Abstract
Herein, we report the first characterization of Shab slow inactivation. Open Shab channels inactivate within seconds, with two voltage-independent time constants. Additionally, Shab presents significant closed-state inactivation. We found that with short depolarizing pulses, shorter than the slowest inactivation time constant, the resulting inactivation curve has a marked U-shape, but as pulse duration increases, approaching steady-state conditions, the U-shape vanishes, and the resulting inactivation curves converge to the classical Boltzmann h∞ curve. Regarding the mechanism of inactivation, we found that external K+ and TEA facilitate both open- and closed-state inactivation, while the cavity blocker quinidine hinders inactivation. These results together with our previous observations regarding the K+-dependent stability of the K+ conductance, suggest the novel hypothesis that inactivation of Shab channels, and possibly that of other Kv channels whose inactivation is facilitated by K+, does not involve a significant narrowing of the extracellular entry of the pore. Instead, we hypothesize that there is only a rearrangement of a more internal segment of the pore that affects the central cavity and halts K+ conduction.
Keywords: Shab, potassium channels, slow inactivation, U-type inactivation, C-type inactivation, Drosophila
Introduction
In response to a sustained depolarization, most voltage-gated ion channels open and thereafter inactivate. Inactivation is customarily divided into fast or slow depending on its mechanism and the time scale in which it develops. Slow inactivation is a complex process that develops from the open state over a time course of hundreds of milliseconds to several seconds. The mechanism of slow inactivation is not fully understood and may vary in different channel types.
At first sight slow inactivation may seem too slow to be physiologically relevant, however it can also develop from closed states and have a slow recovery, producing cumulative inactivation in the time scale of cell signaling.1,2 Additionally, when both fast and slow inactivation mechanisms are present they can be coupled.3,4
Among Kv channels, the most extensively studied form of slow inactivation is that present in the Drosophila Shaker B channel, termed C-type inactivation by Aldrich’s laboratory because of its dependency on residues present toward the C-terminus domain of the protein.3 C-inactivation has also been documented in other Kv channels such as HERG and channels of the mammalian Kv1 family.5
Shaker C-inactivation develops from the open state with a time constant of ~1–2 sec, in physiological [K+].3,6 A hallmark of C-inactivation is its inhibition by external K+ (Ko+) and tetraethylammonium (TEAo) ions.4,6-9 These, along with other observations,10 lead to the paradigm that during C-inactivation there is a localized closing or narrowing on the extracellular vestibule of the pore, around Shaker residue 449,6-13 a key determinant of the TEAo binding site on the channels.9,14,15 Accordingly, this region of the external vestibule of the pore has come to be referred to as the C-type inactivation gate. K+o and TEAo are thought to inhibit C-type inactivation by sterically hindering the closing of this gate.5,10,16
Other observations indicate that along with the localized constriction on the external vestibule of the pore, C-inactivation may recruit a larger conformational change that affects the selectivity filter (e.g., refs. 17–21) as well as locations along the S6 segment (e.g., refs. 3, 22–25), in particular the central cavity of the pore. Regarding the latter, and of particular interest to this study, is the recent demonstration that internal TEA (TEAi) inhibits the slow inactivation of Kv2.1 and Shaker channels.26,27
Another protein domain that has been considered to be engaged in slow inactivation gating is the voltage sensor, since inactivation during a long depolarization, is accompanied with a shift of the gating-charge (Q) vs. voltage (V) curve toward hyperpolarized potentials.28,29 The term P/C inactivation is used to distinguish between the change(s) in the pore and the accompanying change in the voltage sensor.5,30 Nonetheless, recent observations have suggested that inactivation and the shift of the Q-V curve may not be causally related, but that the latter might be an intrinsic property of voltage sensors.31
Some Kv channels present a slow inactivation whose properties differ from those distinctive of the canonical C-inactivation of Shaker. An example is Kv2.1, a mammalian ortholog of Shab, which presents an inactivation that preferentially arises from partially activated closed states, follows a U-shaped inactivation curve, and therefore has been termed U-type inactivation.32,33 In addition to Kv2.1, other Kvs including Kv1.3 and truncated Kv1.5 channels,5,34 have also been reported to present U-type inactivation.
In contrast to its inhibitory effect on C-inactivation, Ko+ slightly accelerates the open-state inactivation (OI) of both Kv2.1 and Kv1.3 channels, although it slows down, and in a more prominent manner, the dominant closed-state inactivation (CI) of Kv2.1 channels.26,32,34 On the other hand, TEAo has no effect either on OI or CI of Kv2.1.26 It is noteworthy that, to our knowledge, no mechanism has been proposed to explain the facilitation of inactivation exerted by Ko+ in these channels.5
In contrast to the extensive studies performed to characterize the Drosophila Shaker channel inactivation, the inactivation of the related Shab channels35,36 has escaped attention. This has occurred in spite of a wealth of evidence indicating significant roles of Shab channels in membrane excitability, for example in action potential repolarization, and repeated spiking in a variety of Drosophila muscle and neural cells, as well as in cardiac beat frequency.37-43 Clearly, in order to best understand the role of Shab in cell physiology, knowledge of its inactivation gating is required. Additionally, this will undoubtedly increase our understanding of inactivation mechanisms and pore dynamics of Kv channels.
Herein we report the first characterization of the slow inactivation gating of delayed rectifier Shab channels, and a critical test for U-type inactivation. Additionally, a novel hypothesis regarding the mechanism of K+-facilitated inactivation process is stated.
Results
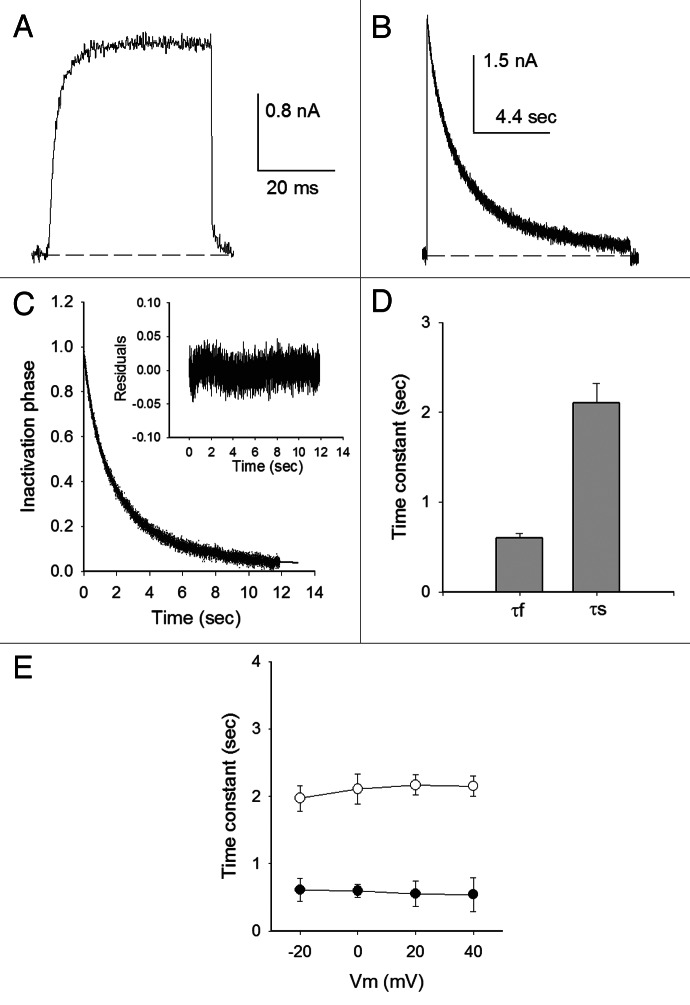
Once open, Shab K+ channels undergo a slow inactivation process that develops over seconds.36 This is illustrated in Figure 1 which presents potassium currents (IK) evoked either by a short, 30-ms, depolarization to 0 mV (Fig. 1A), or by a long, 12 sec, pulse to the same voltage (Fig. 1B), in cells bathed in Nao/Ki control solutions (see Materials and Methods). In contrast to Ik evoked with the short pulse, which does not decay, when the membrane is maintained depolarized for 12 sec, IK gradually decays as channels slowly inactivate from the open state.
Figure 1. Open state inactivation. (A) Ik evoked by a 30 ms pulse to 0 mV (Nao/Ki). (B) IK evoked by a 12 sec pulse as in (A). (C) Normalized inactivation phase of IK in (B), fitted with the equation (solid line): Ik(t)/Io = AfNa*exp(−t/τf,OI,Na) + AsNa*exp(−t/τs,OI,Na) + CNa; where: Io is peak IK; AfNa = 0.39, τf,OI,Na = 0.83sec; AsNa = 0.55, τs,OI,Na = 3.2sec; and CNa = 0.03 (see Text). The inset shows the residuals of the fit. (D) Inactivation time constants at 0 mV, obtained as in (B). (E) Fast (closed symbols) and slow (open symbols) time constants vs. Vm. n = 8
The time course of Shab open-sate inactivation (OI) is best fitted with the sum of two exponentials than with a simpler single exponential model (p < 0.001). This is illustrated in Figure 1C which shows the fit of the normalized inactivation phase of Ik in B (solid line), with a fast time constant τf,OI,Na = 0.84 sec (average, 0.6 ± 0.1 sec; see Fig. 1D), with relative amplitude AfNa = 0.42 (average, 0.3 ± 0.04); and a slow time constant τs,OI,Na = 3.3 sec (average, 2.1 ± 0.2 sec), with relative amplitude AsNa = 0.6 (average, 0.68 ± 0.03), plus a constant CNa = 0.03 term (average, 0.05 ± 0.01, n = 8). The inset shows the residuals of the fit. As commonly found in several types of inactivation, both fast and slow, Figure 1E shows that at fully activating potentials (≥ −20 mV) both time constants are not appreciably voltage-dependent.
Shab τs,OI,Na has a value similar to the time constant of the Shaker C-type inactivation in equivalent ionic conditions,6 and faster than that of its mammalian ortholog Kv2.1 channel.32 Considering that the latter presents a U-type inactivation, our next goal was to determine if Shab presents its defining characteristic, namely a U-shaped inactivation curve.
Shab inactivation curve: A test for U-type inactivation
In order to determine if Shab presents U-type inactivation the Shab inactivation curve was analyzed, applying depolarizing pulses of variable duration, using both a modified three-pulse protocol and a standard two-pulse protocol. In the first one, a control pulse was initially applied (Vcontrol = +40mV/30ms). Thereafter, a pulse (pre-pulse) of variable amplitude and duration was delivered. Finally, a test pulse was applied (Vtest = Vcontrol), and inactivation was evaluated as the ratio of the currents evoked by the last and the first pulse, IK,test/IK,control.
The standard protocol lacked the first control pulse, and inactivation was assessed as IK(V)/IK,max, where IK,max is the maximal IK measured during the second test pulse that was applied immediately after delivering the first pre-pulse of the indicated amplitude and durations. Both protocols yielded identical results (see below) due to the lack of variation of IK in the first pulse of the three-pulse protocol (not shown, but noticeable in Fig. 2A right panel).
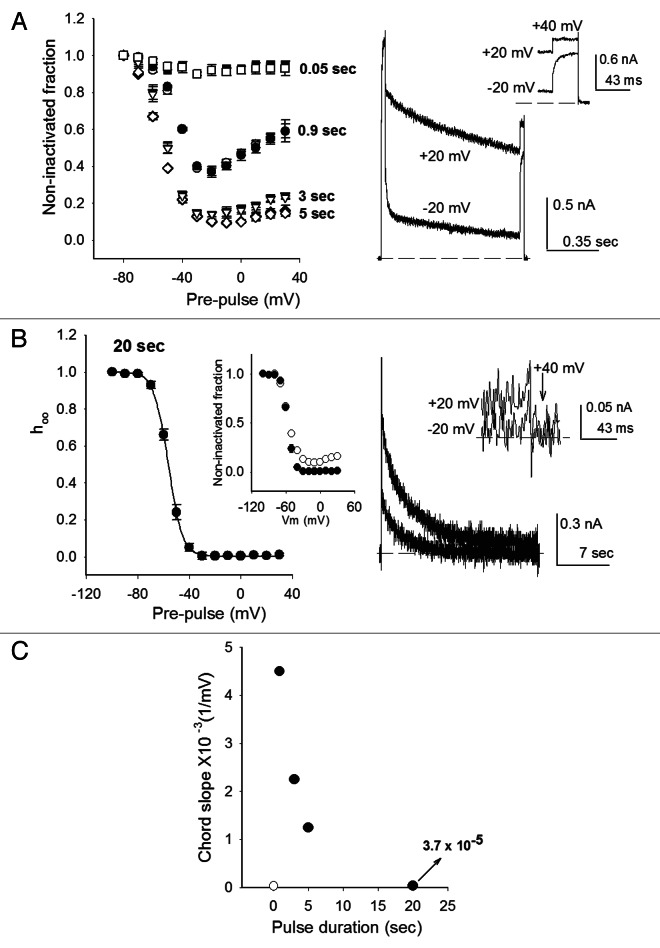
Figure 2. Inactivation curve under far and near steady-state conditions. (A) Left panel, inactivation promoted with short depolarizing pulses, as indicated. Inactivation was assessed with either a three (closed symbols) or a two pulse protocol (open symbols) (see text). Right panel, superimposed IK, obtained with the three-pulse protocol, applying a −20 or a +20 mV pre-pulse of 0.9 sec duration, as indicated. The inset shows IK evoked by the final test pulse in an expanded scale. (B) Left panel, h∞ inactivation curves (data from ref. 44). The curve was obtained applying 20-sec pre-pulses of the indicated amplitude in Nao solutions. The line is the fit of the points with the Boltzmann equation h∞ = 1/(1+exp[(zF/RT)(Vm-V1/2)]), with parameters V1/2 = -56.3mV, z = 4.6. Inset: inactivation after either 3- (open circles) or 20- sec pulses (closed circles). Right panel, superimposed IK recorded as in (A) right panel, but applying either −20 or +20 mV pulses of 20 sec duration, as indicated. The inset shows IK evoked by the final, +40 mV, test pulse (arrow) in an expanded scale. (C) Inactivation curve chord slope, between −20 and +20 mV, as a function of pre-pulse duration. Points are mean ± SEM of at least four experiments.
Figure 2A left panel is a plot of the extent of inactivation (non-inactivated fraction) reached with the delivery of inactivating pulses of increasing duration (0.05, 0.9, 3 and 5 sec, as indicated), applying either the two (closed symbols) or the three-pulse (open symbols) protocols. As expected, very short depolarizations, lasting only 0.05 sec, produce a quite small inactivation at all of the voltages tested. Nonetheless, it is seen that the points of the resulting inactivation curve show a slight hint of a U-shape.
When pulse duration is increased to 0.9 sec the extent of inactivation increases and the points in the curve acquire a marked U-shape,5,32-34 with a well-defined minimum at −20 mV. The latter is illustrated by the traces in the right panel which depict two superimposed IK obtained with the three-pulse protocol with pre-pulse of either −20 mV/0.9s or +20 mV/0.9s, as indicated. Note that as observed in the curve, IK,test at +40 mV following the +20 mV pre-pulse is notably larger than IK,test following the −20 mV pre-pulse. Finally, and importantly: observe that because of the slow kinetics of inactivation (Fig. 1), a 0.9 sec pulse is clearly not sufficiently long to approach steady-state conditions.
When pulse duration is further increased to 3 and 5 sec, and thus steady-state inactivation is progressively approached, two facts are observed: first, the extent of inactivation increases, and second, and more interesting, the marked U-shape of the points observed with the shorter 0.9 sec pulses, becomes less evident (Fig. 2A).
The U-shape completely disappears when the pulse duration is significantly increased (Fig. 2B, left panel) to 20 sec (~10 times the slowest inactivation time constant, Fig. 1 and see below), yielding near steady-state conditions, under which the inactivation curve follows the classical h∞ Boltzmann equation. This is also noted in the inset, which compares the curves obtained with 3 sec (duration ~τs,OI,Na) and 20 sec pre-pulses (duration > > τs,OI,Na). The same h∞ Boltzmann-curve is obtained both with Nao or 100 mM Ko solutions.44 The right panel serves to illustrate IK behavior with 20 sec pre-pulses, by showing two superimposed IK evoked with the 3-pulse protocol applying either −20 or +20 mV. Notice that the corresponding IK, at the final test pulse of +40 mV, have a size difference that is hardly distinguishable from the noise, as clearly seen with the expanded scale of the inset (indicated by the arrow).
In contrast to U-type curves, which present a sizable positive slope at positive potentials, the Boltzmann equation, which fits Shab inactivation under near steady-state conditions, approaches 0 when V > > V1/2. This is illustrated in Figure 2C, which plot the chord slope obtained with the average relative currents following pre-pulses of −20 and +20 mV {[Irel(+20 mV)-Irel(-20 mV)]/40}, of the indicated duration, as in Figure 2A and B. The magnitude of the slope provides a measurement of departure from a Boltzmann relation, in other words it serves to indicate how marked is the U-shape character of the corresponding curve. Note that as pre-pulse duration increases, approaching near steady-state conditions, the slope falls steeply toward 0, as contained in the Boltzmann equation. The open circle departs from the general tendency of the remaining points (closed circles). This point corresponds to 0.05 sec pre-pulses, too short to produce a significant inactivation (Fig. 2A).
It is clear that the U-shape of the inactivation curves as in Figure 2A are due to kinetic processes, which are the result of being far from steady-state conditions (see ref. 45 and Discussion). After rejecting U-inactivation, as the type of inactivation present in Shab channels, our next goal was to determine whether Shab presents the characteristic features of C-type inactivation.
Shab inactivation is not C-type: Effects of TEAo and Ko+ on OI
In order to determine if inactivation follows a C-type mechanism, the effects of TEAo and Ko+ on open state inactivation (OI) were determined by applying 7 sec pulses, that open and inactivate the channels, to cells bathed in either TEA or K+-containing external solutions. Figure 3A presents the outcome of adding TEA to the external solution. The traces in the left panel compare representative IK recorded either in the reference Nao solution (larger trace, labeled Na) or with 15 mM TEAo (smaller trace, labeled TEA), a concentration that inhibits ~50% of channels.
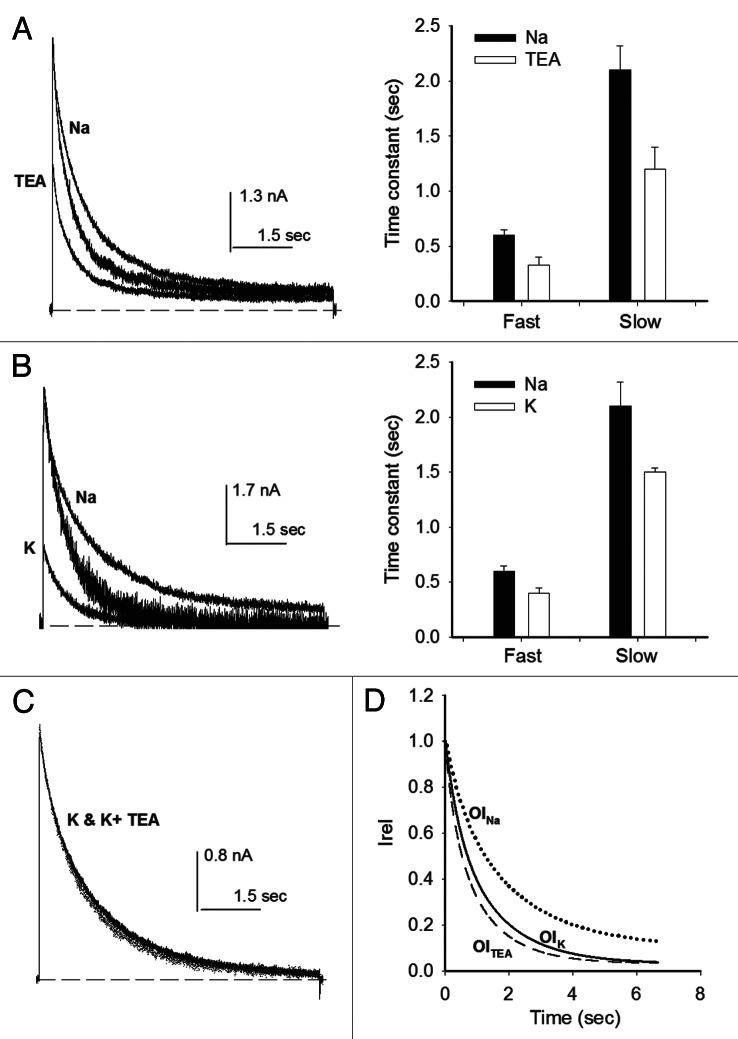
Figure 3. Effects of TEAo and Ko+ on OI. (A) Left panel, IK evoked by 0 mV/7sec pulse applied either in the absence (Nao) or in the presence of 15 mM TEAo, as indicated. Middle trace: IK with TEA scaled to control peak IK. OI follows the time course (Fig. 1): Ik(t)/Io = Af*exp(−t/τf,OI) + As*exp(−t/τs,OI) + C; Io is peak IK; τf,OI,TEA = 0.33 ± 0.07, τs,OI,TEA = 1.2 ± 0.22, AfTEA = 0.28 ± 0.07, AsTEA = 0.65 ± 0.07, CTEA = 0.06 ± 0.02. Right panel, inactivation time constants measured either in Nao or in 15 mM TEAo. TEAo significantly decreases both constants (p < 0.05). (B) Left panel, IK evoked by a +20mV/7sec pulse applied in either Nao or 100 Ko solutions, as indicated. Middle trace: IK in 100 Ko scaled to control peak IK. Right panel, inactivation time constants, as indicated. (C) Superposed IK recorded in either 100 mM K+o or in 100 K+o plus 15 mM TEAo. (D) OI time course. The lines are the least squares fit of OI of traces as in (A and B): 15TEA (dotted line, parameters as above, n = 6); 100 Ko (dashed line, n = 6): τf,OI,K = 0.4 ± 0.05 sec, τs,OI,K = 1.5 ± 0.04 sec, AfK = 0.34 ± 0.13, AsK = 0.64 ± 0.12, CK = 0.02 ± 0.008; Nao (solid line): τf,OI,Na = 0.6 ± 0.05sec, τs,OI,Na = 2.1 ± 0.22, AfNa = 0.3 ± 0.02, AsNA = 0.68 ± 0.03, CNa = 0.05 ± 0.01.
In order to facilitate its comparison, IK with TEAo was scaled to the peak IK in 0 TEAo (middle trace). As observed in Nao solutions (Fig. 1), OI in the presence of TEAo also follows a two exponential time course (right panel), but, it is seen that in this condition, Shab OI becomes noticeably faster. This is quantified in the histogram on the right panel, which compares inactivation time constants in Nao, with those obtained with 15 mM TEAo. Notice that TEAo significantly decreases (~44%) both the fast (p = 0.02, n = 4) and the slow (p = 0.023, n = 4) OI time constants, without significantly changing their relative amplitudes.
TEAo facilitation of Shab inactivation is in clear contrast to its opposite, inhibitory, effect on C-inactivation,6-9 and to its lack of effect on the inactivation of the Kv2.1 channel.26
Next we investigated the role of Ko+ on Shab OI. Figure 3B left panel compares IK evoked by a +20mV/7sec pulse with the cell bathed in 100 Ko+ (smaller IK, labeled K) against IK recorded in the same cell bathed in 0 Ko solution (trace labeled Na). IK in 100 Ko is much smaller than in 0 Ko, because with 100 mM Ko+ the pulse voltage is near the K+ equilibrium potential. Therefore, to compare their kinetics the trace in 100 Ko+ was scaled to peak IK in 0 Ko+ (middle trace). Note that, in contrast to its known inhibitory effect on C-type inactivation, Ko+ clearly speeds Shab OI. Quantitation is reported in the right panel which shows that Ko+ significantly decreases (~30%) both the fast (p = 0.027) and the slow (p = 0.019) time constants. This speeding-up occurred without a significant change in the relative amplitudes of their corresponding OI phases (see figure legend), as seen with TEAo.
TEAo blocks Ik in 100 Ko+ as effectively as in 0 Ko+ (not shown), therefore we wondered whether the individual effects of 15 mM TEAo and 100 mM K+o could be additive. The superimposed traces in Figure 3C illustrate that, unexpectedly, they are not (the same result was observed in at least two other cells, see Discussion). The above observations are summarized in Figure 3D which, for clarity, presents the plot of the least squares curves that fit OI, in the indicated conditions.
The former observations show that Shab inactivation presents properties opposite to those characteristic of the C-type inactivation mechanism. Also, it is important to point out that: the facilitation exerted by TEAo and Ko+ does not by itself suggest an inactivation mechanism involving a closing or significant narrowing of the extracellular entry of the pore.
Our next goal was to determine if Shab presented closed state inactivation (CI), and whether the pore had to open for K+ and TEA to be able to affect inactivation.
Inactivation from closed states
We determined the extent and kinetics of closed-state inactivation by assessing the cumulative inactivation produced by short 20-ms repeated pulses of either 0 or +20 mV, which activate the channels without allowing the development of significant OI, applied at a rate that precluded significant recovery from inactivation between consecutive pulses (1.3 x10−4 Hz).
Figure 4A illustrates IK obtained with the above protocol, in the reference Nao/Ki solutions. For clarity only the first IK recorded are shown. Note that, although none of the currents presents a noticeable OI, IK decreased with each consecutive pulse, therefore indicating the presence of closed state inactivation (CINa). This is best seen in Figure 4B which presents the average relative IK as a function of the number of pulses.
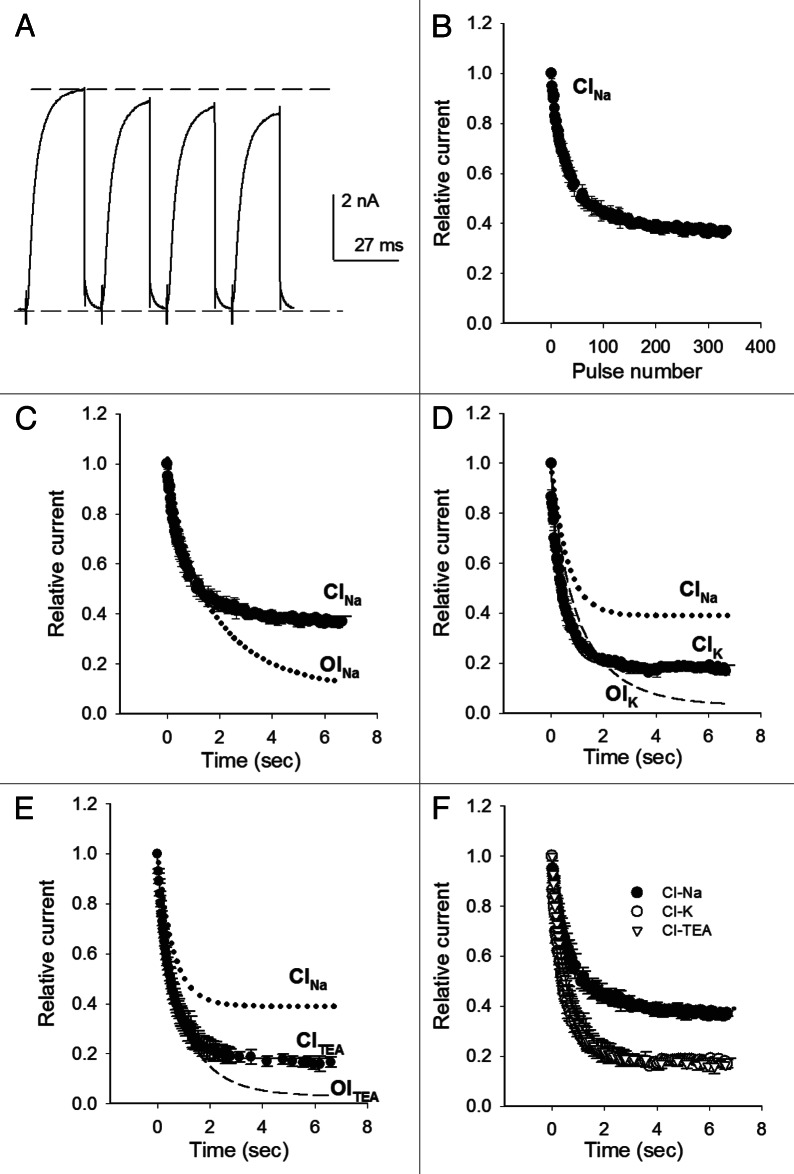
Figure 4. Closed state inactivation. (A) IK evoked by four consecutive 0mV/20ms pulses, applied at 1.3 x10−4 Hz (Nao/Ki). (B) Average relative IK as a function of the number of applied pulses, as in (A). (C) Points in (B) plotted as Relative IK vs. time (CINa, closed circles). The solid line is the fit of the points with the equation: I/Io = Aexp(−t/τ)+ C, where I is IK at time t; Io is IK at t = 0; τCI,Na = 0.61 sec, ANa = 0.61, CNa = 0.39. The dotted line is the curve that fits OINa time course. (D) CI time course with 100 Ko+ (closed circles, CIK). The solid line is the least squares fit of the points with τCI,K = 0.4 sec, AK = 0.8 and CK = 0.19. Dashed line: OIK time course; dotted line: CINa time course. (E) CITEA: Average relative IK vs. time, assessed with 15 mM TEAo (closed circles). The solid line is the fit of the points with τCI,TEA = 0.5 sec, CTEA = 0.18 and ATEA = 0.82. Dashed line: OITEA time course; dotted line: CINa time course. (F) CI time course in the indicated conditions. HP = −80 mV. The points are the mean ± SEM of at least four experiments on each condition.
In order to determine CI kinetics, the points in Figure 4B were plotted as the average relative IK vs. time (Fig. 4C). CINa follows a single exponential time course (solid line), with time constant τCI,Na = 0.61sec, plus a constant term CNa = 0.39, which accounts for the fraction of channels that do not manage to inactivate during their transit along closed states, at this voltage. Note that, interestingly, τCI,Na at −80 mV is basically identical to the fast OINa time constant (Fig. 1), which accounts for ~30% of OINa. This is easily observed in Figure 4C which for comparison also presents the curve that describes OINa time course (dashed line).
The former observations show that, in contrast to Shaker which mainly inactivates from the open state, Shab inactivates from both open and closed states, but in contrast to its ortholog Kv2.1, Shab channels do not preferentially inactivate from closed states.
Next we investigated whether channels have to open for its inactivation to be modulated by Ko+ and TEAo. Figure 4D shows the plot of the average relative IK vs. time, obtained with the above protocol in cells bathed in 100 Ko+ (closed circles). IK falls along an exponential time course (solid line), as channels inactivate from closed states, with time constant τCI,K = 0.4 sec, plus a constant term Ck = 0.2. Importantly, CIK is significantly faster and therefore more complete (p < 0.01), than CINa. The latter is visually appreciated in the figure which, for a reference, also presents the time course of CINa (dotted line). Finally, notice that τCI,K at −80 mV is basically equal to τf,OI,K (dashed line), as previously also observed in Nao (Fig. 4C).
The role of TEAo on closed state inactivation is presented in Figure 4E, which shows CI in cells bathed in 15 mM TEAo (closed circles). CITEA follows an exponential time course (solid line through the points) with time constant τCI,TEA = 0.5 sec, plus a constant term CTEA = 0.19. Notice that CITEA is faster, and hence more complete, than CI in the absence of TEAo (CINa, dotted line, p < 0.01). Also, notice that, as previously found with both Nao and 100 Ko solutions, τCI,TEA is basically identical to τf,OI,TEA (dashed line).
Figure 4F compares CI in the ionic solutions tested. It is easily appreciated that CITEA and CIK are indistinguishable (Fig. 4F), and faster and more complete than CINa. These data shows that, at the tested concentrations, Ko+ and TEAo exert similar effects on both open (Fig. 3) and closed state inactivation.
The above observations indicate that TEAo and Ko+ facilitate Shab inactivation, regardless of the state of the activation gate. This suggest the hypothesis that Shab reaches the same inactivated conformation from either open or closed states, and further indicates that Shab inactivation does not obey a C-type inactivation mechanism.
Interestingly, and related to the latter, recent observations point to the involvement of the central cavity in the slow inactivation gating of several Kv channels, exhibiting both C- and non-C-type inactivation mechanisms. Therefore, we decided to test the effect of adding an intracellular pore blocker that binds to the central cavity on Shab inactivation.
Inactivation inhibition by the intracellular pore blocker quinidine
It has been reported that inactivation properties may change upon establishing the inside-out configuration of the patch-clamp technique.27,46 Therefore, we decided to use quinidine (Qd), as this molecule added to the extracellular solution rapidly equilibrates across the membrane blocking the Shab open pore from the intracellular side of the membrane,47 allowing tests of Qd effect on inactivation in the whole-cell recording configuration.
In order to evaluate the effect of Qd on Shab inactivation, a 0mV/7sec pulse was applied to channels bathed in a control 5 Ko+ solution.47 Thereafter, Qd was added at either 0.03 or 0.1 mM concentrations in the 5 Ko+ solution, and once it equilibrated across the membrane (not shown), its effect on inactivation was tested.
Figure 5A shows two superimposed IK recorded before (larger IK) and after the addition of 0.1 mM Qd (smaller IK). Qd blocked Shab, markedly reducing IK amplitude and, more interesting for the present discussion, slowing inactivation when compared with control IK. This is best appreciated in the right panel which presents IK with Qd normalized to control peak IK. In these superimposed traces, Qd inhibition of inactivation is easily appreciated by comparing IK size at pulse-end in control vs. in Qd-blocked channels.
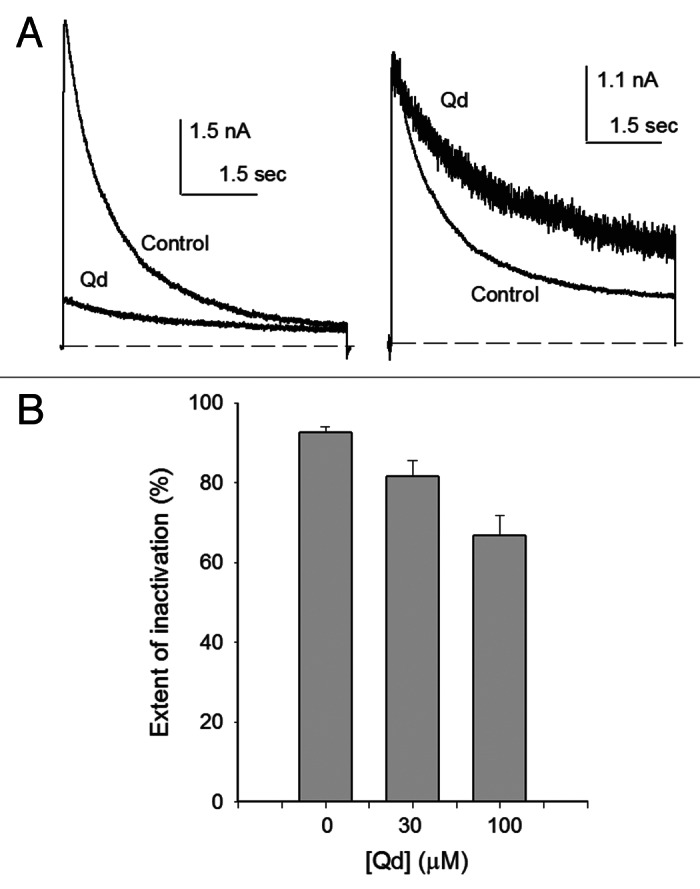
Figure 5. Quinidine blockage hinders inactivation. (A) Left panel, superimposed Ik evoked by a 0mV/7sec pulse either in control 5 Ko+ or in 5 Ko+ + 0.03 mM Qd (trace labeled Qd) solutions. Right panel: IK with Qd scaled to control peak IK. (B) Extent of inactivation vs. [Qd]. The extent of inactivation was evaluated from traces as in (A), as: 1-(Iend/Ipeak), where Ipeak is peak IK and Iend is IK at pulse end.
The above observation is quantitatively presented in Figure 5B, which illustrates the extent of inactivation reached at the end of the inactivating pulse, as in A (see figure legend). Note that in the absence of Qd the extent of inactivation (92.6 ± 1.4%, n = 5) is significantly larger than that observed in channels bathed in either 0.03mM (81.6 ± 4%, n = 5; p = 0.0318), or 0.1 mM Qd solutions (66.8 ± 5%, n = 5; p = 0.0011). Although further work is needed to determine the mechanism by which Qd hinders inactivation, this observation points to the participation of the central cavity of the pore in the slow inactivation gating of Shab channels.
A kinetic model of Shab inactivation
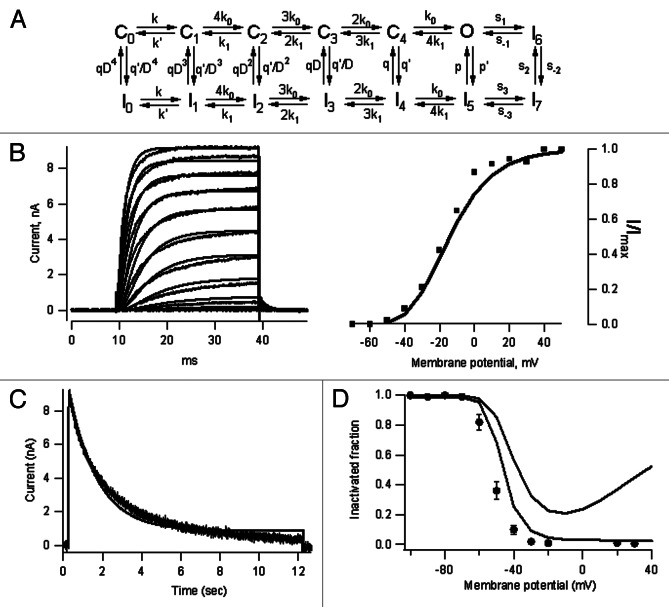
The observations reported in this study were synthesized in a kinetic model (Fig. 6A). The model is an extension of a kinetic scheme developed to explain the effect of the anti-inflammatory drug celecoxib on Shab channels.48 The model consists of five closed (Co to C4) and a single open state (O).49 Inactivation (I) develops from both open and closed states, in a coupled fashion (see Materials and Methods). As a result of the different values of inactivation rate constants along the activation pathway (Table 1), the probability of inactivation increases as channels approach the open conformation.
Figure 6. A kinetic model of Shab inactivation. (A) Kinetic model of Shab inactivation, C, I and O have their standard meaning (see Materials andMethods and Table 1). (B) Comparison of the model predictions with the voltage-dependent activation of Shab channels. The left panel shows good agreement between the model and the data for short activating pulses from −50 to +50 mV. The G-V curve, plotted as a fractional activated current in the right panel is also well explained by the model. (C) Model prediction of slow inactivation. For long activating pulses, the model also provides a reasonable good fit of inactivation time course, although it predicts a somewhat larger remaining current at pulse end (Vm = 0mV). (D) Inactivation curve. For long inactivating pulses (12 sec, black trace) the model predicts a monotonically increasing inactivation at depolarized potentials, as contained in the Boltzmann curve, while for shorter pulses (5 sec) a U-shaped inactivation is expected as a consequence of the channel not having reached steady-state during the pulse.
Table 1. Kinetic model rate constants.
| Rate constants in s−1 @ 0 mV | Charge (in eo) |
|---|---|
| ko = 700 k1 = 50 q = 2.2 q’ = 0.1 p = 0.05 p’ = 0.06 s2 = 35 s−2 = 0.3 k = 240 k’ = 130 s1 = 0.03 s−1 = 0.1 s3 = 3 s−3 = 7 |
0.7 −0.9 0.7 −0.6 0.1 0.2 0 0 0.5 −0.5 0.1 −0.1 0 0 |
Model rate constants. (A) The voltage-dependence of rate constants is implemented in the model as exponential equations of the form: ki(V)=ki(0)exp(ziV / kT), where ki(V) is the rate constant at voltage V, ki(0) is its value at 0 mV, zi is the charge associated with the transition and k and T are Boltzmann’s constant and the temperature in °K. (B) The value of the allosteric factor D is taken to be 0.3.
Although in the model they are labeled with different numbers, the state I reached from the open and the different closed states should be taken to represent the same inactivated conformation of the protein. Also, as channels do not appreciably conduct during recovery from inactivation, the transition I6 to O was endowed with a small rate (Table 1). The model uses data not shown of recovery from inactivation (manuscript in preparation). Note that the model fairly well reproduces channel activation (Fig. 6B), as well as inactivation (Fig. 6C). Finally, note that the model reproduces both, the apparent U-shaped inactivation curve (Fig. 6D), observed with depolarizations that are too short to approach steady-state conditions (0.9 sec), and the Boltzmann h∞-curve, obtained under near steady-state conditions (20 sec pulses).
Discussion
Shab channels present a slow inactivation process that develops from the open state with two voltage-independent time constants. In the reference Nao solution the slower constant has a magnitude (~2 sec) similar to that of Shaker C-type inactivation,6 and much faster than that of OI of the related Kv2.1 channels,26,32 under comparable recording conditions.
In addition to OI, Shab also undergoes closed state inactivation. CI develops with a time constant equal to that of the fast OI component (~0.6 sec). Considering that the latter accounts for ~30% of IK decay during a pulse, and that ~40% of channels do not undergo CI upon delivery of a moderate, 0 mV, pulse from −80 mV, we conclude that although Shab has significant CI it does not have the so called preferential closed state inactivation seen in other Kvs whose inactivation is also facilitated by Ko+.5,32,50
This demonstrates that Ko+ facilitation is a property not strictly associated with inactivation processes that preferentially develop from closed states. Additionally, as Shab presents significant CI, our observations also show that the term U-type inactivation is inadequate to denote inactivation process significantly arising from pre-open closed states.
Importantly, we demonstrate that the inactivation curve presents an apparent U-shape when pulse durations are too short to approach steady-state conditions. In contrast, when the inactivating pulse durations are much longer than the slowest inactivation time constant, which allows the channels to approach near steady-state conditions, the inactivation curve ceases to be U-shaped, and it is fitted by the classical h∞ Boltzmann curve.
The U-shape of the inactivation curves obtained when pulse durations are too short (duration ≤ slowest inactivation time constant) are the result of kinetic processes arising from the relative values of activation and inactivation rates. At more depolarized voltages channel activation is faster, and since inactivation is voltage-independent, and can be reached from both open and closed states (see below), delivering pulses too short to approach steady-state inactivation causes the fewer of these channels to inactivate from closed states during the activating pulse and, because pulse duration is relatively short, they will not have sufficient time to inactivate fully from the open state, yielding the U-shaped curve.
On the other hand, as steady-state conditions are approached channels have enough time to reach a time-invariant extent of inactivation, both from closed and open states, and the U-shape of the curve vanishes. Based on this, and on the extensive discussion of Yue and coworkers,45 we consider that only in limiting conditions, such as when inactivation arises only from closed states, would a U-shaped curve be observed, near steady-state conditions. On the other hand, when channels inactivate from the open state, but with a very slow rate, approaching near steady-state conditions may be technically difficult to achieve, but this by itself should not vindicate the identification of a particular inactivation as U-type.
Shab inactivation is faster in the presence of either external K+ or TEA. Additionally, we found that when both ions are present, their individual effects are not additive, despite the fact that TEAo blocks IK in 100 mM K+o as effectively as in Nao. A possible explanation of these observations is that the individual effect of the ions may have reached saturation at the concentrations tested, and that TEAo and Ko+ speed inactivation by binding to different sites. TEAo on its blocking site in the external vestibule of the pore,14 and K+ within the selectivity filter51 This proposal is in agreement with the observation that Ko+, but not TEAo, increases the OI rate of Kv2.1 channels.26,32
As mentioned before, TEAo significantly speeds both OI and CI of Shab, and in contrast it does not affect at all the inactivation of the Shab mammalian ortholog Kv2.1.26 A possible explanation for this difference is that Shab presents a cysteine at the Shaker position 449, a key determinant of the external TEA binding site of Kv channels,9,14,15 while Kv2.1 presents a tyrosine (Y), at the equivalent position. Tyrosine residues yield a high affinity TEAo blocking-site,14 where, interestingly, TEAo binds without affecting inactivation.9 This is thought to occur because the Y-binding site seems to hold TEA in a relatively external position, outside the voltage drop across the membrane, as inferred from the voltage-independent TEAo blockage of pore.9 In other words, it seems that TEAo needs to bind deep enough to sense the voltage drop across the membrane, as it does in Shab (data not shown), to either down or upregulate inactivation, as it does in for example Shaker6 and Shab, respectively.
External K+ significantly speeds Shab OI while in Kv2.1 the same effect is observed although it is less pronounced.26,32 This similarity suggests that the K+ affinity of the relevant K+ binding site(s) may only be slightly different in both channels, as expected from their conserved signature sequence, and the presence in both channels of the same aminoacid (cysteine) at the Shaker position 463 on S6, a position known to influence K+ binding to the selectivity filter24 (see below).
As it does on OI, Ko+ also facilitates Shab CI, but in contrast it inhibits the dominant closed state inactivation of Kv2.1.26 Whatever the cause of this difference may be, it suggests that the relative weights of CI/OI of these channels may not simply be due to their different relative values of inactivation and activation rate constants, but to a qualitative difference on its corresponding CI; even though further work is needed to clarify this point, the differential effects of K+ and TEA on OI/CI of Kv2.1 suggest that inactivation of the latter may be a more complex process than that operating on Shab, as it is also suggested by the regulation of Kv2.1 inactivation by modulatory subunits.26
Role of the central cavity on Shab inactivation
Quinidine is a lipophilic cation that blocks Shab IK,47 upon binding to the central cavity of the pore.47,52-54 Here we show that Qd block hinders Shab inactivation.
Although Qd competes with pore K+ ions, mutually destabilizing their binding,47 and this effect by itself should be expected to slow down inactivation, the observation that Qd inhibits OI in the presence of only 5 mM Ko+, a [K+] rather low to significantly speed inactivation, suggest that Qd inhibition of inactivation is not the result of a reduced K+ occupancy of the pore. Thus, it appears that Qd binding to the central cavity somehow hinders the Shab inactivation conformational change.
An opposite effect of Qd is observed in C-type inactivating Kv1.4 channels, in which Qd speeds inactivation through an allosteric effect.55,56 Although further work is needed to fully characterize Qd role on Shab inactivation, its effect appears to be similar to the inhibitory effect of internal TEA on Shaker inactivation and on Kv2.1 OI,26,27 and hence suggests that involvement of the central cavity may be a common theme in different slow inactivation mechanisms.
Comparison with C-type inactivation
Shab inactivation (OI/CI) is accelerated by Ko+ and TEAo. This feature stands in contrast to the characteristic inhibitory effect that these ions exert on C-inactivation,6-9 which is thought to arise from a steric impediment of a local narrowing of the extracellular entry of the pore that accompanies inactivation,9,10,12 referred to as the closing of the C-inactivation gate.5,10,16 In the case of Shab, as well as those Kvs whose inactivation is facilitated by TEAo and Ko+, the up-modulation effect of these ions is not easily reconciled with a mechanism presenting the C-inactivation narrowing of the extracellular entry of the pore, nor a general significant constriction of the selectivity filter.
Another characteristic of C-inactivation is its dependency on the residues present at the Shaker positions 449 and 463. As mentioned before, Shab presents a cysteine while Shaker has a threonine at position 499. It is known that the mutation T449C on Shaker preserves the hallmarks of C-inactivation.11 Therefore, it seems unlikely that the differential effects of TEAo and K+o on inactivation of these channels are due to these different residues, at that position on the external vestibule of the pore.
Shaker B presents an alanine at position 463 on S6. It has been reported that the mutation A463C reduces the K+ affinity of the most internal K+ site (s4) of the selectivity filter, and that, as a consequence, K+ occupancy of the externally located site that regulates C-inactivation increases, rendering Shaker inactivation rather insensitive to [Ko+] changes.24 Shab presents a cysteine at the Shaker 463 equivalent position and its inactivation is appreciably modulated by Ko+ although, as we have shown, in an opposite direction to that in Shaker. This suggests that the K+ site(s) that up-modulate Shab inactivation might not be saturated at low Ko+, as is the case in Shaker A463C. The latter would mean that the presence of a cysteine in Shab does not affect K+ affinity of the pore in the way that it does in Shaker. Alternatively, it might be that Ko+ speeds Shab inactivation in a site(s) different from that at which it inhibits Shaker C-inactivation.
Shab inactivation: a novel hypothesis regarding slow inactivation mechanisms
In this study we have shown that Ko+ and TEAo speed Shab inactivation. On the other hand, we have previously demonstrated that upon exposure to 0 K+ solutions across the membrane the Shab K+ conductance irreversibly collapses with a kinetics that is basically indistinguishable when the channels are either deactivated (Vm = −80 mV) or inactivated (Vm = 0 mV) before their exposure to 0 K+ solutions.44 It is important to emphasize that in these experiments channels are either deactivated or inactivated in control Ko+-containing solutions, before its subsequent exposure to 0 K+, and that throughout the latter the channels are kept undisturbed at either −80 (deactivated) or 0 mV (inactivated). In other words, this indicates that inactivation does not impede the leak of the stabilizing K+ ion(s) from the Shab pore toward the external solution.44
Taken together, the above observations cannot be reconciled, in an obvious manner, with an inactivation mechanism involving a significant narrowing or closing of the extracellular portion of the pore, as it is considered to occur in C-inactivation. Hence, we propose that when Shab inactivates there is only a rearrangement or conformational change of the intracellular side of the pore, affecting the central cavity, which somehow halts K+ conduction. The latter suggests that Ko+ and TEAo may speed inactivation by allosterically favoring the internally located conformational change of the pore. Moreover, we hypothesize that a similar mechanism may operate in other channels whose slow inactivation is facilitated by external K+, such as Kv2.1 and Kv1.3.
Materials and Methods
Cell culture and channel expression
Sf9 cells grown at 27°C in Grace’s media (Gibco) were infected, at a multiplicity of infection of ~10, with a recombinant baculovirus containing Shab K+-channel cDNA (dShab 11), as reported.57 Experiments were conducted 48 h after the infection.
Electrophysiological recordings
Currents were recorded under whole-cell patch-clamp with an Axopatch 1D amplifier (Axon Instruments). The currents were filtered online and sampled at frequencies set to fulfill the Nyquist criteria, with a Digidata 1322A interface. The near steady-state inactivation curve of Figure 2 was obtained using the gear-shift sampling facility of pClamp 7 using a Digidata 1200 interface (Axon Instruments). Electrodes were made of borosilicate glass (KIMAX 51) pulled to a 1–1.5 MΩ resistance, 80% of the series resistance was compensated. The holding potential (HP) was −80 mV.
Solutions
Solutions will be named by their main, test, cation and location across the membrane. The internal Ki solution contained (mM): 30 KCl, 90 KF, 2 MgCl2, 10 EGTA-K, 10 Hepes-K. The Nai solution contained (mM): 30 NaCl, 90 NaF, 2 MgCl2, 10 EGTA-Na, 10 Hepes-Na. External solutions contained (mM): Nao (0 Ko+): 145 NaCl, 10 CaCl2, 10 Hepes-Na. XKo: X KCl, 145-X NaCl, 10 CaCl2, 10 Hepes-Na. Fifteen TEAo: 15 TEA-Cl, 130 NaCl, 10 CaCl2, 10 Hepes-Na. The pH of all solutions was 7.2. Quinidine was added to the external 5 Ko solution at the indicated concentrations.
Data analysis
The model depicted in Figure 6 was solved using Runge-Kutta integration implemented in programs written in-house in Igor Pro (Wavemetrics). The time and voltage-dependent occupancy of the open state (po) was transformed to current according to:
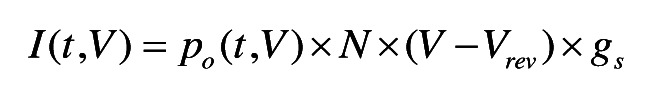 |
Where V is the applied voltage, Vrev is the reversal potential estimated from the Nernst equation for potassium, N is the number of channels and gs is the single channel conductance, which we set to be 20 pS.57
Points are mean ± SEM of the indicated number of independent experiments. Curves were compared with the extra-sum-of-squares F-test using GraphPad Prism version 5.00 (GraphPad Software, www.graphpad.com). Significance level was set to 0.05.
Acknowledgments
This study was performed in partial fulfillment of the requirements of the Doctorate degree in Biomedical Sciences of EC at the UNAM. The authors thank J.M. Hernandez for cell culture. This work was supported by DGAPA_UNAM grant IN229712 and CONACYT grant 153504.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
† These authors contributed equally to this work.
Previously published online: www.landesbioscience.com/journals/channels/article/23569
References
- 1.Aldrich RW. Inactivation of voltage-gated delayed potassium current in molluscan neurons. A kinetic model. Biophys J. 1981;36:519–32. doi: 10.1016/S0006-3495(81)84750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–65. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- 3.Hoshi T, Zagotta WN, Aldrich RW. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron. 1991;7:547–56. doi: 10.1016/0896-6273(91)90367-9. [DOI] [PubMed] [Google Scholar]
- 4.Baukrowitz T, Yellen G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 1995;15:951–60. doi: 10.1016/0896-6273(95)90185-X. [DOI] [PubMed] [Google Scholar]
- 5.Kurata HT, Fedida D. A structural interpretation of voltage-gated potassium channel inactivation. Prog Biophys Mol Biol. 2006;92:185–208. doi: 10.1016/j.pbiomolbio.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 6.López-Barneo J, Hoshi T, Heinemann SH, Aldrich RW. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1993;1:61–71. [PubMed] [Google Scholar]
- 7.Grissmer S, Cahalan M. TEA prevents inactivation while blocking open K+ channels in human T lymphocytes. Biophys J. 1989;55:203–6. doi: 10.1016/S0006-3495(89)82793-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi KL, Aldrich RW, Yellen G. Tetraethylammonium blockade distinguishes two inactivation mechanisms in voltage-activated K+ channels. Proc Natl Acad Sci U S A. 1991;88:5092–5. doi: 10.1073/pnas.88.12.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molina A, Castellano AG, López-Barneo J. Pore mutations in Shaker K+ channels distinguish between the sites of tetraethylammonium blockade and C-type inactivation. J Physiol. 1997;499:361–7. doi: 10.1113/jphysiol.1997.sp021933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yellen G. The moving parts of voltage-gated ion channels. Q Rev Biophys. 1998;31:239–95. doi: 10.1017/S0033583598003448. [DOI] [PubMed] [Google Scholar]
- 11.Yellen G, Sodickson D, Chen TY, Jurman ME. An engineered cysteine in the external mouth of a K+ channel allows inactivation to be modulated by metal binding. Biophys J. 1994;66:1068–75. doi: 10.1016/S0006-3495(94)80888-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Jurman ME, Yellen G. Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron. 1996;16:859–67. doi: 10.1016/S0896-6273(00)80106-3. [DOI] [PubMed] [Google Scholar]
- 13.De Biasi M, Hartmann HA, Drewe JA, Taglialatela M, Brown AM, Kirsch GE. Inactivation determined by a single site in K+ pores. Pflugers Arch. 1993;422:354–63. doi: 10.1007/BF00374291. [DOI] [PubMed] [Google Scholar]
- 14.Heginbotham L, MacKinnon R. The aromatic binding site for tetraethylammonium ion on potassium channels. Neuron. 1992;8:483–91. doi: 10.1016/0896-6273(92)90276-J. [DOI] [PubMed] [Google Scholar]
- 15.Luzhkov VB, Aqvist J. Mechanisms of tetraethylammonium ion block in the KcsA potassium channel. FEBS Lett. 2001;495(3):191–6. doi: 10.1016/s0014-5793(01)02381-x. [DOI] [PubMed] [Google Scholar]
- 16.Rasmusson RL, Morales MJ, Wang S, Liu S, Campbell DL, Brahmajothi MV, et al. Inactivation of voltage-gated cardiac K+ channels. Circ Res. 1998;82:739–50. doi: 10.1161/01.RES.82.7.739. [DOI] [PubMed] [Google Scholar]
- 17.Starkus JG, Kuschel L, Rayner MD, Heinemann SH. Ion conduction through C-type inactivated Shaker channels. J Gen Physiol. 1997;110:539–50. doi: 10.1085/jgp.110.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiss L, LoTurco J, Korn SJ. Contribution of the selectivity filter to inactivation in potassium channels. Biophys J. 1999;76:253–63. doi: 10.1016/S0006-3495(99)77194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Hesketh JC, Fedida D. A high-Na+ conduction state during recovery from inactivation in the potassium channel Kv1.5. Biophys J. 2000;89:23–45. doi: 10.1016/S0006-3495(00)76486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cordero-Morales JF, Cuello LG, Zhao Y, Jogini V, Cortes DM, Roux B, et al. Molecular determinants of gating at the potassium-channel selectivity filter. Nat Struct Mol Biol. 2006;13:311–8. doi: 10.1038/nsmb1069. [DOI] [PubMed] [Google Scholar]
- 21.Cordero-Morales JF, Jogini V, Lewis A, Vásquez V, Cortes DM, Roux B, et al. Molecular driving forces determining potassium channel slow inactivation. Nat Struct Mol Biol. 2007;14:1062–9. doi: 10.1038/nsmb1309. [DOI] [PubMed] [Google Scholar]
- 22.Castle NA, Fadous SR, Logothetis DE, Wang GK. 4-Aminopyridine binding and slow inactivation are mutually exclusive in rat Kv1.1 and Shaker potassium channels. Mol Pharmacol. 1994;46:1175–81. [PubMed] [Google Scholar]
- 23.Fedida D, Bouchard R, Chen FS. Slow gating charge immobilization in the human potassium channel Kv1.5 and its prevention by 4-aminopyridine. J Physiol. 1996;494:377–87. doi: 10.1113/jphysiol.1996.sp021499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogielska EM, Aldrich RW. Functional consequences of a decreased potassium affinity in a potassium channel pore. Ion interactions and C-type inactivation. J Gen Physiol. 1999;113:347–58. doi: 10.1085/jgp.113.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang XJ, Bett GCL, Li XY, Bondarenko VE, Rasmusson RL. C-type inactivation involves a significant decrease in the intracellular aqueous pore volume of Kv1.4 K+ channels expressed in Xenopus oocytes. J Physiol. 2003;549:683–95. doi: 10.1113/jphysiol.2002.034660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerschensteiner D, Monje F, Stocker M. Structural determinants of the regulation of the voltage-gated potassium channel Kv2.1 by the modulatory α-subunit Kv9.3. J Biol Chem. 2003;278:18154–61. doi: 10.1074/jbc.M213117200. [DOI] [PubMed] [Google Scholar]
- 27.González-Pérez V, Neely A, Tapia C, González-Gutiérrez G, Contreras G, Orio P, et al. Slow inactivation in Shaker K channels is delayed by intracellular tetraethylammonium. J Gen Physiol. 2008;132:633–50. doi: 10.1085/jgp.200810057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olcese R, Latorre R, Toro L, Bezanilla F, Stefani E. Correlation between charge movement and ionic current during slow inactivation in Shaker K+ channels. J Gen Physiol. 1997;110:579–89. doi: 10.1085/jgp.110.5.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loots E, Isacoff EY. Molecular coupling of S4 to a K(+) channel’s slow inactivation gate. J Gen Physiol. 2000;116:623–36. doi: 10.1085/jgp.116.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Biasi M, Hartmann HA, Drewe JA, Taglialatela M, Brown AM, Kirsch GE. Inactivation determined by a single site in K+ pores. Pflugers Arch. 1993;422:354–63. doi: 10.1007/BF00374291. [DOI] [PubMed] [Google Scholar]
- 31.Villalba-Galea CA, Sandtner W, Starace DM, Bezanilla F. S4-based voltage sensors have three major conformations. Proc Natl Acad Sci U S A. 2008;105:17600–7. doi: 10.1073/pnas.0807387105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klemic KG, Shieh CC, Kirsch GE, Jones SW. Inactivation of Kv2.1 potassium channels. Biophys J. 1998;74:1779–89. doi: 10.1016/S0006-3495(98)77888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng YM, Azer J, Niven CM, Mafi P, Allard CR, Qi J, et al. Molecular determinants of U-type inactivation in Kv2.1 channels. Biophys J. 2011;101:651–61. doi: 10.1016/j.bpj.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klemic KG, Kirsch GE, Jones SW. U-type inactivation of Kv3.1 and Shaker potassium channels. Biophys J. 2001;81:814–26. doi: 10.1016/S0006-3495(01)75743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butler A, Wei AG, Baker K, Salkoff L. A family of putative potassium channel genes in Drosophila. Science. 1989;243:943–7. doi: 10.1126/science.2493160. [DOI] [PubMed] [Google Scholar]
- 36.Wei A, Covarrubias M, Butler A, Baker K, Pak M, Salkoff LK. K+ current diversity is produced by an extended gene family conserved in Drosophila and mouse. Science. 1990;248:599–603. doi: 10.1126/science.2333511. [DOI] [PubMed] [Google Scholar]
- 37.Singh S, Wu CF. Properties of potassium currents and their role in membrane excitability in Drosophila larval muscle fibers. J Exp Biol. 1990;152:59–76. doi: 10.1242/jeb.152.1.59. [DOI] [PubMed] [Google Scholar]
- 38.Quattrocki EA, Marshall J, Kaczmarek LK. A Shab potassium channel contributes to action potential broadening in peptidergic neurons. Neuron. 1994;12:73–86. doi: 10.1016/0896-6273(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 39.Tsunoda S, Salkoff L. Genetic analysis of Drosophila neurons: Shal, Shaw, and Shab encode most embryonic potassium currents. J Neurosci. 1995;15:1741–54. doi: 10.1523/JNEUROSCI.15-03-01741.1995. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsunoda S, Salkoff L. The major delayed rectifier in both Drosophila neurons and muscle is encoded by Shab. J Neurosci. 1995;15:5209–21. doi: 10.1523/JNEUROSCI.15-07-05209.1995. b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ueda A, Wu CF. Distinct frequency-dependent regulation of nerve terminal excitability and synaptic transmission by IA and IK potassium channels revealed by Drosophila Shaker and Shab mutations. J Neurosci. 2006;26:6238–48. doi: 10.1523/JNEUROSCI.0862-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng IF, Wu CF. Differential contributions of Shaker and Shab K+ currents to neuronal firing patterns in Drosophila. J Neurophysiol. 2007;97:780–94. doi: 10.1152/jn.01012.2006. [DOI] [PubMed] [Google Scholar]
- 43.Frolov RV, Berim IG, Singh S. Inhibition of delayed rectifier potassium channels and induction of arrhythmia: a novel effect of celecoxib and the mechanism underlying it. J Biol Chem. 2008;283:1518–24. doi: 10.1074/jbc.M708100200. [DOI] [PubMed] [Google Scholar]
- 44.Gómez-Lagunas F. Stability of the Shab K+ channel conductance in 0 K+ solutions: the role of the membrane potential. Biophys J. 2007;93:4197–208. doi: 10.1529/biophysj.106.095794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patil PG, Brody DL, Yue DT. Preferential closed-state inactivation of neuronal calcium channels. Neuron. 1998;20:1027–38. doi: 10.1016/S0896-6273(00)80483-3. [DOI] [PubMed] [Google Scholar]
- 46.Chen J, Avdonin V, Ciorba MA, Heinemann SH, Hoshi T. Acceleration of P/C-type inactivation in voltage-gated K(+) channels by methionine oxidation. Biophys J. 2000;78:174–87. doi: 10.1016/S0006-3495(00)76583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomez-Lagunas F. Quinidine interaction with Shab K+ channels: pore block and irreversible collapse of the K+ conductance. J Physiol. 2010;588:2691–706. doi: 10.1113/jphysiol.2010.193128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arias-Olguin II, Carrillo E, Meza-Torres B, Barriga-Montoya C, Balleza D, Gomez-Lagunas F. Induction of a fast inactivation gating on delayed rectifier Shab K(+) channels by the anti-inflammatory drug celecoxib. Channels (Austin) 2011;5:56–64. doi: 10.4161/chan.5.1.13972. [DOI] [PubMed] [Google Scholar]
- 49.Zagotta WN, Hoshi T, Aldrich RW. Shaker potassium channel gating. III: Evaluation of kinetic models for activation. J Gen Physiol. 1994;103:321–62. doi: 10.1085/jgp.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bähring R, Covarrubias M. Mechanisms of closed-state inactivation in voltage-gated ion channels. J Physiol. 2011;589:461–79. doi: 10.1113/jphysiol.2010.191965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 52.Yeola SW, Rich TC, Uebele VN, Tamkun MM, Snyders DJ. Molecular analysis of a binding site for quinidine in a human cardiac delayed rectifier K+ channel. Role of S6 in antiarrhythmic drug binding. Circ Res. 1996;78:1105–14. doi: 10.1161/01.RES.78.6.1105. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Zhu B, Yao JA, Tseng GN. Differential effects of S6 mutations on binding of quinidine and 4-aminopyridine to rat isoform of Kv1.4: common site but different factors in determining blockers’ binding affinity. J Pharmacol Exp Ther. 1998;287:332–43. [PubMed] [Google Scholar]
- 54.Caballero R, Pourrier M, Schram G, Delpón E, Tamargo J, Nattel S. Effects of flecainide and quinidine on Kv4.2 currents: voltage dependence and role of S6 valines. Br J Pharmacol. 2003;138:1475–84. doi: 10.1038/sj.bjp.0705199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang S, Morales MJ, Qu YJ, Bett GC, Strauss HC, Rasmusson RL. Kv1.4 channel block by quinidine: evidence for a drug-induced allosteric effect. J Physiol. 2003;546:387–401. doi: 10.1113/jphysiol.2002.029512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bett GC, Rasmusson RL. Inactivation and recovery in Kv1.4 K+ channels: lipophilic interactions at the intracellular mouth of the pore. J Physiol. 2004;556:109–20. doi: 10.1113/jphysiol.2003.055012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Islas LD, Sigworth FJ. Voltage sensitivity and gating charge in Shaker and Shab family potassium channels. J Gen Physiol. 1999;114:723–42. doi: 10.1085/jgp.114.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]