L-type calcium channel (LTCC) function is critical for electrical and cellular signaling in a diverse range of cell types. In cardiac muscle LTCC function (ICa,L) provides trigger Ca2+ for Ca2+-induced Ca release, and ICa,L kinetics is a critical determinant of action potential duration.1 LTCC blockade is an effective antihypertensive regimen, likely owing to contributions of ICa,L in vascular smooth muscle for maintenance of vasotone. Cardiac and vascular smooth muscle finely grade function. In part, precise regulation of ICa,L contributes to such graded contraction.
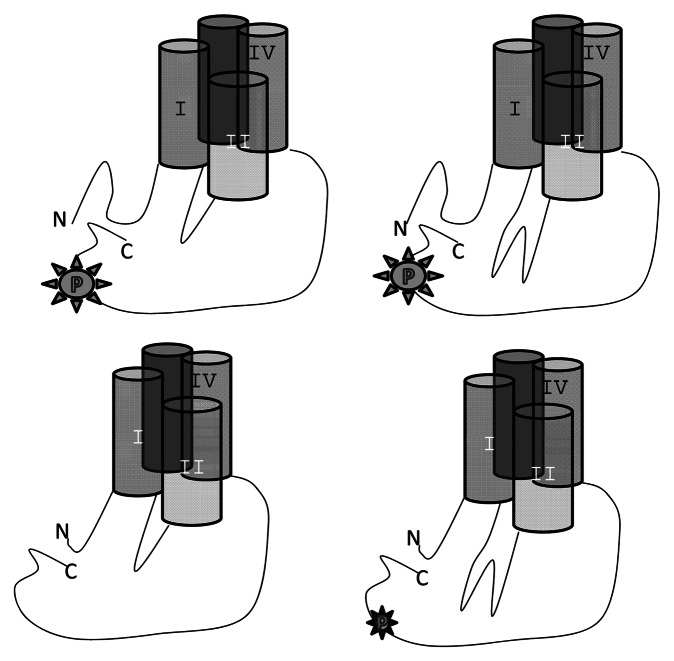
The CaV1.2 pore-forming Ca2+-channel protein is encoded by up to ~50 exons, though there is substantial heterogeneity of CaV1.2 exon usage. Three key determinants of ICa,L modulation have been associated with: the cytosolic localized N-terminus, connecting linker between homologous repeats I and II (LI-II), and the C-terminus. For example, LI-II splice variants prominent in vascular smooth muscle confer higher diltiazem sensitivity.2 Angiotensin II (AngII) is an important regulator of vasotone and contributes to cardiomyocyte signaling. In the heart AngII elicits a biphasic response.3 AngII receptor activation via Gq-containing trimeric G-proteins elicits a variety of effects including activation of PKC and ICa,L modulation. However, the consequence of Gq-signaling of ICa,L is controversial. Discrepant literature can arise from studies of diverse CaV1.2 splice variants, and from intricacies of Gq signaling, including, but not limited to differential effects of PKC and Gβγ subunits following receptor activation. In a recent report in Channels the Dascal laboratory sheds new light on the complex regulation of ICa,L by Gq-signaling pathways.4 Their new study4 uses Xenopus oocytes as a ‘null’ background to heterologously express CaV1.2 containing four prevalent combinations of exons expressed in human vasculature or heart. Activated-receptors elicited a biphasic response with an early increase followed by decline of ICa,L about 10 min after the stimulus. Addition of Gβγ scavenger or Gβγ alone reveals a relatively slowly accumulating inhibition of ICa,L by Gβγ. The cardiac expressed long N-terminal CaV1.2 recapitulates native biphasic response to Gq-receptor activation. The early increase requires the relatively long cardiac-expressed N-terminus and is mediated by PKC in opposition to the Gβγ diminution of ICa,L. From here the story gets more complicated. The C-terminus contains a PKC substrate site at Ser1928, and the new study shows a requirement for S1928 to mediate the PKC enhancement of increase of ICa,L (independently of the Gβγ decrease); however, the N-terminus binds to PKC and Gβγ suggesting N- and C-terminal communication reminiscent, for example of CaM signaling.5 In a nutshell this new study separates Gq-PLC vs. Gβγ modulation of CaV1.2 in an exon-specific fashion (Fig. 1).
Figure 1. Exon usage influences PKC-mediated C-terminal phosphorylation (note that phosphorylation was not assayed in this study, but was inferred from a Ser to Ala mutation analysis); in turn, C-terminal phosphorylation increases current in long N-terminal splice variants. Four combinations shown: long vs. short N-terminus or LI-II. PKC and Gβγ bind to all N-terminal variants, and oppose PKC effects albeit more efficaciously in short N-terminal variants. Long LI-II partially compensates for short N-terminal.
There are a few caveats also worth noting. First, recapitulation of native ICa,L modulation is notoriously difficult to achieve. As the authors note, some of these limitations are uniquely overcome in the X. oocytes (as opposed to mammalian cells, such as HEK 293 cells). The LTCC is a complex of multiple proteins in mammalian cells. The finding that specific N- and C-termini must communicate to capture native modulation suggests a complicated folding pattern that might encompass interactions with other proteins such as CaM, CaMKII, CaVβ subunits, and RGK proteins. A second issue is that Ba2+, not Ca2+ is used as the charge carrier. In x. oocytes Ca2+ induces a large contaminating Cl- current. Aside from the well-established Ca2+-CaM influence on LTCC kinetics, a recent study showed that the dogmatic auto-inhibition by the distal C-terminus is relieved when Ca2+ is the charge carrier,6 and the accessory protein Rem interacts with CaV1.2 C-terminus in a Ca2+-CaM dependent fashion.7 Therefore, caution must be used interpreting the data. This new study is an interesting step forward, but follow-up studies in native systems are of critical importance to verify the complex interplay of channel exon usage and Gβγ and PKC modulation of L-type Ca2+ channel function.
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/24147
References
- 1.Alseikhan BA, DeMaria CD, Colecraft HM, Yue DT. Engineered calmodulins reveal the unexpected eminence of Ca2+ channel inactivation in controlling heart excitation. Proc Natl Acad Sci U S A. 2002;99:17185–90. doi: 10.1073/pnas.262372999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang HY, Liao P, Wang JJ, Yu J, Soong TW. Alternative splicing modulates diltiazem sensitivity of cardiac and vascular smooth muscle Ca(v)1.2 calcium channels. Br J Pharmacol. 2010;160:1631–40. doi: 10.1111/j.1476-5381.2010.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang W, Oudit GY, Patel MM, Shah AM, Woodgett JR, Tsushima RG, et al. Role of phosphoinositide 3-kinase alpha, protein kinase C, and L-type Ca2+ channels in mediating the complex actions of angiotensin II on mouse cardiac contractility. Hypertension. 2010;56:422–9. doi: 10.1161/HYPERTENSIONAHA.109.149344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss S, Keren-Raifman T, Oz S, Ben Mocha A, Haase H, Dascal N. Modulation of distinct isoforms of L-type calcium channels by G(q)-coupled receptors in Xenopus oocytes: antagonistic effects of Gβγ and protein kinase C. Channels (Austin) 2012;6:426–37. doi: 10.4161/chan.22016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dick IE, Tadross MR, Liang H, Tay LH, Yang W, Yue DT. A modular switch for spatial Ca2+ selectivity in the calmodulin regulation of CaV channels. Nature. 2008;451:830–4. doi: 10.1038/nature06529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crump SM, Andres DA, Sievert G, Satin J. The Cardiac L-type Calcium Channel Distal Carboxyl-Terminus Auto-inhibition Is Regulated by Calcium. Am J Physiol Heart Circ Physiol. 2012 doi: 10.1152/ajpheart.00396.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pang C, Crump SM, Jin L, Correll RN, Finlin BS, Satin J, et al. Rem GTPase interacts with the proximal CaV1.2 C-terminus and modulates calcium-dependent channel inactivation. Channels (Austin) 2010;4:192–202. doi: 10.4161/chan.4.3.11867. [DOI] [PMC free article] [PubMed] [Google Scholar]