1128/MCB.23.8.2680-2698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci U S A. 2002;99:7090–5. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–53. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waymire KG, Mahuren JD, Jaje JM, Guilarte TR, Coburn SP, MacGregor GR. Mice lacking tissue non-specific alkaline phosphatase die from seizures due to defective metabolism of vitamin B-6. Nat Genet. 1995;11:45–51. doi: 10.1038/ng0995-45. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Lee YJ, Holm JB, Terry MD, Oswald RE, Horne WA. The Ca2+ channel beta4c subunit interacts with heterochromatin protein 1 via a PXVXL binding motif. J Biol Chem. 2011;286:9677–87. doi: 10.1074/jbc.M110.187864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Restituito S, Cens T, Rousset M, Charnet P. Ca(2+) channel inactivation heterogeneity reveals physiological unbinding of auxiliary beta subunits. Biophys J. 2001;81:89–96. doi: 10.1016/S0006-3495(01)75682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spafford JD, Van Minnen J, Larsen P, Smit AB, Syed NI, Zamponi GW. Uncoupling of calcium channel alpha1 and beta subunits in developing neurons. J Biol Chem. 2004;279:41157–67. doi: 10.1074/jbc.M403781200. [DOI] [PubMed] [Google Scholar]
- 23.Sandoz G, Lopez-Gonzalez I, Grunwald D, Bichet D, Altafaj X, Weiss N, et al. Cavbeta-subunit displacement is a key step to induce the reluctant state of P/Q calcium channels by direct G protein regulation. Proc Natl Acad Sci U S A. 2004;101:6267–72. doi: 10.1073/pnas.0306804101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hidalgo P, Gonzalez-Gutierrez G, Garcia-Olivares J, Neely A. The alpha1-beta-subunit interaction that modulates calcium channel activity is reversible and requires a competent alpha-interaction domain. J Biol Chem. 2006;281:24104–10. doi: 10.1074/jbc.M605930200. [DOI] [PubMed] [Google Scholar]
- 25.Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch TP, Campbell KP. Calcium channel beta-subunit binds to a conserved motif in the I-II cytoplasmic linker of the alpha 1-subunit. Nature. 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- 26.Walker D, Bichet D, Campbell KP, De Waard M. A beta 4 isoform-specific interaction site in the carboxyl-terminal region of the voltage-dependent Ca2+ channel alpha 1A subunit. J Biol Chem. 1998;273:2361–7. doi: 10.1074/jbc.273.4.2361. [DOI] [PubMed] [Google Scholar]
- 27.Bidaud I, Mezghrani A, Swayne LA, Monteil A, Lory P. Voltage-gated calcium channels in genetic diseases. Biochim Biophys Acta. 2006;1763:1169–74. doi: 10.1016/j.bbamcr.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 28.Subramanyam P, Obermair GJ, Baumgartner S, Gebhart M, Striessnig J, Kaufmann WA, et al. Activity and calcium regulate nuclear targeting of the calcium channel beta4b subunit in nerve and muscle cells. Channels (Austin) 2009;3:343–55. doi: 10.4161/chan.3.5.9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc, B. 1995;57:289–300. [Google Scholar]
- 30.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:H0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]